Abstract
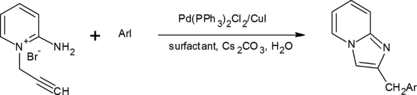
The reaction of 2-amino-1-(2-propynyl)pyridinium bromide with various iodobenzenes, catalyzed by Pd-Cu, in the presence of sodium lauryl sulfate as the surfactant and cesium carbonate as the base, in water, leads to the formation of 2-substituted imidazo[1,2-α]pyridines.
INTRODUCTION
The Sonogashira reaction cocatalyzed by palladium and copper is a powerful and straightforward method for the construction of arylated internal alkyne compounds,[ Citation 1 ] which have a C(sp2)–C(sp) bond and are important intermediates in organic synthesis, including natural products,[ Citation 2 ] biologically active molecules,[ Citation 3 ] molecular electronics,[ Citation 4 ] and polymers.[ Citation 5 ] The original Sonogashira reaction was generally performed in the presence of large amounts of palladium and copper(I) iodide as cocatalyst in organic solvents, which were economically and environmentally malignant. This protocol has recently been improved by several modifications such as reaction in aqueous media, in ionic liquid, or under microwave irradiation[ Citation 6 ] and the use of promoters (Zn, Mg, Sn) or effective ligands.[ Citation 7 ] However, in most of the catalytic processes, organic solvents are usually employed as the reaction media, often creating a great deal of safety, health, and environmental issues because of their flammability, toxicity, and volatility. From an economic and environmental standpoint, it is desirable to avoid any use of hazardous and expensive organic solvents. The use of water or aqueous solution represents one of the most economically and environmentally viable alternatives to organic solvents formetal-catalyzed reactions.[ Citation 8 ] Several examples of Pd-catalyzed Sonogashira reactions in aqueous media have been reported.[ Citation 9 ]
In continuation of our recent studies[ Citation 10 ] on the Pd-catalyzed reaction of acetylenes leading to heterocyclic compounds of biological significance, we became interested in developing a synthetic route to substituted imidazo[1,2-α]pyridines[ Citation 11 ] in water.
RESULTS AND DISCUSSION
In this communication, we report that treatment of 2-amino-1-(2-propynyl) pyridinium bromide 1 [ Citation 11 ] with aryl halides 2a–i in water and in the presence of bis(triphenylphosphine)palladium chloride(II), copper iodide, sodium laurylsulfate, and cesium carbonate at 60 °C. The 2-substituted imidazo[1,2-α]pyridines 3a–i were obtained in moderate to high yields (Scheme , Table ). The reactions were carried out under an argon atmosphere, and water was degassed prior to use.
Table 1. Yields of 2-benzylimidazo[1,2-α]pyridines Footnote a
For optimization of the reaction conditions, we chose the reaction of 2-amino-1-(2-propynyl) pyridinium bromide 1 with 2-iodo-5-nitrotuluene 2a as the model reaction, and the effects of the base, surfactant, and catalyst on the reaction were examined. First, several bases were screened for the reaction in the presence of a catalytic amount of Pd(PPh3)2Cl2. As shown in Table , the reaction is significantly influenced by the base employed. The reaction works very well when inorganic bases such as K2CO3 and Cs2CO3 are used (entries 2 and 3 in Table ), with the best result obtained in the case of cesium carbonate as the base (entry 3 in Table ).
Table 2. Effect of base on the heterocyclization during Sonogashira coupling of compound with 2-iodo-5-nitrotoluene in waterFootnote a
The influences of the amounts of copper(I) iodide, surfactant, and the catalyst were investigated using reaction of compound 1 with 2a. The results are shown in Table . Increasing the amount of the palladium catalyst could shorten the reaction time but does not increase the yield (entry 3). Low palladium concentration often prolonged the reaction time and decreased the yield (entry 1).
Table 3. Effects of catalyst, cocatalyst, and surfactant on the heterocyclization during Sonogashira coupling of compound with 2-iodo-5-nitritoluene in waterFootnote a
The use of surfactant is also critical for the success of the reaction: without a surfactant/phase-transfer reagent, the yield dropped from 95% to 10% (compare entries 2 and 8). We also found that with an increase in the amount of surfactant, the reaction yield did not increase (entry 7). Low surfactant concentration often decreased the yield (entry 6). No reaction was observed when either Cu(II) alone or Pd(II) alone was used as the catalyst (entries 4 and 5).
Subsequently, the reaction of a variety of aryl iodides 2a–i and 2-amino-1-(2-propynyl) pyridinium bromide 1 were studied under the optimal conditions. As shown in Table , the presence of electron-withdrawing groups such as NO2, Cl, and CN on the aryl iodide seems to be essential. When iodobenzene or p-iodoanisole was used as the aryl iodide, Sonogashira coupling could not be achieved.
In conclusion, we have developed a successful palladium-catalyzed reaction for the synthesis of 2-aryl-substituted imidazo[1,2-α]pyridines in the presence of sodium lauryl sulfate as the surfactant and cesium carbonate as the base in water.
EXPERIMENTAL
General Procedure for Synthesis of 2-Substituted Imidazo[2,1-α]Pyridines 3a–i
A mixture of the aryl iodide (1 mmol), (PPh3)2PdCl2 (3 mol%), CuI (7 mol%), sodium lauryl sulfate (7 mol%), and cesium carbonate (3 mmol) was stirred in water (5 mL) at 60 °C for 30 min under an argon atmosphere. 2-Amino-1-(2-propynyl)pyridinium bromide[ Citation 11 ] (1 mmol) was then added, and the mixture was stirred at 60 °C for 10 h. After completion of the reaction, the resulting solution was concentrated in vacuo, and the crude product was subjected to silica-gel column chromatography using CHCl3–CH3OH (95:5) as eluent to afford the pure product (Table ).
Data
Compound 3a
Mp 262–263 °C (lit.[ Citation 11 ] 263–264 °C); 1H NMR (500 MHz, DMSO-d6): δ = 2.35 (s, 3H, CH3), 4.28 (s, 2H, CH2), 7.00–8.02 (m, 7H, PyH, ArH), 8.58 (s, 1H, CH of imidazole); 13C NMR (125 MHz, DMSO-d6): δ = 20.03, 33.95, 114.86, 116.46, 121.75, 121.97, 125.30, 128.06, 130.83, 131.38, 133.05, 136.21, 139.13, 146.35, 147.86; IR, ν (KBr disc): 1520, 1340 cm−1; MS m/z 267 (M+). Anal. calcd. for C15H13N3O2: C, 67.40; H, 4.90; N, 15.72. Found: C, 66.88; H, 4.74; N, 15.89.
Compound 3b
Mp 251–252 °C (lit.[ Citation 11 ] 250–251 °C); 1H NMR (500 MHz, DMSO-d6): δ = 4.46 (s, 2H, CH2), 6.98–8.00 (m, 8H, PyH, ArH), 8.66 (s, 1H, CH of imidazole); 13C NMR (125 MHz, DMSO-d6): δ = 34.25, 114.60, 116.24, 122.85, 123.15, 129.10, 130.22, 130.95, 132.15, 134.27, 136.23, 139.12, 146.25, 148.33; IR, ν (KBr disc): 1510, 1340 cm−1; MS m/z 253 (M+). Anal. calcd. for C14H11N3O2: C, 66.40; H, 4.38; N, 16.59. Found: C, 66.12; H, 4.11; N, 16.65.
Compound 3c
Mp 293–294 °C (lit.[ Citation 11 ] 294–295 °C); 1H NMR (500 MHz, DMSO-d6): δ = 4.29 (s, 2H, CH2), 6.97–8.32 (m, 8H, PyH, ArH), 8.58 (s, 1H, CH of imidazole); 13C NMR (125 MHz, DMSO-d6): δ = 33.92, 114.35, 116.55, 122.30, 127.95, 128.26, 130.15, 130.90, 133.17, 133.86, 135.86, 138.60, 146.20, 147.31; IR, ν (KBr disc): 1500, 1335 cm−1; MS m/z 253 (M+). Anal. calcd. for C14H11N3O2: C, 66.40; H, 4.38; N, 16.59. Found: C, 66.51; H, 4.42; N, 16.37.
Compound 3d
Mp 255–257 °C (lit.[ Citation 11 ] 256–257 °C); 1H NMR (500 MHz, DMSO-d6): δ = 4.31 (s, 2H, CH2), 6.96–8.31 (m, 8H, PyH, ArH), 8.65 (s, 1H, CH of imidazole); 13C NMR (125 MHz, DMSO-d6): δ = 34.21, 114.60, 116.76, 123.25, 128.30, 131.10, 131.87, 132.90, 135.82, 138.86, 145.27, 147.32; IR, ν (KBr disc): 1510, 1340 cm−1; MS m/z 253 (M+). Anal. calcd. for C14H11N3O2: C, 66.40; H, 4.38; N, 16.59. Found: C, 66.22; H, 4.26; N, 16.41.
Compound 3e
Mp 232–233 °C (lit.[ Citation 11 ] 231–232 °C); 1H NMR (500 MHz, DMSO-d6): δ = 4.41 (s, 2H, CH2), 6.94–8.32 (m, 7H, PyH, ArH), 8.56 (s, 1H, CH of imidazole); 13C NMR (125 MHz, DMSO-d6): δ = 33.81, 114.16, 116.32, 123.30, 128.36, 130.04, 130.64, 131.24, 132.43, 134.27, 137.46, 138.87, 144.30, 148.68; IR, ν (KBr disc): 1510, 1350 cm−1; MS m/z 287 (M+). Anal. calcd. for C14H10ClN3O2: C, 58.45; H, 3.50; N, 14.61. Found: C, 58.30; H, 3.41; N, 14.50.
Compound 3f
Mp 245–246 °C (lit.[ Citation 11 ] 246–247 °C); 1H NMR (500 MHz, DMSO-d6): δ = 4.25 (s, 2H, CH2), 6.95–8.03 (m, 7H, PyH, ArH), 8.59 (s, 1H, CH of imidazole); 13C NMR (125 MHz, DMSO-d6): δ = 33.75, 114.37, 116.86, 123.80, 126.58, 127.72, 128.23, 130.85, 131.47, 132.36, 135.30, 137.65, 141.11, 148.15, IR, ν (KBr disc): 1510, 1345 cm−1; MS m/z 287 (M+). Anal. calcd. for C14H10ClN3O2: C, 58.45; H, 3.50; N, 14.61. Found: C, 58.26; H, 3.41; N, 14.27.
Compound 3g
Mp 256–257 °C; 1H NMR δ (500 MHz, DMSO-d 6): δ = 4.24 (s, 2H, CH2), 6.80–8.11 (m, 7H, ArH), 8.55 (s, 1H, CH of imidazole); 13C NMR (125 MHz, DMSO-d6): δ = 33.80, 114.21, 116.67, 123.09, 125.97, 127.20, 128.32, 129.98, 131.70, 132.12, 135.15, 136.98, 140.23, 149.02, IR, ν (KBr disc): 1510, 1340 cm−1; MS m/z 287 (M+). Anal. calcd. for C14H10ClN3O2: C, 58.45; H, 3.50; N, 14.61. Found: C, 58.34; H, 3.44; N, 14.47.
Compound 3h
Mp 236–237 °C (lit.[ Citation 11 ] 236–237 °C); 1H NMR (500 MHz, DMSO-d6): δ = 2.51 (s, 3H, CH3), 4.22 (s, 2H, CH2), 6.96–7.87 (m, 8H, PyH, ArH), 8.60 (s, 1H, CH of imidazole); 13C NMR (125 MHz, DMSO-d6): δ = 27.49, 34.20, 114.33, 116.12, 127.51, 129.26, 130.09, 130.77, 133.27, 135.98, 137.35, 138.49, 145.30, 198.26; IR, ν (KBr disc): 1690 cm−1; MS m/z 250 (M+). Anal. calcd. for C16H14N2O: C, 76.78; H, 5.64; N, 11.19. Found: C, 76.60; H, 5.46; N, 11.25.
Compound 3i
Mp 261–262 °C (lit.[ Citation 11 ] 260–261 °C); 1H NMR (500 MHz, DMSO-d6): δ = 3.81 (s, 3H, CH3), 4.24 (s, 2H, CH2), 7.01–8.32 (m, 8H, PyH, ArH), 8.64 (s, 1H, CH of imidazole); 13C NMR (125 MHz, DMSO-d6): δ = 33.89, 51.76, 114.54, 116.28, 128.36, 129.15, 130.05, 130.68, 132.84, 136.98, 138.08, 139.31, 148.93, 167.35; IR, ν (KBr disc): 1710 cm−1; MS m/z 266 (M+). Anal. calcd. for C16H14N2O2: C, 72.16; H, 5.30; N, 10.52. Found: C, 71.74; H, 5.45; N, 10.30.
ACKNOWLEDGMENTS
The authors thank the Research Council of Shahrood University of Technology for the support of this work.
Notes
a Reaction conditions: 1 (1 mmol), 2a–h (1 mmol), Cs2CO3 (3 mmol), Pd(PPh)3Cl2 (3 mol%), CuI (7 mol%), Sodium laurylsulfate (7 mol%), and degassed water (5 mL) at 60 °C for 10 h.
a Reaction conditions: 1 (1 mmol), 2a (1 mmol), base (3 mmol), Pd(PPh)3Cl2 (3 mol%), CuI (7 mol%), sodium laurylsulfate (7 mol%), and degassed water (5 mL) at 60 °C for 10 h.
b Isolated yields.
a Reaction conditions: 1 (1 mmol), 2a (1 mmol), Cs2CO3 (3 mmol), and degassed water (5 mL) at 60 °C for 10 h.
b Isolated yields.
c Reaction time: 12 h.
d Reaction time: 7 h.
REFERENCES
- (a) Sonogashira , K. ; Tohda , Y. ; Hagihara , N. A convenient synthesis of acetylenes: Catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes, and bromopyridines . Tetrahedron Lett. 1975 , 16 , 4467 – 4470 ; (b) Sonogashira , K. ; Yatake , T. ; Tohda , Y. ; Takahashi , S. ; Hagihara , N. Novel preparation of σ-alkynyl complexes of transition metals by copper(I) iodide-catalysed dehydrohalogenation . J. Chem. Soc., Chem. Commun. 1977 , 9 , 291 ; (c) Takahashi , S. ; Kuroyama , Y. ; Sonogashira , K. ; Hagihara , N. A convenient synthesis of ethynylarenes and diethynylarenes . Synthesis . 1980 , 627 .
- (a) Nicolaou , K. C. ; Dai , W. M. Chemistry and biology of the enediyne anticancer antibiotics . Angew. Chem., Int. Ed. Engl. 1991 , 30 , 1387 – 1416 ; (b) Paterson , I. ; Davies , R. D. M. ; Marquez , R. Total synthesis of the callipeltoside aglycon . Angew. Chem., Int. Ed. 2001 , 40 , 603 – 607 .
- (a) Kort , M. ; Correa , V. ; Valentijn , A. R. P. M. ; Marel , G. A. ; Potter , B. V. L. ; Taylor , C. W. ; Boom , J. H. Synthesis of potent agonists of the D-myo-inositol 1,4,5-trisphosphate receptor based on clustered disaccharide polyphosphate analogues of adenophostin . A. J. Med. Chem. 2000 , 43 , 3295 – 3303 ; (b) Cosford , N. D. P. ; Tehrani , L. ; Roppe , J. ; Schweiger , E. ; Smith , N. D. ; Anderson , J. ; Bristow , L. ; Brodkin , J. ; Jiang , X. ; McDonald , I. ; Rao , S. ; Washburn , M. ; Varney , M. A. 3-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]-pyridine: A potent and highly selective metabotropic glutamate subtype 5 receptor antagonist with anxiolytic activity . J. Med. Chem. 2003 , 46 , 204 – 206 .
- (a) Brunsveld , L. ; Meijer , E. W. ; Prince , R. B. ; Moore , J. S. Self-assembly of folded m-phenylene ethynylene oligomers into helical columns. J. Am. Chem. Soc. 2001, 123, 7978–7984; (b) Mongin , O. ; Porres , L. ; Moreaux , L. ; Mertz , J. ; Blanchard-Desce , M. Synthesis and photophysical properties of new conjugated fluorophores designed for two-photon-excited fluorescence. Org. Lett. 2002, 4, 719–722.
- (a) Mongin , O. ; Papamicaël , C. ; Hoyler , N. ; Gossauer , A. Modular synthesis of benzene-centered porphyrin trimers and a dendritic porphyrin hexamer . J. Org. Chem. 1998 , 63 , 5568 – 5580 ; (b) Tobe , Y. ; Utsumi , N. ; Nagano , A. ; Naemura , K. Synthesis and association behavior of [4.4.4.4.4.4]metacyclophanedodecayne derivatives with interior binding groups . Angew. Chem., Int. Ed. 1998 , 37 , 1285 – 1287 ; (c) Onitsuka , K. ; Fujimoto , M. ; Ohshiro , N. ; Takahashi , S. Convergent route to organometallic dendrimers composed of platinum-acetylide units . Angew. Chem., Int. Ed. 1999 , 38 , 689 – 692 .
- (a) Genet , J. P. ; Blart , E. ; Savignac , M. Palladium-catalyzed cross-coupling reactions in a homogeneous aqueous medium . Synlett 1992 , 715 ; (b) Kabalka , G. W. ; Wang , L. ; Namboodiri , V. ; Pagni , R. M. Rapid microwave-enhanced, solventless Sonogashira coupling reaction on alumina . Tetrahedron Lett. 2000 , 41 , 5151 – 5154 ; (c) Fukuyama , T. ; Shinmen , M. ; Nishitani , S. ; Sato , M. ; Ryu , I. A copper-free sonogashira coupling reaction in ionic liquids and its application to a microflow system for efficient catalyst recycling . Org. Lett. 2002 , 4 , 1691 – 1694 .
- (a) Powell , N. A. ; Rychnovsky , S. D. Iodide acceleration in the Pd-catalyzed coupling of aromatic 1,2-ditriflates with alkynes: Synthesis of enediynes . Tetrahedron Lett. 1996 , 37 , 7901 – 7904 ; (b) Crisp , G. T. ; Turner , P. D. ; Stephens , K. A. Palladium-catalysed coupling of terminal alkynes with aryl halides aided by catalytic zinc . J. Organomet. Chem. 1998 , 570 , 219 – 224 ; (c) Nakamura , K. ; Okubo , H. ; Yamaguchi , M. Low temperature Sonogashira coupling reaction . Synlett . 1999 , 549 ; (d) Choudary , B. M. ; Madhi , S. ; Chowdari , N. S. ; Kantam , M. L. ; Sreedhar , B. Layered double hydroxide supported nanopalladium catalyst for Heck-, Suzuki-, Sonogashira-, and Stille-type coupling reactions of chloroarenes . J. Am. Chem. Soc. 2002 , 124 , 14127 – 14136 ; (e) Köllhofer , A. ; Pullmann , T. ; Plenio , H. A versatile catalyst for the Sonogashira coupling of aryl chlorides . Angew. Chem., Int. Ed. 2003 , 42 , 1056 – 1058 ; (f) Faller , J. W. ; Kultyshev , R. G. ; Parr , J. Alkynyl germatranes as alternative reagents for the preparation of biarylethynes . Tetrahedron Lett. 2003 , 44 , 451 – 453 .
- (a) Lubineau , A. ; Auge , J. In Modern Solvents in Organic Synthesis ; P. Knochel (Ed.); Springer : Berlin , 1999 ; (b) Cornils, B. Aqueous-Phase Organometallic Catalysis: Concepts and Applications; W. A. Herrmann (Eds.).; Wiley-VCH: Weinheim, 1998.
- (a) Casalnuovo , A. L. ; Calabrese , J. C. Palladium-catalyzed alkylations in aqueous media . J. Am. Chem. Soc. 1990 , 112 , 4324 – 4330 ; (b) DeVasher , R. B. ; Moore , L. R. ; Shaughnessy , K. H. Aqueous-phase, palladium-catalyzed cross-coupling of aryl bromides under mild conditions, using water-soluble, sterically demanding alkylphosphines . J. Org. Chem. 2004 , 69 , 7919 – 7927 ; (c) Anderson , K. W. ; Buchwald , S. L. General catalysts for the Suzuki-Miyaura and Sonogashira coupling reactions of aryl chlorides and for the coupling of challenging substrate combinations in water . Angew. Chem., Int. Ed. 2005 , 44 , 6173 – 6177 ; (d) Mio , M. J. ; Kopel , L. C. ; Braun , J. B. ; Gadzikwa , T. L. ; Hull , K. L. ; Brisbois , R. G. ; Markworth , C. J. ; Grieco , P. A. One-pot synthesis of symmetrical and unsymmetrical bisarylethynes by a modification of the Sonogashira coupling reaction . Org. Lett. 2002 , 4 , 3199 – 3202 ; (e) Bumagin , N. A. ; Sukhomlinova , L. I. ; Luzikova , E. V. ; Tolstaya , T. P. ; Beletskaya , I. P. Catalytic coupling of terminal acetylenes with iodoarenes and diaryliodonium salts in water . Tetrahedron Lett. 1996 , 37 , 897 – 900 ; (f) Bhattacharya , S. ; Sengupta , S. Palladium catalyzed alkynylation of aryl halides (Sonogashira reaction) in water . Tetrahedron Lett. 2004 , 45 , 8733 – 8736 ; (g) López-Deber , M. P. ; Castedo , L. ; Granja , J. R. Synthesis of N-(3-arylpropyl)amino acid derivatives by Sonogashira types of reaction in aqueous media . Org. Lett. 2001 , 3 , 2823 – 2826 ; (h) Raju , S. ; Mukkanti , K. ; Annamalai , P. ; Pal , M. Facile synthesis of substituted thiophenes via Pd/C-mediated sonogashira coupling in water . Bioorg. Med. Chem. Lett. 2006 , 16 , 6185 – 6189 .
- (a) Heravi , M. M. ; Keivanloo , A. ; Rahimizadeh , M. ; Bakavoli , M. ; Ghassemzadeh , M. Pd–Cu catalyzed heterocyclization during Sonogashira coupling: Synthesis of 3-benzylthiazolo[3,2-a]benzimidazole . Tetrahedron Lett. 2004 , 45 , 5747 – 5749 ; (b) Heravi , M. M. ; Kivanloo , A. ; Rahimzadeh , M. ; Bakavoli , M. ; Ghassemzadeh , M. ; Neumüller , B. Regioselective synthesis of 6-benzylthiazolo-[3,2-b]1,2,4-triazoles during Sonogashira coupling . Tetrahedron Lett. 2005 , 46 , 1607 – 1610 ; (c) Heravi, M. M. ; Keivanloo, A. ; Rahimizadeh, M. ; Bakavoli, M. ; Ghassemzadeh, M. Synthesis of thiazolo[3,2-d][1,2,4] triazines through palladium-catalyzed heteroannulation of acetylenic compounds . J. Heterocycl. Chem. 2007 , 44 , 1 – 3 .
- Bakherad , M. ; Isfahani , H. N. ; Keivanloo , A. ; Doostmohammadi , N. Pd-Cu catalyzed heterocyclization during Sonogashira coupling: Synthesis of 2-benzylimidazo[1,2-α]pyridine . Tetrahedron Lett. 2008 , 49 , 3819 – 3822 .