Abstract
4-Aryl-3-cyano-2,5-dihydro-1H-indeno[1,2-b]pyridin-2-one and 4-aryl-3-cyano-1,2,5,6-tetrahydrobenzo[h]quinolin-2-one have been prepared from indan-1-one or 3,4-dihydronaphthalen-1(2H)-one, aromatic aldehydes, 2-cyanoacetamide, in the presence of sodium hydroxide under solvent-free conditions. The rapid and facile method produced products in high yields.
There has been a gradual change from classical reaction conditions to more environmentally friendly routes.[ Citation 1 ] This growth of green chemistry holds significant potential for reduction of the by-products, waste production and lowering of energy costs. As one of efficient synthetic methods of green chemistry, solvent-free synthesis is now used in a lot of chemical transformations for the synthesis of various compounds.[ Citation 2 ]The most evident improvements are reduced reaction times and cleaner reactions because of fewer side products.
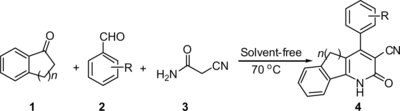
By virtue of the convergence of productivity, facile execution, and generally high yield of products, one-pot multicomponent reactions (MCRs) have attracted considerable attention from the point of view of ideal synthesis.[ Citation 3 ] However, if the one-pot MCRs could be carried out under solvent-free conditions, it would be the most efficient synthetic methods of organic synthesis. As part of our continued interest[ Citation 4 ] in the development of facile methods for the synthesis of organic compounds, herein we report a very simple and highly efficient method for the synthesis of 4-aryl-3-cyano-2,5-dihydro-1H-indeno[1,2-b]pyridin-2-one and 4-aryl-3-cyano-1,2,5,6-tetrahydro-benzo[h]quinolin-2-one derivatives via a three-component cyclocondensation reaction under solvent-free conditions.
Substituted 3-cyano-2-pyridones derivatives are important intermediates in the pharmaceutical, dye, and photo industries. Synthesis of these compounds have been widely studied using conventional heating in the presence of various catalysts and usually in polar solvents.[ Citation5-7 ] However, in reported literature, the organic solvent was necessary. In our recent research, we found these compounds also could be synthesized under solvent-free conditions. In the typical experimental procedure, indan-1-one, aromatic aldehydes, and 2-cyanoacetamide were mixed thoroughly with 0.2 g sodium hydroxide. The reaction mixture was heated at 70 °C for about 10–15 min and the 4-aryl-3-cyano-2,5-dihydro-1H-indeno[1,2-b]pyridin-2-one was afforded in excellent yields. To expand this reaction, 3,4-dihydronaphthalen-1(2H)-one was chosen to react with aromatic aldehydes and 2-cyanoacetamide under similar conditions. The reactions also could be carried out smoothly, and 4-aryl-3-cyano-1,2,5,6-tetrahydrobenzo[h ]quinolin-2-one could be obtained with high yields (Scheme ).
Scheme 1 The reaction of indan-1-one or 3,4-dihydronaphthalen-1(2H)-one, aromatic aldehydes, and 2-cyano-acetamide.
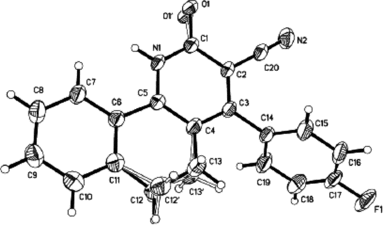
All structures of the compounds were confirmed from IR, 1H NMR, and elemental analyses. The x-ray diffraction analysis of crystal 4 h [ 8 ]further confirmed the structures (Fig. 1). Our observations are recorded in Table .
Table 1. The reaction results for compounds
In conclusion, we have demonstrated a novel, one-pot, three-component reaction under solvent-free conditions that offers a facile and efficient route for the synthesis of 4-aryl-3-cyano-2,5-dihydro-1H-indeno[1,2-b]pyridin-2-one and 4-aryl-3-cyano-1,2,5,6-tetrahydro-benzo[h]quinolin-2-one derivatives. This method has some advantages, such as shorter reaction times, milder conditions, simplicity of the reaction, and good product yields.
EXPERIMENTAL
Melting points were uncorrected. IR spectra were recorded on a Tensor 27 spectrometer in KBr. 1H NMR spectra were obtained in DMSO-d 6 solution with Me4Si as internal standard using a Bruker-400 spectrometer. Elemental analyses were carried out using Perkin-Elmer 240 II analyzer. X-ray diffraction was measured on a Siemens P4 diffractometer.
General Procedure for the Syntheses of 4-Aryl-3-cyano-2,5-dihydro-1H-indeno[1,2-b]pyridin-2-one and 4-Aryl-3-cyano-1,2,5,6-tetrahydro-benzo[h]quinolin-2-one
The general procedure is represented as follows: indan-1-one (or 3,4-dihydronaphthalen-1(2H)-one) 1 (2 mmol), aromatic aldehyde 2 (2 mmol), 2-cyanoacetamide 3 (2 mmol), and NaOH (5 mmol) were added to a flask at 70 °C. The reaction could be completed within 10–15 min, and the reaction mixture was poured into water. The product was filtered, dried, and recrystallized from 25–30 mL 95% ethanol.
Data
Compound 4a
Mp > 300 °C; IR (KBr) v: 3241 (NH), 2222 (CN), 1636 (CO) cm−1; 1H NMR (400 MHz, DMSO-d 6) (δ, ppm): 3.66 (2H, s, CH2), 7.50–7.53 (2H, m, ArH), 7.63 (3H, t, J = 7.8, 8.4 Hz, ArH), 7.70 (2H, d, J = 7.8 Hz, ArH), 8.17 (1H, d, J = 2.0 Hz, ArH), 13.65 (1H, s, NH). Anal. calcd. for C19H11BrN2O: C, 62.83; H, 3.05; N, 7.71. Found: C, 62.62; H, 3.10; N, 7.87.
Compound 4b
Mp > 300 °C; IR (KBr) v: 3287 (NH), 2221 (CN), 1636 (CO) cm−1; 1H NMR (400 MHz, DMSO-d 6) (δ, ppm): 3.67 (2H, s, CH2), 7.50 (2H, t, J = 3.6, 3.6 Hz, ArH), 7.63 (1H, d, J = 6.0 Hz, ArH), 7.67 (4H, t, J = 8.4, 8.4 Hz, ArH), 7.17 (1H, d, J = 6.0 Hz, ArH), 13.64 (1H, s, NH). Anal. calcd. for C19H11FN2O: C, 75.49; H, 3.67; N, 9.27. Found: C, 75.60; H, 3.57; N, 9.34.
Compound 4c
Mp > 300 °C; IR (KBr) v: 3337 (NH), 2222 (CN), 1698 (CO) cm−1; 1H NMR (400 MHz, DMSO-d 6) (δ, ppm): 3.50 (2H, dd, J = 22.4 Hz, J = 22.4 Hz, CH2), 7.54 (2H, t, J = 3.6. 3.6 Hz, ArH), 7.58 (2H, br, ArH), 7.62 (2H, t, J = 4.0, 4.0 Hz, ArH), 7.72 (1H, d, J = 7.2 Hz, ArH), 8.20 (1H, d, J = 4.0 Hz, ArH), 13.68 (1H, s, NH). Anal. calcd. for C19H11ClN2O: C, 71.59; H, 3.48; N, 8.79. Found: C, 71.39; H, 3.56; N, 8.84.
Compound 4d
Mp > 300 °C; IR (KBr) v: 3296 (NH), 2219 (CN), 1637 (CO) cm−1; 1H NMR (400 MHz, DMSO-d 6) (δ, ppm): 3.68 (2H, s, CH2), 7.53 (2H, t, J = 5.6. 5.6 Hz, ArH), 7.64 (1H, d, J = 5.6 Hz, ArH), 7.70 (4H, dd, J = 8.4 Hz, J = 8.4 Hz, ArH), 8.19 (1H, d, J = 5.6 Hz, ArH), 13.68 (1H, s, NH). Anal. calcd. for C19H11ClN2O: C, 71.59; H, 3.48; N, 8.79. Found: C, 71.36; H, 3.58; N, 8.86.
Compound 4e
Mp > 300 °C; IR (KBr) v: 3291 (NH), 2223 (CN), 1635 (CO) cm−1; 1H NMR (400 MHz, DMSO-d 6) (δ, ppm): 4.13 (2H, s, CH2), 7.50–7.54 (1H, m, ArH), 7.52 (2H, t, J = 6.4, 6.4 Hz, ArH), 7.84 (2H, t, J = 6.4, 6.4 Hz, ArH), 7.89 (1H, d, J = 6.8 Hz, ArH), 8.00 (1H, s, ArH), 13.85 (1H, s, NH). Anal. calcd. for C19H10Cl2N2O: C, 64.61; H, 2.85; N, 7.93. Found: C, 64.91; H, 2.75; N, 7.83.
Compound 4f
Mp > 300 °C; IR (KBr) v: 3339 (NH), 2219 (CN), 1658 (CO) cm−1; 1H NMR (400 MHz, DMSO-d 6) (δ, ppm): 3.71 (2H, s, CH2), 7.54 (2H, t, J = 6.0 Hz, J = 7.2 Hz, ArH), 7.66 (1H, d, J = 7.2 Hz, ArH), 7.69 (1H, d, J = 8.4 Hz, ArH), 7.89 (1H, d J = 8.0 Hz, ArH), 8.04 (1H, d, J = 7.6 Hz, ArH), 8.19–8.21 (1H, br, ArH), 13.76 (1H, s, NH). Anal. calcd. for C19H10Cl2N2O: C, 64.61; H, 2.85; N, 7.93. Found: C, 64.47; H, 2.91; N, 7.86.
Compound 4g
Mp > 300 °C; IR (KBr) v: 3292 (NH), 2219 (CN), 1637 (CO) cm−1; 1H NMR (400 MHz, DMSO-d 6) (δ, ppm): 3.72 (2H, s, CH2), 3.87 (3H, s, OCH3), 7.14 (2H, t, J = 8.8, 8.8 Hz, ArH), 7.52 (2H, t, J = 3.6, 3.6 Hz, ArH), 7.66 (1H, m, ArH), 7.89 (2H, d, J = 8.8 Hz, ArH), 8.17 (1H, br, ArH), 13.59 (1H, s, NH). Anal. calcd. for C20H14N2O2: C, 76.42; H, 4.49; N, 8.91. Found: C, 76.62; H, 4.41; N, 8.80.
Compound 4h
Mp > 300 °C; IR (KBr) v: 3131 (NH), 2220 (CN), 1634 (CO) cm−1; 1H NMR (400 MHz, DMSO-d 6) (δ, ppm): 2.33 (2H, br, CH2), 2.74 (2H, t, J = 7.2, 7.2 Hz, CH2), 7.35 (1H, d, J = 6.4 Hz, ArH), 7.39–7.46 (4H, m, ArH), 7.52 (2H, dd, J = 5.2 Hz, J = 5.6 Hz, ArH), 8.07 (1H, br, ArH), 12.77 (1H, s; NH). Anal. calcd. for C20H13FN2O: C, 75.94; H, 4.14; N, 6.01. Found: C, 75.63; H, 4.07; N, 6.12.
Compound 4i
Mp > 300 °C; IR (KBr) v: 3124 (NH), 2221 (CN), 1638 (CO) cm−1; 1H NMR (400 MHz, DMSO-d 6) (δ, ppm): 2.36 (2H, t, J = 6.0, 6.0 Hz, CH2), 2.76 (2H, t, J = 6.8, 6.8 Hz, CH2), 7.35 (1H, d, J = 6.8 Hz, ArH), 7.43 (4H, dd, J = 8.4 Hz, J = 8.4 Hz, ArH), 7.78 (2H, d, J = 8.8 Hz, ArH), 8.07 (1H, d, J = 7.6 Hz, ArH), 12.62 (1H, s, NH). Anal. calcd. for C20H13BrN2O: C, 63.68; H, 3.47; N, 7.43. Found: C, 63.40; H, 3.51; N, 7.54.
Compound 4j
Mp > 300 °C; IR (KBr) v: 3132 (NH), 2221 (CN), 1638 (CO) cm−1; 1H NMR (400 MHz, DMSO-d 6) (δ, ppm): 2.37 (2H, br, CH2), 2.76 (2H, t, J = 6.8, 6.8 Hz, CH2), 7.35 (1H, d, J = 7.2 Hz, ArH), 7.44 (4H, dd, J = 8.0 Hz, J = 8.0 Hz, ArH), 7.78 (2H, d, J = 8.4 Hz, ArH), 8.07 (1H, d, J = 7.6 Hz, ArH), 12.66 (1H, s, NH). Anal. calcd. for C20H13ClN2O: C, 72.18; H, 3.94; N, 8.42. Found: C, 72.40; H, 3.92; N, 8.52.
Compound 4k
Mp > 300 °C; IR (KBr) v: 3130 (NH), 2223 (CN), 1637 (CO) cm−1; 1H NMR (400 MHz, DMSO-d 6) (δ, ppm): 2.37 (2H, br, CH2), 2.76 (2H, t, J = 6.8, 6.8 Hz, CH2), 7.35 (1H, d, J = 7.2 Hz, ArH), 7.43 (2H, dd, J = 7.2 Hz, J = 7.2 Hz, ArH), 7.49 (2H, d, J = 8.0 Hz, ArH), 7.64 (2H, d, J = 8.4 Hz, ArH), 8.07 (1H, d, J = 7.6 Hz, ArH), 12.62 (1H, s, NH). Anal. calcd. for C20H13ClN2O: C, 72.18; H, 3.94; N, 8.42. Found: C, 72.40; H, 3.85; N, 8.55.
Compound 4l
Mp 287–289 °C; IR (KBr) v: 3123 (NH), 2218 (CN), 1635 (CO) cm−1; 1H NMR (400 MHz, DMSO-d 6) (δ, ppm): 2.28 (1H, t, J = 7.2, 6.8 Hz, CH2), 2.38 (1H, t, J = 6.8, 7.2 Hz, CH2), 2.75 (2H, t, J = 6.8 Hz, J = 8.0 Hz, CH2), 7.23–7.30 (1H, m, ArH), 7.35–7.48 (3H, m, ArH), 7.69–7.85 (2H, m, ArH), 8.03–8.09 (1H, m, ArH), 12.54 (1H, s, NH). Anal. calcd. for C20H12Cl2N2O: C, 65.41; H, 3.29; N, 7.63. Found: C, 65.66; H, 3.22; N, 7.51.
Compound 4m
Mp > 300 °C; IR (KBr) v: 3128 (NH), 2221 (CN), 1634 (CO) cm−1; 1H NMR (400 MHz, DMSO-d 6) (δ, ppm): 2.37 (2H, br, CH2), 2.40 (3H, s, CH3), 2.76 (2H, t, J = 7.2, 7.2 Hz, CH2), 7.31 (2H, d, J = 8.4 Hz, ArH), 7.36 (3H, d, J = 8.4 Hz, ArH), 7.43 (2H, t, J = 6.4 Hz, J = 7.2 Hz, ArH), 8.07 (1H, d, J = 8.4 Hz, ArH), 12.65 (1H, s, NH). Anal. calcd. for C21H16N2O: C, 80.75; H, 5.16; N, 8.97. Found: C, 80.36; H, 5.21; N, 8.87.
Compound 4n
Mp > 300 °C; IR (KBr) v: 3120 (NH), 2219 (CN), 1636 (CO) cm−1; 1H NMR (400 MHz, DMSO-d 6) (δ, ppm): 2.41 (2H, t, J = 6.0, 7.2 Hz, CH2), 2.75 (2H, t, J = 6.0, 7.2 Hz, CH2), 3.84 (3H, s, CH3O), 7.11 (2H, d, J = 8.8 Hz, ArH), 7.34 (1H, d, J = 6.8 Hz, ArH), 7.38 (3H, d, J = 8.8 Hz, ArH), 7.43 (1H, t, J = 7.2, 7.2 Hz, ArH), 8.06 (1H, d, J = 7.2 Hz, ArH), 12.58 (1H, s, NH). Anal. calcd. for C21H16N2O2: C, 76.81; H, 4.91; N, 8.53. Found: C, 76.55; H, 4.86; N, 8.62.
ACKNOWLEDGMENTS
We thank the Natural Science Foundation of Jiangsu Education Department (No. 08KJB150017), the National Natural Science Foundation of China (No. 20772103), and the PeiYu Foundation (No. 07PYL06) of Xuzhou Normal University for financial support.
REFERENCES
- (a) Varma , R. S. Clay and clay-supported reagents in organic synthesis . Tetrahedron 2002 , 58 , 1235 – 1255 ; (b) Tanaka, K. Solvent-Free Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2003 .
- Tanaka , K. ; Toda , F. Solvent-free organic synthesis. Chem. Rev. 2000, 100, 1025–1074.
- (a) Weber , L. ; Illegen , K. ; Almstetter , M. Discovery of new multi component reactions with combinatorial methods . Synlett 1999 , 366 – 374 ; (b) Armstrong , R. W. ; Combs , A. P. ; Tempest , P. A. ; Brown , S. D. ; Keating , T. A. Multiple-component condensation strategies for combinatorial library synthesis . Acc. Chem. Rev. 1996 , 29 , 123 – 131 .
- (a) Rong , L. C. ; Li , X. Y. ; Wang , H. Y. ; Shi , D. Q. ; Tu , S. J. An efficient and facile synthesis of 2-amino-4,6-diarylbenzene-1,3-dicarbonitrile and 1,2-dihydro-2-oxo-4,6-diarylpyridine-3-carbonitrile under solvent-free conditions . Chem. Lett. 2006 , 35 , 1314 – 1315 ; (b) Rong , L. C. ; Wang , H. Y. ; Shi , J. W. ; Yang , F. ; Yao , H. ; Tu , S. J. ; Shi , D. Q. An efficient and facile procedure for the synthesis of 4,6-Diaryl-2(1H)-pyridones under solvent-free conditions . J. Heterocycl. Chem. 2007 , 44 ( 10 ), 1505 – 1508 ; (c) Rong , L. C. ; Han , H. X. ; Yang , F. ; Yao , H. ; Jiang , H. ; Tu , S. J. An efficient one-pot synthesis of 2-amino-4,6-diarylbenzene-1,3-dicarbonitrile under solvent-free conditions . Synth. Commun. 2007 , 37 ( 21 ), 3767 – 3772 .
- Siedel , M. C. ; Viste , K. L. ; Yih , R. Y. Plant growth inhibition with N-aryl-pyrid-2-ones. U. S. Patent 3,761,240, September 25 , 1973 .
- Misic-Vukovic , M. ; Mijin , D. ; Radojkovic-Velickovic , M. ; Valentic , N. ; Krstic , V. Condensation of 1,3-diketones with cyanoacetamide: 4,6-Disubstituted-3-cyano-2-pyridones . J. Serb. Chem. Soc. 1998 , 63 ( 8 ), 585 – 599 .
- Paulvannan , K. ; Chen , T. Solid-phase synthesis of 1,2,3,4-tetrahydro-2-pyridones via aza-annulation of enamines . J. Org. Chem. 2000 , 65 , 6160 – 6166 ; (b) Dave , C. G. ; Shah , D. A. ; Agrawal , Y. K. A simple and convenient synthesis of 4,6-disubstituted 3-cyanopyridin-2(1H)-ones under solvent-free microwave conditions . Ind. J. Chem. 2004 , 43B ( 4 ), 885 – 887 ; (c) Alberola , A. ; Calvo , L. A. ; Ortega , A. G. ; Ruiz , M. C. S. ; Yustos , P. Regioselective synthesis of 2(1H)-pyridinones from â-aminoenones and malononitrile: Reaction mechanism . J. Org. Chem. 1999 , 64 , 9493 – 9498 .
- X-ray crystallography for 4h: empirical formula C20H13FN2O, Fw = 316.32, T = 298(2) K, triclinic, space group p–1, a = 8.116 (10) Å, b = 9.278 (12) Å, c = 11.263 (14) Å, α = 98.674 (19)°, β = 105.095 (17)°, γ = 104.846 (18)°, V = 769.7 (16) Å3, Z = 2, Dcalcd. = 1.365 Mg/m3, λ (MoK) = 0.71073 Å, µ = 0.094 mm−1, F(000) = 328. 2.34° < θ < 25.001°, R = 0.0482, wR = 0.1149, s = 0.997, largest diff. peak and hole: 0.149 and −0.168 e Å−3.