Abstract
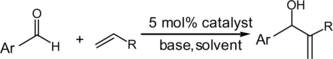
A novel and efficient catalytic system of the Morita–Baylis–Hillman (MBH) reaction between aromatic aldehyde and activated alkenes has been developed. The novel system of a combination of Sc(OTf)3 and 3-hydroxyquinuclidine (3-HQD) showed a high catalytic activity for the MBH reaction.
INTRODUCTION
The Morita–Baylis–Hillman (MBH) reaction,[ Citation1-6 ] an important reaction, has been added to the list of the useful carbon–carbon bond-forming reactions. Since the MBH reaction possesses the two most important requirements, atom economy and generation of functional groups, it qualifies to be in the list of efficient synthetic reactions.[ Citation 7 ] However, the major drawback of the MBH reaction is its slow reaction rate (e.g., the reaction of tert-butyl acrylate with benzaldehyde using 10 mol% DABCO catalyst takes 28 days to complete).[ Citation 8 ] So, it was very significant to develop a novel efficient synthesis for the reaction. A number of physical and chemical methods have been developed to accelerate the notoriously slow MBH reaction.[ Citation 9 ] For example, the Aggarwal group has found that the use of Sc(OTf)3 (5 mol%) in the MBH reaction with 1,4-dimethylaminopyridine (DABCO) (100 mol%) as the catalyst resulted in an acceleration of the reaction [Krel (DABCO) = 3.3], but the yield can increase up to only 11.6% after 24 h when the reaction was conducted using tert-butyl acrylate and benzaldehyde.[ Citation 10 ] Then, after a few years, they found that 3-HQD with GnCl can also caused an increase in the rate of the reaction in water [Krel (DABCO) = 9.5]. In contrast to not using Lewis acid, no additional rate acceleration was observed with Sc(OTf)3 in water.[ Citation 11 ] Herein, we report anovel and efficient condition for the MBH reaction using Sc(OTf)3 and 3-HQD as cocatalysts and dimethylformamide (DMF) as the solvent.
RESULTS AND DISCUSSION
To establish the reaction conditions, we first examined the reaction of p-nitrobenzaldehyde and methyl acrylate in CH3CN and investigated the effect of different amine catalysts on reaction rate as shown in Scheme , and the results are summarized in Table . We found that no reaction occurred when using pyridine as a Lewis base (Table , entry3). As expected, no product was observed after stirring for 24 h at 20 °C when Sc(OTf)3 alone was employed as the catalyst. DMAP has been compared with imidazole and found to be superior in the reaction between p-nitrobenzaldehyde and methyl acrylate in CH3CN. We found that DMAP was indeed better than imidazole, but with reactions conducted neat, 3-HQD gave the highest yield and fastest rate. Therefore, we selected 3-HQD as the base in MBH reaction for further investigation.
Table 1. Effect of base on the MBH reactionFootnote a
It is well-known that the MBH reaction is affected by solvents. Auge has shown that the MBH reaction between acrylonitrile and various aldehydes can be accelerated in water.[ Citation 12 ] However, they had chosen to study reactions involving acrylonitrile, as this is already a relatively fast-reacting substrate in the MBH reaction. Besides this, we also focused on slower reacting substrates: acrylates. We found that water did not work well in these reactions, but to our delight, the use of DMF as a solvent allowed for a dramatic increase in the yield of the MBH reaction (Table , entries 4–10). Also, we found that raising the temperature accelerated the reaction and led to increase in yield. The optimal reaction temperature for the reaction was 40 °C (Table , entries 11 and 12); higher reaction temperature did not significantly improve the result.
Table 2. MBH reactions of p-nitrobenzaldehyde (1.0 equiv.) with methyl acrylate (3.0 equiv.) in the presence of Sc(OTf)3 and 3-HQD
Based on these results, the reaction was best conducted in DMF at 40 °C with 5 mol% of catalyst in a 1:4 molar ratio of Sc(OTf)3 to 3-HQD. Naturally, with optimized conditions in hand, we examined a set of aromatic aldehydes coupled with activated alkenes (methyl acrylate, ethyl acrylate, and acrylonitrile). The experimental results are listed in Table .
Table 3. Sc(OTf)3/3-HQD cocatalyzed the MBH reactionFootnote a
As shown in Table , all aromatic aldehydes were converted to their corresponding MBH products in moderate to high yields in reaction times as short as 10 min. It is worth mentioning that the system was tested synthetically on various substrates and found to give good rate accelerations with aromatic aldehydes with acceptor groups and lower rate accelerations with aromatic aldehydes with donor groups. Notable examples from Table include a fast reaction with 2-furaldehyde and acrylonitrile (entry 17) and a very rapid reaction with p-nitrobenzaldehyde (entry 11). Acrylamide is not a suitable substrate with 3-HQD; no reaction was observed with it.
CONCLUSIONS
In conclusion, we have developed a new condition that accelerates the MBH reaction. The novel catalytic system of a combination of Sc(OTf)3 and 3-HQD showed high catalytic activity for the MBH reaction.
EXPERIMENTAL
All chemicals and resin were obtained from commercial suppliers and were used without further purification. Infrared (IR) spectra were recorded on a Perkin-Elmer 983 Fourier transform infrared (FT-IR) spectrometer, and 1H NMR spectra were made on a Bruker Avance DMX 300 instrument. Mass spectral (MS) analyses were performed on an HP-5973 spectrometer.
General Procedure for the Synthesis of 1–17
Aromatic aldehyde (1 mmol) was mixed with activated alkenes (3 mmol) in a 25-mL flask. Sc(OTf)3 (5 mol%), 3-HQD (20 mol%), and DMF (4 mL) were added to the mixture. The vessel was immersed in a preheated oil bath and stirred vigorously until thin-layer chromatography (TLC) and/or gas chromatography (GC) of the crude reaction mixture indicated that the aromatic aldehyde had been completely consumed. The reaction mixture was allowed to cool to room temperature, Et2O was added to thereaction mixture, and the organic phase was separated. The filtratewas concentrated, and the resulting residue was purified by flash chromatography (hexane–ethyl acatate) to provide the desired product.
Data
Methyl 2-[1-Hydroxy-1-(4-nitrophenyl)Methyl]Acrylate (Table , Entry 1)
A yellowish oil; 1H NMR (CDCl3, 300 MHz): δ 3.46 (brs, 1H), 3.67 (s, 3H), 5.57 (d, H, J = 6.52 Hz), 5.84 (s, 1H), 6.33 (s, 1H), 7.50 (d, 2H, J = 8.52 Hz), 8.12 (d, 2H, J = 8.51 Hz); 13C NMR (CDCl3, 75 MHz): δ51.86, 72.1, 123.25, 126.86, 127.02, 140.63, 147.04, 148.34, 166.02; IR (CHCl3) 3490, 1715 cm−1.
Ethyl 2-[1-Hydroxy-1-(4-nitrophenyl)Methyl]Acrylate (Table , Entry 2)
A yellowish oil; 1H NMR (CDCl3, 300 MHz): δ 1.32 (t, 3H, J = 7.14 Hz), 3.46 (brs, 1H), 4.24 (q, 2H, J = 7.14 Hz), 5.67 (d, H, J = 6.15 Hz), 5.91 (s, 1H), 6.45 (s, 1H), 7.63 (d, 2H, J = 8.64 Hz), 8.25 (d, 2H, J = 8.64 Hz); 13C NMR (CDCl3, 75 MHz): δ 13.63, 60.60, 65.36, 72.02, 123.17, 126.49, 140.94, 146.93, 148.59, 165.54; IR (CHCl3) 3485, 1720 cm−1.
2-[1-Hydroxy-1-(4-nitrophenyl)Methyl]Acrylonitrile (Table , Entry3)
A yellowish oil; 1H NMR (CDCl3, 300 MHz): δ 2.98 (brs, 1H), 5.38 (d, 1H, J = 6.3 Hz), 6.04 (s, 1H), 6.13 (s, 1H), 7.54 (d, 2H, J = 8.40 Hz), 8.19 (d, 2H, J = 8.40 Hz); 13C NMR (CDCl3, 75 MHz): δ 72.85, 116.02, 123.70, 125.05, 127.04, 130.78, 145.82, 147.60; IR (CHCl3) 3480, 1720 cm−1.
Methyl 2-[1-Hydroxy-1-(2,4-dichlorophenyl)Methyl]Acrylate (Table ,Entry 4)
A colorless oil; 1H NMR (CDCl3, 300 MHz): δ3.52 (brs, 1H), 3.76 (s, 1H), 5.58 (d, 1H, J = 6.87 Hz), 5.89 (s, 1H), 6.33 (s, 1H), 7.26 (d, 1H, J = 8.07 Hz), 7.36 (s, 1H), 7.48 (d, 1H, J = 8.31 Hz); 13C NMR (CDCl3, 75 MHz): δ 51.86, 68.54, 126.78, 128.82, 133.07, 136.63, 139.88, 144.66, 166.50; IR (CHCl3) 3490, 1715 cm−1.
Ethyl 2-[1-Hydroxy-1-(2,4-dichlorophenyl)Methyl]Acrylate (Table , Entry 5)
A colorless oil; 1H NMR (CDCl3, 300 MHz): δ 1.30 (t, 3H, J = 6.40 Hz), 3.58 (brs, 1H), 4.19 (q, 2H, J = 6.40 Hz), 5.19 (d, 1H, J = 5.80 Hz), 5.51 (s, 1H), 6.11 (s, 1H), 7.07 (d, 1H, J = 8.31 Hz), 7.11 (s, 1H), 7.21 (d, 1H, J = 8.31 Hz); 13C NMR (CDCl3, 75 MHz): δ 13.67, 60.85, 65.13, 68.36, 126.94, 128.79, 133.67, 140.27, 142.85, 165.30; IR (CHCl3) 3485, 1720 cm−1.
2-[1-Hydroxy-1-(2,4-dichlorophenyl)Methyl]Acrylonitrile (Table , Entry 6)
A colorless oil; 1H NMR (CDCl3, 300 MHz): δ 3.12 (brs, 1H), 5.70 (s, 1H), 6.07 (s, 1H), 6.08 (s, 1H), 7.35 (d, 1H, J = 7.74 Hz), 7.41 (s, 1H), 7.57 (d, 1H, J = 7.74 Hz); 13C NMR (CDCl3, 75 MHz): δ 69.75, 117.32, 123.64, 127.59, 128.58, 131.29, 134.62, 139.81; IR (CHCl3) 3480, 1720cm−1.
Methyl 2-[1-Hydroxy-1-(4-chlorophenyl)Methyl]Acrylate (Table , Entry 7)
A colorless oil; 1H NMR (CDCl3, 300 MHz): δ 3.11 (brs, 1H), 3.74 (s, 1H), 5.52 (d, 1H, J = 5.58 Hz), 5.83 (s, 1H), 6.34 (s, 1H), 7.28 (d, 2H, J = 8.41 Hz), 7.33 (d, 2H, J = 8.37 Hz); 13C NMR (CDCl3, 75 MHz): δ51.74, 72.38, 120.64, 127.61, 129.71, 132.12, 138.41, 144.65, 165.11; IR (CHCl3) 3490, 1715 cm−1.
Ethyl 2-[1-Hydroxy-1-(4-chlorophenyl)Methyl]Acrylate (Table , Entry 8)
A colorless oil; 1H NMR (CDCl3, 300 MHz): δ1.27 (t, 3H, J = 7.07 Hz), 3.34 (brs, 1H), 4.16 (q, 2H, J = 6.96 Hz), 5.51 (d, 1H, J = 5.37 Hz), 5.83 (s, 1H), 6.34 (s, 1H), 7.31 (d, 2H, J = 8.47 Hz), 7.38 (d, 2H, J = 8.47 Hz);13C NMR (CDCl3, 75 MHz): 1720 cm−1.
2-[1-Hydroxy-1-(4-chlorophenyl)Methyl]Acrylonitrile (Table , Entry 9)
A colorless oil; 1H NMR (CDCl3, 300 MHz): δ 3.57 (brs, 1H), 5.20 (d, 1H, J = 6.74 Hz), 5.99 (s, 1H), 6.05 (s, 1H), 7.27 (d, 2H, J = 8.41 Hz), 7.34 (d, 2H, J = 8.37 Hz); 13C NMR (CDCl3, 75 MHz): δ 72.96, 116.48, 125.50, 127.59, 128.69, 130.26, 134.27, 137.33; IR (CHCl3) 3480, 1720 cm−1.
Methyl 2-[1-Hydroxy-1-(phenyl) Methyl] Acrylate (Table , Entry 10)
A colorless oil; 1H NMR (CDCl3, 300 MHz): δ 3.19 (brs, 1H), 3.71 (s, 3H), 5.54 (d, 1H, J = 6.18 Hz), 5.83 (s, 1H), 6.32 (s, 1H), 7.27–7.33 (m, 5H); 13C NMR (CDCl3, 75 MHz): δ 51.63, 72.79, 125.76, 126.27, 127.49, 128.08, 140.93, 141.60, 166.42; IR (CHCl3) 3490, 1715 cm−1.
Ethyl 2-[1-Hydroxy-1-(phenyl)Methyl]Acrylate (Table , Entry 11)
A colorless oil; 1H NMR (CDCl3, 300 MHz): δ 2.96 (brs, 1H), 5.30 (d, 1H, J = 5.10 Hz), 6.09 (s, 1H), 6.14 (s, 1H), 6.35 (d, 1H, J = 4.98 Hz), 6.37 (d, 1H, J = 4.72 Hz), 7.39 (d, 1H, J = 4.50 Hz); 13C NMR (CDCl3, 75 MHz): δ 74.31, 108.34, 110.47, 117.25, 123.61, 131.36, 143.20, 154.61; IR (CHCl3) 3485, 1720 cm−1.
2-[1-Hydroxy-1-(phenyl)Methyl]Acrylonitrile (Table , Entry 12)
A colorless oil; 1H NMR (CDCl3, 300 MHz): δ 2.62 (brs, 1H), 5.52 (d, 1H, J = 5.36 Hz), 6.01 (s, 1H), 6.09 (s, 1H), 7.36–7.41 (m, 5H); 13C NMR (CDCl3, 75 MHz): δ73.85, 116.59, 125.86, 126.20, 128.62, 128.65, 129.51, 138.83; IR (CHCl3) 3480, 1720 cm−1.
Methyl 2-[1-Hydroxy-1-(furyl)Methyl]Acrylate (Table , Entry 13)
A colorless oil; 1H NMR (CDCl3, 300 MHz): δ 3.31 (brs, 1H), 3.73 (s, 3H), 5.56 (d, 1H, J = 4.92 Hz), 5.93 (s, 1H), 6.22 (s, 1H), 6.30 (d, 1H, J = 5.12 Hz), 6.36 (d, 1H, J = 4.99 Hz), 7.34 (d, 1H, J = 5.31 Hz); 13C NMR (CDCl3, 75 MHz): δ 52.31, 74.10, 108.29, 110.69, 122.74, 140.31, 154.28; IR (CHCl3) 3490, 1715 cm−1.
Ethyl 2-[1-Hydroxy-1-(furyl)Methyl]Acrylate (Table , Entry 14)
A colorless oil; 1H NMR (CDCl3, 300 MHz): δ 1.23 (t, 3H, J = 7.17 Hz), 3.70 (brs, 1H), 4.16 (q, 2H, J = 7.18 Hz), 5.55 (d, 1H, J = 6.01 Hz), 5.93 (d, 1H, J = 2.67 Hz), 6.18 (d, 1H, J = 3.21 Hz), 6.26 (d, 1H, J = 2.83 Hz), 6.33 (d, 1H, J = 2.71 Hz), 7.31 (d, 1H, J = 2.05 Hz); 13C NMR (CDCl3, 75 MHz): δ13.62, 60.60, 66.29, 106.74, 109.98, 125.90, 139.50, 141.86, 153.98, 165.65; IR (CHCl3) 3485, 1720 cm−1.
2-[1-Hydroxy-1-(furyl)Methyl]Acrylonitrile (Table , Entry 15)
A colorless oil; 1H NMR (CDCl3, 300 MHz): δ 2.96 (brs, 1H), 5.30 (d, 1H, J = 5.10 Hz), 6.09 (s, 1H), 6.14 (s, 1H), 6.35 (d, 1H, J = 4.98 Hz), 6.37 (d, 1H, J = 4.72 Hz), 7.39 (d, 1H, J = 4.50 Hz); 13C NMR (CDCl3, 75 MHz): δ 74.31, 108.34, 110.47, 117.25, 123.61, 131.36, 143.20, 154.61; IR (CHCl3) 3480, 1720 cm−1.
Methyl 2-[1-Hydroxy-1-(4-methoxyphenyl)Methyl]Acrylate (Table , Entry 16)
A colorless oil; 1H NMR (CDCl3, 300 MHz): δ 2.91 (brs, 1H), 3.76 (s, 3H), 3.84 (s, 3H), 5.57 (d, 1H, J = 4.79 Hz), 5.88 (s, 1H), 6.36 (s, 1H), 6.90 (d, 2H, J = 8.64 Hz), 7.32 (d, 2H, J = 8.58 Hz); 13C NMR (CDCl3, 300 MHz): δ 52.30, 55.91, 74.21, 114.35, 122.96, 128.32, 133.62, 140.67, 159.30, 167.38; IR (CHCl3) 3490, 1715 cm−1.
Ethyl 2-[1-Hydroxy-1-(4-methoxyphenyl)Methyl]Acrylate (Table , Entry 17)
A colorless oil; 1H NMR (CDCl3, 300 MHz): δ 1.23 (t, 3H, J = 7.14 Hz), 3.24 (brs, 1H), 3.85 (s, 3H), 4.14 (q, 2H, J = 6.93 Hz), 5.50 (d, 1H, J = 6.20 Hz), 5.85 (s, 1H), 6.31 (s, 1H), 6.86 (d, 2H, J = 8.61 Hz), 7.27 (d, 2H, J = 8.58 Hz); 13C NMR (CDCl3, 75 MHz): δ 13.7, 54.89, 60.53, 72.36, 113.41, 124.99, 127.57, 133.21, 142.06, 158.81, 166.02; IR (CHCl3) 3485, 1720 cm−1.
ACKNOWLEDGMENTS
The authors thank the National Natural Science Foundation of China (Grant No. 20872001) and the Natural Science Foundation of Education Administration of Anhui Province (KJ2008A064).
Notes
a Reaction conditions: 3.0 mmol of methyl acrylate, 1.0 mmol of p-nitrobenzaldehyde, 100 mmol% of Lewis base using CH3CN as solvent.
b Isolated yield based on p-nitrobenzaldehyde.
a Isolated yield based on p-nitrobenzaldehyde.
a Reaction conditions: 3.0 mmol of α,β-unsaturated ester, 1.0 mmol of aromatic aldehydes, 5 mmol% of Sc(OTf)3, and 20 mmol% of Lewis base using DMF as solvent.
b Isolated yield based on aromatic aldehyde.
REFERENCES
- Baylis , A. B. ; Hillman , M. E. D. German Patent 2155113, 1972 . Chem. Abstr. 1972 , 77 , 34174q .
- Carey , F. A. ; Sundberg , R. J. Advanced Organic Chemistry, , 3rd ed. ; Plenum : New York , 1990 ; parts A & B.
- March , J. Advanced Organic Chemistry, , 4th ed. ; Wiley : New York , 1992 .
- Ciganek , E. Organic Reactions ; L. A. Paquette ( Ed. ); Wiley : New York , 1997 ; vol. 51 .
- Trost , B. M. ; Fleming , I. ( Eds. ). Comprehensive Organic Synthesis ; Pergamon : New York , 1991 ; vols. 1–9 .
- Hassner , A. ; Stumer , C. Organic Synthesis based on Name Reactions and Unnamed Reactions ; Tetrahedron Organic Chemistry Series; J. E. Baldwin , P. D. Magnus ( Eds.); Pergamon : New York , 1998 ; vol. 11.
- Basavaiah , D. ; Rao , A. J. ; Satyanarayana , T. Recent advances in the Baylis–Hillman reaction and applications . Chem. Rev. 2003 , 103 , 811 – 892 .
- Fort , Y. ; Berthe , M. C. ; Caubere , P. The Baylis–Hillman reaction mechanism and applications revisited . Tetrahedron 1992 , 48 , 6371 – 6384 .
- For reviews, see (a) Basavaiah , D. ; Rao , P. D. ; Hyma , R. S. The Baylis–Hillman reaction: A novel carbon–carbon bond forming reaction . Tetrahedron 1996 , 52 , 8001 – 8062 ; (b) Drewes , S. E. ; Roos , G. H. P. Synthetic potential of the tertiary-amine-catalysed reaction of activated vinyl carbanions with aldehydes . Tetrahedron 1988 , 44 , 4653 – 4670 ; (c) Langer , P. New strategies for the development of an asymmetric version of the Baylis–Hillman reaction . Angew. Chem., Int. Ed. 2000 , 39 , 3049 – 3052 .
- Aggarwal , V. K. ; Mereu , A. ; Tarver, G. J. McCague , R. Metal- and ligand-accelerated catalysis of the Baylis–Hillman reaction . J. Org. Chem. 1998 , 63 , 7183 – 7189 .
- Aggarwal , V. K. ; Dean , D. K. ; Mereu, A: Williams , R. Rate acceleration of the Baylis–Hillman reaction in polar solvents (water and formamide): Dominant role of hydrogen bonding, not hydrophobic effects, is implicated . J. Org. Chem. 2002 , 67 , 510 – 514 .
- Augé , J. ; Lubin , N. ; Lubineau , A. Acceleration in water of the Baylis–Hillman reaction . Tetrahedron Lett. 1994 , 35 , 7947 – 7948 .