Abstract
A green, efficient, and large-scale method for tetrahydropyranylation of alcohols in the presence of a catalytic amount of pyridinium chloride at room temperature under solvent-free conditions is reported.
INTRODUCTION
Protection of hydroxy groups plays an essential role in multistep organic synthesis including natural products and biologically active compounds. Among the protecting groups for protection of alcohols, tetrahydropyranylation is one of the most frequently used processes for protecting hydroxyl groups, because tetrahydropyrane (THP) ethers are less expensive, easy to deprotect, and stable enough under strong basic media such as Grignard and alkyl lithium reagents, oxidative conditions, and alkylating and acylating reagents.[ Citation 1 ] A variety of reagents have been reported for tetrahydropyranylation of alcohols including using protic acids,[ Citation 2 ] Lewis acids,[ Citation 3 ] ion-exchange resins,[ Citation 4 ] LiOTf,[ Citation 5 ] K5CoW12O4 · 3H2O,[ Citation 6 ] CuSO4 · 5H2O,[ Citation 7 ] In(OTf)3,[ Citation 8 ] PdCl2(CH3CN)2,[ Citation 9 ] ZnCl2,[ Citation 10 ] I2,[ Citation 11 ] silica chloride,[ Citation 12 ] tetrabutylammonium tribromide,[ Citation 13 ] zirconium sulfophenyl phosphonate,[ Citation 14 ] ionic liquids,[ Citation 15 ] zeolites,[ Citation 16 ] and K-10 clay.[ Citation 17 ]
Although some of these methods are convenient protocols for tetrahydropyranylation of alcohols with good to high yields, the majority of these methods suffer from at least one disadvantage, such as stronglyacidic conditions, long reaction times, high temperature, poor selectivity, expensive reagents, toxicity, and need for excessive amounts of reagents. Therefore, there is still demand for the introduction of mild and chemoselective methods for this purpose.
RESULTS AND DISCUSSION
In recent years, there has been an increasing interest on reactions that proceed in the absence of solvent because of the reduced pollution, low cost, and straightforward workup.[ Citation 18 ] In continuation of our effort to develop new methods in organic transformations,[ Citation18–21 ] herein we report an efficient method for tetrahydropyranylation of alcohols in the presence of a catalytic amounts of pyridinium chloride (20 mol%) and DHP. The reaction was carried out easily at room temperature under solvent-free conditions (Scheme ).
To optimize the reaction conditions, initially we tried the conversion of benzyl alcohol (2 mmol) to the corresponding THP ether with pyridinium chloride (0.4 mmol, 0.05 g) and DHP (2.4 mmol) in various solvents and also grinding under solvent-free conditions at room temperature. As shown in Table , in comparison to conventional methods, the yields of the reaction under solvent-free conditions are higher and the reaction time is shorter. To evaluate the effect of pyridinium chloride in this reaction, we did observe that the reaction in the absence of pyridinium chloride did not occur at all even for longer reaction time and refluxing conditions. We believed that pyridinium chloride has a dual effect in this procedure (ionic liquid and acid catalyst).
Table 1. Conversion of benzyl alcohol to the corresponding THP ether under different conditions in the presence of pyridinium chloride
To examine the possibility of a larger scale reaction, we mixed the alcohols (50 mmol), 3,4-dihydro-2H-pyran (60 mmol), and a catalytic amount of pyridinium chloride (2 mmol%) in a mortar and ground with a pestle for the time specified in Table to produce the corresponding THP ether. Using this catalyst, a variety of alcohols including primary, benzylic, and allylic alcohols underwent tetrahydropyranylation in good to excellent yields and reasonable reaction times (Table ). Even with a bulky molecule like benzhydrol (entry 2l), the reactions were fast. As shown in Table , because of the low amount catalyst and mild reaction conditions, allylic alcohols were also converted to their corresponding tetrahydropyranylation ethers in good yields without formation of any elimination product or isomerization of the double bond (entry 14). The reaction is clean, and the catalyst also can be used several times without losing its activity.
Table 2. Tetrahydropyranylation of alcohols using pyridinium chloride at room temperature under solvent-free conditionsFootnote a
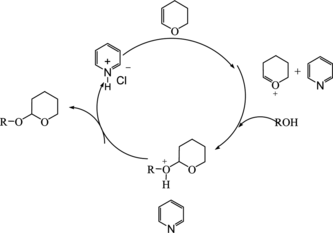
Based on our results, the possible mechanism is shown in Scheme .
CONCLUSION
In conclusion, we have developed a simple, large-scale, and efficient method for tetrahydropyranylation of various alcohols using pyridinium chloride. The mild reaction conditions, short reaction times, good to high yields, low cost, easy preparation, easy handling of pyridinium chloride as ionic liquid and acid catalyst, straightforward isolation of product and catalyst, and reusability of catalyst are the advantages of this method.
EXPERIMENTAL
General
All starting materials were purchased from Merck and Aldrich and used without further purification. All yields refer to isolated products after purification. Products were characterized by comparison with authentic samples and by spectroscopic data (IR, 1H NMR and mass spectroscopy). 1H NMR spectra were recorded at 300 MHz. The spectra were measured in CDCl3 unless otherwise stated, relative to tetramethylsilane (TMS) (0.00 ppm).
General Procedure for Tetrahydropyranylation of Alcohols
Pyridinium chloride (2 mmol, 0.23 g) was added to a mixture of alcohols (10 mmol) and DHP (12 mmol, 1.0 g) in a mortar and was ground with a pestle for the time specified in Table . The progress of the reaction was followed by thin-layer chromatography (TLC). After disappearance of the starting alcohols, the reaction mixture was treated with diethyl ether (2 × 70 ml), and after vigorous stirring, the solids were filtered through a sintered glass funnel. The solvent was evaporated under vacuum. The crude THP ether was purified through a short column of silica gel (EtOAc–cyclohexane, 1:4) to obtain the pure products.
ACKNOWLEDGMENT
We gratefully acknowledge the funding support received for this project from the Isfahan University of Technology (IUT), IR Iran (ARH), and Grant GM 33138 (AER) from the National Institutes of Health, USA. Further financial support from Center of Excellence in Sensor and Green Research (IUT) is gratefully acknowledged.
Notes
a The reaction was carried out in 5 ml of solvent.
b Yields based on the isolated products.
a Yields based on the isolated pure products after chromatography and confirmed by comparison with authentic samples (TLC, IR, NMR) and mass spectroscopy).
REFERENCES
- (a) Green , T. W. ; Wuts , P. G. M. Protective Groups in Organic Synthesis , , 3rd ed. ; John Wiley : New York , 1999 ; 306 ; (b)Caine, D.; Deutsch, H. Total synthesis of (−)-axisonitrile-3: An application of the reductive ring opening of vinylcyclopropanes. J. Am. Chem. Soc. 1978, 100, 8031; (c) Isobe, M.; Iio, H.; Kawai, T.; Goto, T. Synthesis of sesquiterpene antitumor lactones, 2: A new stereocontrolled total synthesis of (±)-vernolepin. J. Am. Chem. Soc. 1978, 100, 1942 .
- (a) Boom , J. H. V. ; Herschied , J. D. M. ; Reese , C. B. p-Toluenesulfonic acid as a catalyst for the rapid tetrahydropyranylation and methoxytetrahydropyranylation of steroidal alcohols . Synthesis 1973 , 167 ; (b) Miyashita , M. ; Yoshikoshi , A. ; Grieco , P. A. Pyridinium p-toluenesulfonate: A mild and efficient catalyst for the tetrahydropyranylation of alcohols . J. Org. Chem. 1977 , 42 , 3772 ; (c) Shimizu , K. ; Hayashi , E. ; Hatamachi , T. ; Kodama , T. ; Kitayama , Y. SO3H-functionalized silica for acetalization of carbonyl compounds with methanol and tetrahydropyranylation of alcohols . Tetrahedron Lett. 2004 , 45 , 5135 .
- (a) Tamami , B. ; Parvanak , K. Chemoselective tetrahydropyranylation of alcohols and phenols using polystyrene supported aluminium chloride as a catalyst . Tetrahedron Lett. 2004 , 45 , 715; (b) Namboodiri , V. V. ; Varma , R. S. Solvent-free tetrahydropyranylation (THP) of alcohols and phenols and their regeneration by catalytic aluminum chloride hexahydrate . Tetrahedron Lett. 2002 , 43 , 1143 ; (c) Babu , B. S. ; Balasubramanian , K. Mild and efficient tetrahydropyranylation of alcohols—Catalysis by lithium perchlorate in diethyl ether . Tetrahedron Lett. 1998 , 39 , 9287 .
- (a) Bongini , A. ; Cardillo , G. ; Orena , M. ; Sandri , S. A simple and practical method for tetrahydropyranylation of alcohols and phenols . Synthesis 1979 , 618 ; (b) Olah , G. A. ; Husain , A. ; Singh , B. P. Catalysis by solid superacids, 191: Simplified and improved polymeric perfluorinated resin aulfonic acid (Nafion-H) catalyzed protection—deprotection . Synthesis 1983 , 892 .
- Karimi , B. ; Maleki , J. Lithium triflate (LiOTf)–catalyzed efficient and chemoselective tetrahydropyranylation of alcohols and phenols under mild and neutral reaction conditions . Tetrahedron Lett. 2002 , 43 , 5353 .
- Habibi , M. H. ; Tangestaninejad , S. ; Mohamadpoor , I. ; Mirkhani , V. ; Yadollahi , B. Potassium dodecatangestocobaltate trihydrate (K5CoW12O40 · 3H2O): A mild and efficient catalyst for the tetrahydropyranylation of alcohols and their detetrahydropyranylation . Tetrahedron Lett. 2001 , 42 , 2851 .
- Khan , A. T. ; Choudhury , L. H. ; Ghosh , S. Cupric sulfate pentahydrate (CuSO4 · 5H2O): A mild and efficient catalyst for tetrahydropyranylation/depyranylation of alcohols and phenols . Tetrahedron Lett. 2004 , 45 , 7891 .
- Mineno , T. A fast and practical approach to tetrahydropyranylation and depyranylation of alcohols using indium triflate . Tetrahedron Lett. 2002 , 43 , 7975 .
- Wang , Y. G. ; Wu , X. X. ; Jiang , Z. Y. A mild and efficient selective tetrahydropyranylation of primary alcohols and deprotection of THP ethers of phenols and alcohols using PdCl2 (CH3CN)2 as catalyst . Tetrahedron Lett. 2004 , 45 , 2973 .
- Ranu , B. C. ; Saha , M. A simple, efficient, and selective method for tetrahydropyranylation of alcohols on a solid phase of alumina impregnated with zinc chloride . J. Org. Chem. 1994 , 59 , 8269 .
- Deka , N. ; Sarma , J. C. Microwave-mediated selective monotetrahydropyranylation of symmetrical diols catalyzed by iodine . J. Org. Chem. 2001 , 66 , 1947 .
- Ravindranath , N. ; Ramesh , C. ; Das , B. Simple, facile, and highly selective tetrahydropyranylation of alcohols using silica chloride . Synlett 2001 , 1777 .
- Naik , S. ; Gopinath , R. ; Patel , B. K. Tetrabutylammoniumtribromide (TBATB)–promoted tetrahydropyranylation/depyranylation of alcohols . Tetrahedron Lett. 2001 , 42 , 7679
- Curini , M. ; Epifano , F. ; Marcotullio , M. C. ; Rosati , O. Zirconium sulfophenyl phosphonate as a heterogeneous catalyst in tetrahydropyranylation of alcohols and phenols . Tetrahedron Lett. 1998 , 39 , 8159 .
- Branco , L. C. ; Afonso , C. A. M. Ionic liquids as recyclable reaction media for the tetrahydropyranylation of alcohols. Tetrahedron 2001, 57, 4405.
- Kumar , P. ; Dinesh , C. U. ; Reddy , R. S. ; Pandey , B. H-Y zeolite: A mild and efficient catalyst for the tetrahydropyranylation of alcohols . Synthesis 1993 , 1069 .
- Hoyer , S. ; Laszlo , P. ; Orlovic , M. ; Polla , E. Catalysis by acidic clay of the protective tetrahydropyranylation of alcohols and phenols . Synthesis 1986 , 655 .
- Hajipour , A. R. ; Kooshki , B. ; Ruoho , A. E. Nitric acid in the presence of supported P2O5 on silica gel: An efficient and novel reagent for oxidation of sulfides to the corresponding sulfoxides . Tetrahedron Lett. 2005 , 46 , 5503 .
- (a) Hajipour , A. R. ; Arbabian , M. ; Ruoho , A. E. Tetramethylammonium dichloroiodate: An efficient and environmentally friendly iodination reagent for iodination of aromatic compounds under mild and solvent-free conditions . J. Org. Chem. 2002 , 67 , 8622 ; (b) Hajipour , A. R. ; Ruoho , A. E. Iodination of aromatic compounds under mild and solvent-free conditions . Org. Prep. Proced. Int. 2002 , 34 , 647 ; (c) Hajipour , A. R. ; Mazloumi , G. An efficient and simple procedure for preparation of esters and anhydrides from acid chlorides in the presence of 1,4-diazabicyclo[2.2.2]octane (DABCO) under solvent-free conditions . Synth. Commun. 2002 , 32 , 23 ; (d) Hajipour , A. R. ; Ruoho , A. E. Oxidation of thiols to the corresponding disulfides with tetramethylammonium chlorochromate under non-aqueous conditions . J. Chem. Res., Synop. 2002 , 547 ; (e) Hajipour , A. R. ; Adibi , H. ; Ruoho , A. E. Wet silica-supported permanganate for the cleavage of semicarbazones and phenylhydrazones under solvent-free conditions . J. Org. Chem. 2003 , 68 , 4553 ; (f) Hajipour , A. R. ; Bageri , H. ; Ruoho , A. E. Solid state oxidation of alcohols using 1-butyl-4-aza-1-azoniabicyclo[2.2.2]octane chlorochromate . Bull. Korean Chem. Soc. 2004 , 25 , 1238 ; (g) Hajipour , A. R. ; Ruoho , A. E. Deprotection of thioacetals by wet alumina supported Cr(VI) oxide under solvent-free conditions . Sul. Lett. 2002 , 25 , 151 ; (h) Hajipour , A. R. ; Mirjalili , B. F. ; Zarei , A. ; Khazdooz , L. ; Ruoho , A. E. A novel method for sulfonation of aromatic rings with silica sulfuric acid . Tetrahedron Lett. 2004 , 45 , 6607 .
- (a) Hajipour , A. R. ; Mallakpour , E. ; Imanzadeh , G. A rapid and convenient synthesis of oximes in dry media under microwave irradiation . J. Chem. Res. 1999 , 228 ; (b) Hajipour , A. R. ; Mallakpour , E. ; Adibi , H. Benzyltriphenylphosphonium peroxymonosulfate as a novel and efficient reagent for oxidation of alcohols under solvent-free condition . Chem. Lett. 2000 , 460 ; (c) Hajipour , A. R. ; Mallakpour , E. ; Afrousheh , A. A convenient and mild procedure for the synthesis of alkyl p-toluenesulfinates under solvent-free conditions using microwave irradiation . Tetrahedron 1999 , 55 , 2311 ; (d) Hajipour , A. R. ; Islami , F. A convenient and highly diastereoselective synthesis of optically active sulfinate esters from sulfonyl chlorides under solid state conditions . Ind. J. Chem. 1999 , 38B , 461 ; (e) Hajipour , A. R. ; Mallakpour , E. ; Imanzadeh , G. Oxidation of alcohols to carbonyl compounds under solvent-free conditions using permanganate supported on alumina . Chem. Lett. 1999 , 99 ; (f) Hajipour , A. R. ; Hantehzadeh , M. Asymmetric reduction of prochiral cyclic imines to alkaloid derivatives by novel asymmetric redusing reagent in THF or under solid-state conditions . J. Org. Chem. 1999 , 64 , 8475 ; (g) Hajipour , A. R. ; Mallakpour , E. ; Backnejad , H. Benzyltriphenylphosphonium chlorochromate: A mild and novel reagent for oxidation of benzylic and allylic alcohols under non-aqueous and aprotic conditions or microwave conditions . Synth. Commun. 2000 , 30 , 3855 ; (h) Hajipour , A. R. ; Mallakpour , E. ; Afrousheh , A. One-pot and simple reaction for the synthesis of alkyl p-toluenesulfinate esters under solid-phase conditions . Phosphorus, Sulfur Silicon Relat. Elem 2000 , 160 , 67 ; (i) Hajipour , A. R. ; Mallakpour , E. ; Khoee , S. An efficient, fast, and selective oxidation of aliphatic and benzylic alcohols to the corresponding carbonyl compounds under microwave irradiation . Synlett 2000 , 740 ; (j) Hajipour , A. R. ; Baltork , I. M. ; Nikbaghat , K. ; Imanzadeh , G. Solid-phase synthesis of oximes . Synth. Commun. 1999 , 29 , 1697 .
- (a) Hajipour , A. R. ; Mahboubkhah , N. 1-Benzyl-4-aza-1-azoniabicyclo[2.2.2]octane periodate: A mild and efficient oxidation for the cleavage of oxime double bonds under anhydrous conditions . J. Chem. Res., Synop. 1998 , 122 ; (b) Hajipour , A. R. ; Ruoho , A. E. Solid state deprotection of thioacetals and thioketals using 1-benzyl-4-aza-1-azoniabicyclo[2.2.2]octane periodate and aluminum chloride . Org. Prep. Proced. Int. 2005 , 37 , 298 ; (c) Hajipour , A. R. ; Ruoho , A. E. Methyltriphenylphosphonium peroxydisulfate and iodine as mild reagents for the iodination of activated aromatic compounds . Org. Prep. Proced. Int. 2005 , 37 , 279 .