Abstract
Highly selective acylation of the alcoholic hydroxy group can be achieved with (hydroxyalkyl)phenols carrying both alcoholic and phenolic hydroxyls by the use of the most common acylating agents, acid chlorides, under microwave irradiation.
INTRODUCTION
Acylation has been employed as the most generally useful protecting method for the hydroxy functionalities.[ Citation 1 ] In general, acylation is carried out by treatment of an alcohol with an appropriate acyl halide or anhydride. Acid chlorides are often used in pyridine and acid anhydrides in the presence of such acylation promoters as 4-(dimethylamino)pyridine (DMAP) or 4-pyrrolidinopyridine (PPY),[ Citation 2 ] so that even hindered hydroxyls are smoothly acylated. The high catalytic activity of these compounds is attributable to the formation of N-acylpyridinium ions as hyperreactive intermediates. On the other hand, it is usually difficult to conduct a selective acylation of compounds bearing multiple hydroxyls. Therefore, a wide variety of reagents[ Citation 3 ] and auxiliaries[ Citation 4 ] have been developed for selective acylation of polyhydric compounds, which is still a fundamental but important topic in synthetic organic chemistry. The regioselective properties of enzymes have also been exploited for such a purpose. For example, the lipase-catalyzed acylation or deacylation procedure has been applied to the synthesis of selectively protected derivatives of polyhydroxy compounds such as carbohydrates.[ Citation 5 ] Recently, we also have reported on the Candida antarctica lipase B–catalyzed regioselective acylation[ Citation 6 ] of dihydroxybenzenes and deacylation[ Citation 7 ] of them acylated at both phenolic hydroxyls. During the course of our continuing study on the enzyme-catalyzed selective acylation of polyhydric compounds, we needed to prepare authentic samples by chemo-enzymatic methods and found that with (hydroxyalkyl)phenols carrying both alcoholic and phenolic hydroxyls, highly selective acylation of the alcoholic hydroxy group can be achieved by the use of the most common acylating agents, acyl chlorides, under microwave irradiation. This information forms the subject of the present communication.
RESULTS AND DISCUSSION
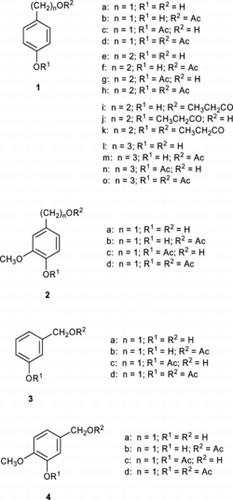
Initially, 2-(4-hydroxyphenyl)ethanol (1e) was chosen as a model compound and its acylation was examined under various reaction conditions (Fig. ). The diol 1e can undergo acylation through two pathways to form either the alkyl monoester (1f) or the phenyl monoester (1g) and finally to afford the diester (1h). The distribution of products was determined by the 1H NMR analysis of the reaction mixture. The most common acylation method employing acetyl chloride (2 mol equiv) in pyridine yielded the diester as the major product, besides a fair amount of the alkyl ester, but no phenyl ester after 45 min needed for the complete consumption of the starting diol at 25 °C (1f, 16.6%; 1g, 0%; 1h, 83.4%). On the other hand, when acetyl chloride (3 mol equiv) alone was used in tetrahydrofuran as a solvent, it was found that the alkyl ester was formed as a sole acylation product, contrary to expectations, though the yield was not very high (entry 1 in Table ). In the presence of pyridine (3 mol equiv), the outcome became different: the starting diol was consumed completely within the same reaction time, and besides the alkyl ester a fair amount of the diester was produced (entry 3). When triethylamine (3 mol equiv) was employed as an additive, the major product was the diester accompanied by a considerable amount of the phenyl ester (entry 5). Next, we examined the effect of microwave irradiation on the acylation reactions. Microwave-assisted organic synthesis has been known to possess a number of advantages:[ Citation 8 ] it is able to substantially shorten reaction times, reduce side reactions to give cleaner reactions, increase yields, and even improve reproducibility. A few examples have revealed also that the chemo- or regioselectivity can be altered under the action of microwave irradiation compared with conventional heating.[ Citation 9 ] In the present case, virtually no effects of microwaves were detected with the reaction using acetyl chloride in the presence of triethylamine (entry 6), and a small increase of the proportion of the diester was observed in the presence of pyridine (entry 4). On the other hand, microwaves exerted a significant influence on the reaction with acyl chloride alone (entry 2): the yield of the alkyl ester was approximately doubled, with the concomitant production of a small amount of the diester.
Table 1. Acetylation of 2-(4-hydroxyphenyl)ethanol () using acetyl chloride in the absence or presence of an additive in tetrahydrofuran under conventional heating (Δ) or microwave irradiation (MW)Footnote a
Stimulated by these facts, we set about examining the effect of microwave irradiation on the acylation of (hydroxyalkyl)phenols depicted in Fig. when an acyl chloride alone was used in the solvent of tetrahydrofuran. The results obtained under microwave irradiation were compared with those of the reactions carried out under conventional heating under otherwise identical conditions (Table ). When the amount of acetyl chloride was reduced to 1 mol equiv, no diester was produced, but the yield of the alkyl ester was very low, though a small increase in yield was observed upon microwave irradiation (entries 1 and 2). When the acid chloride was used in 2 mol equiv, even a prolonged reaction afforded only the alkyl ester in a slightly increased yield under conventional heating (entries 3 and 5); however, its yield was no more than 52% even after 24 h. When microwaves were adapted, the yield of the alkyl ester almost doubled in 40 min, with the formation of the diester being negligible (entry 4). After 60 min, the starting diol was completely consumed, and the alkyl ester was obtained in 94% yield (the remainder was the diester; entry 6). When the acylating agent was replaced by propanoyl chloride, similar results were obtained: the yield of the alkyl monoester (1i) attained under the action of microwave was four times higher than that obtained under the comparable conventional heating (entries 7 and 8). Therefore, the acid chloride was used in 2 mol equiv in the further experiments. Similar yield increase upon microwave irradiation was observed in the acetylation of (hydroxyalkyl)phenols with a shorter (1a) or longer (1l) alkyl chain between the benzene ring and the alcoholic hydroxy group (entries 9 and 10, entries 11 and 12, respectively). The result obtained with 1a was the most satisfactory: only the alkyl ester (1b) was produced quantitatively in 10 min under microwave irradiation, whereas conventional heating afforded it in only a 30% yield in the same reaction time. The diol 1 l gave similar results to those obtained with 1e: the yield of the alkyl ester (1m) increased four times with the action of microwaves, together with the formation of a limited amount (6%) of the diester (1o). With the diol 2a bearing a substituent ortho to the phenolic hydroxyl in 1a, the conventional heating afforded the alkyl ester (2b) in a moderate yield, whereas its yield was augmented to a quantitative level in 30 min under microwave irradiation (entries 13 and 14). With the compound 3a having the meta reciprocal position between a phenolic hydroxyl and the alkyl chain in a benzene ring, acetylation was a little retarded compared to the corresponding compound 1a, whereas the microwave ameliorated the yield of the alkyl ester (3b), though a limited amount of the diester (3d) was produced (entries 15 and 16). The introduction of a substituent into 3a accelerated the acylation reaction, and moreover the microwave irradiation resulted in the sole formation of the alkyl ester (4b) in a quantitative yield (entries 17 and 18).
Table 2. Acylation of (hydroxyalkyl)phenols using acid chlorides in tetrahydrofuran under conventional heating (Δ) or microwave irradiation (MW)Footnote a
In summary, we found that in the acylation of (hydroxyalkyl)phenols carrying both an alcoholic and a phenolic hydroxyl, a highly selective acylation of the alcoholic hydroxy group can be achieved without resorting to the use of specially developed reagents and/or auxiliary compounds, but by using the most conventional reagents, acyl chlorides, under microwave irradiation.
EXPERIMENTAL
Instruments and Reagents
The microwave-assisted reactions were performed using a monomode Green-motif reactor (300 W) from IDX (Japan). The reactor has a single-mode cavity with temperature regulation, and the reaction condition is not expressed by the power generated from the magnetron, thus giving the possibility of operating with rather similar profiles of temperature increases in microwave and conventional heating.
1H and 13C NMR spectra were recorded on a Varian Inova 500 spectrometer at 500 MHz and 125.7 MHz, respectively, with tetramethylsilane (TMS) as an internal standard. Assignments of signals were made by correlation spectroscopy (COSY), DEPT, heteronuclear multiple quantum correlation (HMQC), and heteronuclear multiple bond correlation (HMBC) techniques.
(Hydroxyalkyl)phenols used in this studies were purchased from Tokyo Chemical Industry or Aldrich Chemical Co. Other chemicals were obtained from Wako Pure Chemical Industries, Tokyo Chemical Industry, or Aldrich Chemical Co. All organic solvents were distilled following standard protocols and dried over molecular sieves prior to use.
Authentic Samples of Monoesters and Diesters of (Hydroxyalkyl)phenols
The isomeric monoesters and the diester of each (hydroxyalkyl)phenol were isolated through preparative thin-layer chromatography (TLC) or column chromatography of the reaction products obtained under some different reaction conditions. The methylene protons attached to the benzene ring were mainly employed for the quantification of reaction products by 1H NMR. Selected data for 1f–h are shown as examples.
Selected Data for 1f–h
Compound 1f: Mp 60–61 °C; 1H NMR (DMSO-d 6) δ1.98 (3H, s, CH3CO), 2.75 (2H, t, J = 7.0 Hz, CH 2Ar), 4.12 (2H, t, J = 7.0 Hz, OCH2), 4.90 (1H, s, OH), 6.68 (2H, d, J = 8.5 Hz, H-3 and H-5), 7.03 (2H, d, J = 8.5 Hz, H-2 and H-6), 9.24 (1H, s, OH). Compound 1g: Oil; 1H NMR (DMSO-d 6) δ 2.25 (3H, s, CH3CO), 2.71 (2H, t, J = 6.7 Hz, CH 2Ar), 3.60 (2H, d of t, J = 6.7 and 5.3 Hz, OCH2), 4.67 (1H, t, J = 5.3 Hz, OH), 7.01 (2H, d, J = 8.5 Hz, H-3 and H-5), 7.24 (2H, d, J = 8.5 Hz, H-2 and H-6). Compound 1h: Oil; 1H NMR (DMSO-d 6) δ 1.99 (3H, s, CH 3COOCH2), 2.25 (3H, s, CH 3COOAr), 2.88 (2H, t, J = 7.0 Hz, CH 2Ar), 4.21 (2H, t, J = 7.0 Hz, OCH2), 7.05 (2H, d, J = 8.5 Hz, H-3 and H-5), 7.28 (2H, d, J = 8.5 Hz, H-2 and H-6).
Typical Acylation Procedures
Under Microwave Irradiation
A solution of a (hydroxyalkyl)phenol (0.5 mmol) and acetyl chloride (1.0 mmol) in tetrahydrofuran (7 mL) was subjected to microwave irradiation at 60 °C for the specified time. The solvent was removed at less than ambient temperature under reduced pressure. The residual oil was dissolved in dimethyl sulfoxide-d 6 (DMSO-d 6) and subjected to 1H NMR (500 MHz) analysis for the quantification of the reaction products. The methylene protons attached to the benzene ring were mainly employed for the purpose. The whole content of the reaction mixture was used up for one analysis, and several discrete reaction mixtures were used at different reaction times.
Under Conventional Heating
To clarify the microwave effect, the reactions with conventional heating were carried out under conditions otherwise identical to those with microwave irradiation and not by the conventional manipulation, as follows: A solution of a (hydroxyalkyl)phenol (0.5 mmol) in tetrahydrofuran (7 mL) was preheated at 60 °C (thermostated oil bath) for 5 min, acetyl chloride (1.0 mmol) was added, and then the reaction mixture was heated at this temperature for the specified time. The same workup was adopted for the quantification of the reaction products.
Notes
a Reactions were conducted at 60 °C using 3 mol equiv of acetyl chloride.
b 3 equiv.
a Reactions were conducted at 60 °C. Acetyl chloride (2 mol equiv) was used unless otherwise noted.
b Acetyl chloride (1 mol equiv) was used.
c The product yields after 24 h were as follows: 1f, 52.3%; 1 g, 0%; 1 h, 0%.
d Propanoyl chloride (2 mol equiv) was used.
REFERENCES
- Wuts , P. G. M. ; Greene , T. W. Greene's Protective Groups in Organic Synthesis, , 4th ed. ; Wiley : New York , 2007 .
- Höfle , G. ; Steglich , W. ; Vorbrüggen , H. 4-Dialkylaminopyridines as highly active acylation catalysts . Angew. Chem. Int. Ed. Engl. 1978 , 17 , 569 – 583 .
- For example, see (a) Mukaiyama , T. ; Pai , F.-C. ; Onaka , M. ; Narasaka , K. A useful method for selective acylation of alcohols using 2,2′-bipyridyl-6-yl carboxylate and cesium fluoride . Chem. Lett. 1980 , 563 – 566 ; (b) Paradisi , M. P. ; Zecchini , G. P. ; Torrini , I. Selective acylations of aminophenols and hydroxyalkylphenols with 1-acetyl-v-triazolo[4,5-b]pyridine . Tetrahedron Lett. 1986 , 27 , 5029 – 5032 ; (c) Yamada , S. ; Sugaki , T. ; Matsuzaki , K. Twisted amides as selective acylating agents for hydroxyl groups under neutral conditions: Models for activated peptides during enzymatic acyl transfer reaction . J. Org. Chem. 1996 , 61 , 5932 – 5938 .
- For example, see (a) Breton , G. W. Selective monoacylation of unsymmetrical diols catalyzed by silica gel-supported sodium hydrogen sulfate . J. Org. Chem. 1997 , 62 , 8952 – 8954 ; (b) Orita , A. ; Mitsutome , A. ; Otera , J. Distannoxane-catalyzed highly selective acylation of alcohols . J. Org. Chem. 1998 , 63 , 2420 – 2421 ; (c) Caddick , S. ; McCarroll , A. J. ; Sandham , D. A. A convenient and practical method for the selective benzoylation of primary hydroxyl groups using microwave heating . Tetrahedron 2001 , 57 , 6305 – 6310 ; (d) Clarke , P. A. Selective mono-acylation of meso- and C 2-symmetric 1,3- and 1,4-diols . Tetrahedron Lett. 2002 , 43 , 4761 – 4763 .
- For reviews, see (a) Bornscheuer , U. T. ; Kazlauskas , R. J. Chemo- and regioselective lipase-catalyzed reactions . In Hydrolases in Organic Synthesis, , 2nd ed. ; Wiley-VHC : Weinheim , 2006 ; 141 ; (b) Faber , K. Biotransformations in Organic Chemistry, , 5th ed. ; Springer : Berlin , 2004 ; 363 .
- Miyazawa , T. ; Hamada , M. ; Morimoto , R. ; Murashima , T. ; Yamada , T. Highly regioselective propanoylation of dihydroxybenzenes mediated by Candida antarctica lipase B in organic solvents . Tetrahedron Lett. 2008 , 49 , 175 – 178 .
- Miyazawa , T. ; Hamada , M. ; Morimoto , R. ; Murashima , T. ; Yamada , T. Secondary alcohols act as better nucleophiles than primary alcohols in the lipase-catalyzed regioselective deacylation of dihydroxybenzenes acylated at both phenolic hydroxyls . Tetrahedron Lett. 2007 , 48 , 8334 – 8337 .
- For reviews, see (a) Microwaves in Organic Synthesis, , 2nd ed. ; A. Loupy , (Ed.); Wiley-VCH : Weinheim , 2006 ; (b) Microwaves in Organic and Medicinal Chemistry ; C. O. Kappe , and A. Stadler (Eds.); Wiley-VCH : Weinheim , 2005 .
- de la Hoz , A. ; Díaz-Ortiz , A. ; Moreno , A. Selectivity under the action of microwave irradiation . In Microwaves in Organic Synthesis, , 2nd ed. ; A. Loupy and A. Stadler (Eds.); Wiley-VCH : Weinheim , 2005 .