Abstract
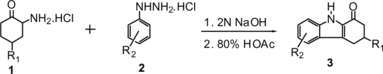
A convenient synthesis of 1-oxo-1,2,3,4-tetra-hydrocarbazoles has been developed by reaction of 2-aminocyclohexanone hydrochlorides with various phenylhydrazine hydrochlorides via Fischer indole synthesis under mild conditions. The method is more satisfactory in terms of the easy availability of starting materials and the simple one-pot operation.
Although 1-oxo-1,2,3,4-tetrahydrocarbazoles rarely occur in nature, they have been increasingly important intermediates in the synthesis of various biologically active heterocyclic compounds because of their unique structures, such as indo[2,3-a]carbazoles,[ Citation 1 ] furo[2,3-a]-carbazoles,[ Citation 2 ] pyrimidino[4,5-a]-carbazoles,[ Citation 3 ] pyrazolino[3,2,1-j,k]carbazoles,[ Citation 4 ] thieno-[2,3-a]carbazoles,[ Citation 5 ] and so on. Many methods for the synthesis of 1-oxo-1,2,3,4-tetrahydrocarbazoles have already been developed. These include, for example, cyclization of diphenylhydrazone of cyclohexane-1,2-dione or 2-phenylhydrazono cyclohexanone via Fischer indole synthesis,[ Citation6-8 ] oxidation of 1,2,3,4-tetrahydro-9H-carbazole with SeO2 [ Citation 9 ] or I2O5,[ Citation 10 ] cyclization of thiohydroxamates with camphorsulfonic acid,[ Citation 11 ] and cyclization of indole-3-butanic acid in refluxing xylene with P2O5, polyphosphoric acid (PPA), or Lewis acid Bi(OTf)3.[12–14] Some of these methods have modest yields, harsh reaction conditions, or tedious procedures. As part of our growing interest in using phenylhydrazines as heterocyclic building blocks in organic synthesis,[ Citation15-17 ] we describe herein a convenient and efficient one-pot synthetic route for the preparation of 1-oxo-1,2,3,4-tetrahydro-carbazoles via Fischer indole synthesis from 2-aminocyclohexanone hydrochlorides and various phenylhydrazine hydrochlorides. The method is more satisfactory in terms of the easy availability of starting materials and the simple one-pot operation, as shown in Scheme .
RESULTS AND DISCUSSION
When an equimolar mixture of 2-aminocyclohexanone hydrochloride 1a and phenylhydrazine hydrochloride 2a was refluxed in glacial acetic acid, two products, 1-oxo-1,2,3,4-tetrahydrocarbazole 3a and indolo[2,3-a]carbazole, were yielded in the ratio of 1:2. The reason was that 3a could proceed in the second Fisher indole synthesis with 2a to give indolo[2,3-a]carbazole. When the amount of 2a was enhanced to 4 equiv, a single product (indolo[2,3-a]carbazole) could be obtained.[ Citation 16 ] In opposition, when increasing the amount of compound 1a, little effect on the yield of 1-oxo-1,2,3,4-tetrahydrocarbazole was exhibited. To seek the general method to get target compound 3a in high yield, various reaction conditions were scrutinized (see Table ), but no promising results could be observed.
Table 1. Acid catalysts screening
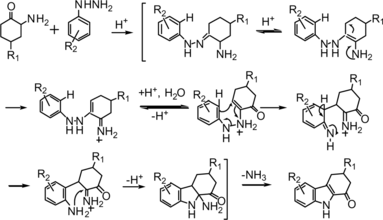
The mechanism of this reaction probably involves a Fisher indole synthesis, as already reported by our laboratory. (Scheme ).[ Citation 16 ] We believed that the presence of the hydrochloric acid in two reagents would be an important factor to speed up the 1-oxo-1,2,3,4-tetrahydrocarbazole converting to indolo-[2,3-a]-carbazole. For that reason, condensation of free 2-aminocyclohexanone with phenyl hydrazine in weak acid medium would be beneficial to the reaction. Because the 2-aminocyclohexanone and phenyl hydrazine are unstable in air, in this procedure, an equimolar of 2 N sodium hydroxide solution was added to the mixture of 1a and 2a to produce 2-aminocyclohexanone and phenyl hydrazine in situ, followed by refluxing in glacial acetic acid for 5 h. A yield of 55% of target compound 3a was obtained. Encouraged by this result, we studied different reaction parameters. The reaction was performed in different acidic solvents such as HOAc, HCOOH, HOAc–H2O, HCOOH–H2O, HOAc–MeOH, and so on. The results disclosed that 80% HOAc was a suitable solvent for the reaction in terms of yield and reaction time. Next, the optimization of the molar ratio of 1a to 2a was tested, and it was found that the ratio of 1.2:1 was sufficient for this reaction with this, the yield of the product 3a could be improved up to 73%, but the excess 1a beyond this ratio did not show further increase in conversion and in yield. Having established the optimized reaction conditions, a series of substituted 1-oxo-1,2,3,4-tetrahydrocarbazoles 3a–3s were synthesized in good to excellent yields, the results are shown in Table . However, reacting 1a with 3-chlorophenylhydrazine hydrochloride 2 m gave a mixture of 5-chloro-1-oxo-1,2,3,4-tetrahydrocarbazoles 3 m and 7-chloro-1-oxo-1,2,3,4-tetrahydrocarbazoles 3m′ in a 1.3:1 ratio (entry 13), which could not be separated.
Table 2. Synthesis of 1-oxo-1,2,3,4-tetrahydrocarbazoles
In summary, the present procedure provides an efficient method for the synthesis of 1-oxo-1,2,3,4-tetrahydrocarbazoles via Fischer indole synthesis. The advantages offered by this method are available starting material, simple operation, insensitivity to air and moisture, high yields of products, and cost-effectiveness. The selected products were characterized by 1H NMR, 13C NMR, elemental analysis, and mass spectroscopic (MS) data.
EXPERIMENTAL
Melting points were obtained on a B-540 Buchi melting-point apparatus and are uncorrected. 1H and 13C NMR spectra were recorded on a Brucker AM 400-MHz spectrometer (at 400 and 100 MHz, respectively) with SiMe4 as the internal standard in CDCl3 or dimethyl sulfoxide (DMSO-d 6). Element analysis were performed on an Eager 300 instrument. Infrared (IR) spectra were recorded on a Brucker Vector-22 spectrophotometer.
General Procedure for the Preparation of 1-Oxo-1,2,3,4-tetrahydrocarbazole (3a)
A solution of 2 N sodium hydroxide (0.48 mL, 0.98 mmol) was added dropwise to the mixture of 2-aminocyclohaxanone hydrochloride (79.3 mg, 0.53 mmol) and phenylhydrazine hydrochloride (63.6 mg, 0.44 mmol) and stirred for 15 min at room temperature. Then, the mixture was refluxed for 5 h followed by addition of 80% HOAc solution (3 mL). After the reaction mixture was cooled to room temperature, it was poured into a saturated NaHCO3 solution (10 mL) and extracted with EtOAc (3 × 10 mL). The combined organic layers were dried over anhydrous Na2SO4 and concentrated in vacuo. The crude product was purified by a silica-gel column chromatography with petroleum ether and EtOAc (5:1) as eluent to give the desired 1-oxo-1,2,3,4-tetrahydrocarbazole (3a).
Previously reported materials were characterized by comparison of their mp, IR, 1H NMR, and MS data with those of authentic samples. All new compounds gave satisfactory spectral data in accordance with their proposed structures.
Data for Selected Compounds
Compound 3g
IR (KBr, cm−1): 3212, 3028, 2939, 1652, 1566, 1298, 1138, 772; 1H NMR (400 MHz, DMSO-d6): δ 2.17 (m, 2H, CH2), 2.60 (m, 2H, CH2), 3.02 (m, 2H, CH2), 3.20 (s, 3H, CH3), 7.58 (d, J = 8.8 Hz, 1H, ArH), 7.79 (dd, J = 8.8 Hz, 1H, ArH), 8.32 (s, 1H, ArH), 12.19 (s, 1H, NH); 13C NMR (100 M, DMSO-d6): 21.0, 24.8, 38.4, 44.6, 114.0, 122.4, 124.1, 124.9, 129.6, 132.5, 133.5, 140.0, 191.2; MS (ESI): m/z = 263 [M+]; Anal. calcd. for C13H13NO3S: C, 59.30, H; 4.98; N, 5.32. Found: C, 59.77; H, 4.69; N, 5.59.
Compound 3o
IR (KBr, cm−1): 3252, 2957, 2872, 1647, 1541, 1457, 1259, 808; 1H NMR (400 MHz, DMSO-d 6): δ 1.12 (d, J = 6.4 Hz, 3H, CH3), 2.36 (m, 2H, CH2), 2.47 (m, 2H, CH2), 3.02 (dd, J = 16.0, 3.2 Hz, 1H, CH), 7.28 (d, J = 8.8 Hz, 1H, ArH), 7.41 (d, J = 8.8 Hz, 1H, ArH), 7.71 (s, 1H, ArH), 11.82 (s, 1H, NH); 13C NMR (100 MHz, DMSO-d 6): 21.4, 29.2, 32.8, 46.6, 114.8, 120.6, 124.6, 126.5, 126.5, 127.1, 132.5, 136.9, 190.7; MS (ESI): m/z = 235 [M + 2], 233 [M+]; (Anal. calcd. for C13H12ClNO: C, 66.81, H: 5.18; N, 5.99. Found: C, 66.77; H, 5.39; N, 5.78.
Compound 3p
IR (KBr, cm−1): 3253, 3060, 2922, 1647, 1538, 1455, 747, 702; 1H NMR (400 MHz, DMSO-d6): δ 2.63 (dd, J = 16.4, 3.2 Hz, 1H, CH2), 2.99 (m, 2H, CH2), 3.25 (dd, J = 16.0, 4.4 Hz, 1H, CH2), 3.56 (m, 1H, CH), 7.06 (t, J = 7.6 Hz, 1H, ArH), 7.24 (t, J = 7.6 Hz, 1H, ArH), 7.30 (m, 3H, ArH), 7.42 (m, 3H, ArH), 7.67 (d, J = 8.0 Hz, 1H, ArH), 11.74 (s, 1H, NH); 13C NMR (100 MHz, DMSO-d6): δ 29.3, 43.3, 45.4, 113.2, 120.1, 121.6, 125.5, 126.7, 127.0, 127.4, 127.5, 128.8, 131.4, 138.7, 144.5, 189.7; MS (ESI): m/z = 261 [M+]; Anal. Calcd. for C18H15NO: C, 82.73; H, 5.79; N, 5.36. Found: C, 83.04; H, 5.52; N, 5.08.
Compound 3q
IR (KBr, cm−1): 3252, 3027, 2919, 1645, 1540, 1467, 809, 729, 698; 1H NMR (400 MHz, DMSO-d 6): δ 2.64 (d, J = 16.0 Hz, 1H, CH2), 3.01 (m, 2H, CH2), 3.27 (d, J = 16.0 Hz, 1H, CH2), 3.60 (t, J = 12.0 Hz, 1H, CH), 7.24 (t, J = 8.0 Hz, 1H, ArH), 7.30 (m, 3H, ArH), 7.41 (t, J = 7.2 Hz, 3H, ArH), 7.79 (s, 1H, ArH), 11.90 (s, 1H, NH); 13C NMR (100 MHz, DMSO-d6): 29.1, 43.0, 45.3, 114.9, 120.8, 124.7, 126.4, 126.7, 126.9, 127.0, 127.4, 128.8, 132.5, 137.0, 144.4, 190.0; MS (ESI): m/z = 297 [M + 2], 295 [M+]; Anal. calcd. for C18H14ClNO: C, 73.10; H, 4.77; N, 4.74. Found: C, 73.06; H, 4.55; N, 4.68.
Compound 3r
IR (KBr, cm−1): 3262, 3078, 2957, 2851, 1643, 1543, 1476, 760; 1H NMR (400 MHz, DMSO-d 6): δ 1.00 (s, 9H, CH3), 2.02 (m, 1H, CH2), 2.38 (m, 2H, CH2), 2.61 (m, 1H, CH2), 3.11 (dd, J = 16.0, 3.6 Hz, 1H, CH), 7.06 (t, J = 7.6 Hz, 1H, ArH), 7.28 (t, J = 7.6 Hz, 1H, ArH), 7.38 (d, J = 8.0 Hz, 1H, ArH), 7.70 (d, J = 8.0 Hz, 1H, ArH), 11.59 (s, 1H, NH); 13C NMR (100 MHz, DMSO-d 6): 28.4, 29.3, 32.1, 40.5, 47.9, 112.6, 118.9, 120.7, 122.8, 123.2, 129.6, 134.1, 137.5, 190.1; MS (EI): m/z = 241 [M+]; Anal. calcd. for C16H19NO: C, 79.63; H, 7.94; N, 5.80. Found: C, 79.73; H, 7.62; N, 5.98.
Compound 3s
IR (KBr, cm−1): 3249, 2962, 2868, 1643, 1542, 1468, 801; 1H NMR (400 MHz, DMSO-d 6): δ 0.99 (s, 9H, CH3), 2.01 (t, J = 12.0 Hz, 1H, CH2), 2.47 (m, 3H, CH2), 3.13 (dd, J = 16.0, 2.0 Hz, 1H, CH), 7.28 (d, J = 9.2 Hz, 1H, ArH), 7.39 (d, J = 9.2 Hz, 1H, ArH), 7.82 (s, 1H, ArH), 11.80 (s, 1H, NH); 13C NMR (100 MHz, DMSO-d 6): 28.2, 29.6, 32.2, 40.3, 47.8, 114.2, 121.0, 124.8, 125.9, 126.7, 127.6, 132.4, 137.2, 190.4; MS (ESI): m/z = 277 [M + 2], 275 [M+]; Anal. calcd. for C16H18ClNO: C, 69.69; H, 6.58; N, 5.08. Found: C, 69.94; H, 6.35; N, 5.38.
Notes
a The ratio of 2-aminocyclohexanone hydrochloride 1a and phenylhydrazine hydrochloride 2a.
b Isolated yield.
c No reaction had occurred.
a Isolated yield.
b A 1.3:1 mixture of 3m and 3m′.
REFERENCES
- Li , X. N. ; Vince , R. Conformationally restrained carbazolone-containing-diketo acids as inhibitors of HIV integrase . Bioorg. Med. Chem. 2006 , 14 , 2942 – 2955 .
- Maertens , F. ; Toppet , S. ; Hoornaert , G. J. ; Compernolle , F. Incorporation of an indole-containing diarylbutylamine pharmacophore into furo[2,3-a]carbazole ring systems . Tetrahedron 2005 , 61 , 1715 – 1722 .
- Vandana , T. ; Prasad , K. J. R. A convenient synthesis of functionalised pyrimido[4,5-a]-carbazoles . Hetercycl. Commun. 2003 , 9 , 579 – 585 .
- Prasad , K. J. R. ; Vijayalakshmi , C. S. ; Sowmithran , D. Synthesis of pyrazolino-[3,2,1-j,k]-carbazole derivatives . Org. Prep. Proced. Int. 1995 , 27 , 678 – 682 .
- Joseph , D. ; Martarello , L. ; Kirsch , G. Tetracyclic compounds from tetrahydrocarbazolones, part 1: Synthesis from 2,3,4,9-tetrahydrocarbazol-1-ones . J. Chem. Res-S. 1995 , 9 , 350 – 351 .
- Strappaghetti , A. ; Rabere , G. ; Fravolini , A. ; Jacquignon , P. Nitrogen-containing carcinogenic compounds, LXXXVII: Synthesis of fluorinated and trifluoromethylated indolo[2,3-a]-carbazoles and indolo[2,3-a]acridines . Heterocycles 1980 , 14 , 935 – 942 .
- Bisagni , E. ; Ducrocq , C. ; Lhoste , J. M. ; Rivalle , C. ; Civier , A. Synthesis of 1-substituted ellipticines by a new route to pyrido[4,3-b]carbazoles . J. Chem. Soc., Perkin. Trans. 1 1979 , 111 , 1706 – 1711 .
- Chakraborty , A. ; Chowdhury , B. K. ; Bhattacharyya , P. Clausenol and clausenine two carbazole alkaloids from Clausena anisata . Phytochem. 1995 , 40 , 295 – 298 .
- Sakai , S. ; Kubo , A. ; Katsuura , K. ; Mochinaga , K. ; Ezaki , M. Oxidation of 2,3-disubstituted indole derivatives with selenium dioxide . Chem. Pharm. Bull. 1972 , 20 , 76 – 81 .
- Yoshida , K. ; Goto , J. ; Ban , Y. Oxidation of cycloalkan[b]indoles with iodine pentoxide (I2O5). Chem. Pharm. Bulletin. 1987, 35, 4700–4704.
- Castagnino , E. ; Corsano , S. ; Mastalia , A. Cyclic ketones from thiohydroxamates . J. Chem. Res. Synop. 1990 , 1 , 30 – 31 .
- Ishizumi , K. ; Shioiri , T. ; Yamada , S. Studies in the indole series, II: General synthesis of cycloalkan[b]indolones . Chem. Pharm. Bull. 1967 , 15 , 863 – 872 .
- Maertens , F. ; Van den Bogaert , A. ; Compernolle , F. ; Hoornaert , G. J. Intramolecular Ritter reactions of 2-(2-cyanoethyl)tetrahydrocyclopenta[b]indole and carbazole derivatives . Eur. J. Org. Chem. 2004 , 22 , 4648 – 4656 .
- Cui , D. M. ; Kawamura , M. ; Shimada , S. ; Hayashi , T. ; Tanaka , M. Synthesis of 1-tetralones by intramolecular Friedel–Crafts reaction of 4-arylbutyric acids using Lewis acid catalysts . Tetrahedron Lett. 2003 , 44 , 4007 – 4010 .
- Luo , Y. ; Hu , Y. Z. A novel and efficient strategy for the preparation of 2,4-disubstituted-1,2,3-triazoles . Synth. Commun. 2003 , 33 , 3513 – 3517 .
- Hu , Y. Z. ; Chen , Y. Q. An efficient and general synthesis of indolo[2,3-a]carbazoles using the Fischer indole synthesis . Synlett 2005 , 1 , 42 – 48 .
- Tang , W. J. ; Hu , Y. Z. Simple and efficient one-pot synthesis of 2,4-diaryl-1,2,3-triazoles . Synth. Commun. 2006 , 36 , 2461 – 2468 .
- Gazengel , J. M. ; Lancelot , J. C. ; Rault , S. ; Robba , M. Access to 6,11-dihydro-5H-pyrimidino-[4,5-a]carbazoles . J. Hetero. Chem. 1990 , 27 ( 7 ), 1947 – 1951 .
- Scott , T. L. ; Yu , X. M. ; Gorugantula , S. P. ; Carrero-Martinez , G. ; Soederberg , B. C. G. Palladium-catalyzed syntheses of tetrahydrocarbazolones as advanced intermediates to carbazole alkaloids . Tetrahedron 2006 , 62 ( 47 ), 10835 – 10842 .