SUMMARY
Tukong is the rumpless indigenous chicken breed of Indonesia. Its features must be described as the basis for their preservation and improvement. The current understanding of the Tukong, including its origin, exterior traits, and production indicators, is presented in this review. As a result, the chicken feather colour varies from greenish-black, reddish-black, bluish-black, brown, and white, with a red pea-shaped comb. The Tukong is a dual-purpose breed categorised as a small-sized bird with a body weight of 1.65 ± 0.82 kg (rooster) and 1.42 ± 0.55 kg (a hen). This breed has the potential to be developed as a meat-producing chicken, which is supported by its high dressing percentage (79.50%). However, the Tukong has low reproduction properties (egg production = 8–14 eggs/6 month period, fertility = 73.51%, hatchability = 81.42–84.26%). The development of the Tukong chicken needs to be increased in order to maintain its sustainability and take advantage of its superiority to other pilot candidates for the formation of new breeds.
Introduction
Indonesia is one of the most biodiverse countries in the world, including livestock genetic resources. More than 31 indigenous chicken breeds with various characteristics have been developed in the country (Sartika and Iskandar Citation2007; Sartika et al. Citation2016; Ulfah et al. Citation2017; Mahardhika et al. Citation2021). These breeds are distributed across different agro-ecological zones of the country with tropical adaptability and better disease resistance than commercial chickens. The majority of indigenous chickens are reared by smallholder farmers under a traditional scavenging system with low input costs for health care, feeding and housing and has been integrated with human livelihoods and food security (Maharani et al. Citation2021). Despite their importance, indigenous chickens are under threat due to unplanned crossbreeding with exotic breeds and changing production systems, while their genetic potential has not been fully characterised. According to the earlier report, approximately 38% of indigenous chicken breeds globally are at serious risk, while another 44% are considered to have an unspecified risk status (FAO Citation2019). Therefore, it is necessary to design and implement effective conservation programmes based on an accurate assessment of genetic diversity, phenotypic attributes, population structure and economically important traits (Manyelo et al. Citation2020) to avoid genetic erosion or even loss of genetic resources (Ulfah et al. Citation2015; Kawabe et al. Citation2014; Zein and Sulandari Citation2009).
Among the indigenous chickens in Indonesia, a rumpless chicken (Gallus gallus domesticus), or locally known as Tukong, has been kept as a valuable genetic resource because it combines the properties of egg-laying, meat production, and fighting ability. It has been reported that Tukong meat is delicious and lean, while its egg production can reach approximately 8–14 eggs per clutch (Tribudi et al. Citation2020). The breed lacks a fleshy portion of the rump (rumplessness) and is kept free-range by smallholder farmers in West Kalimantan. Li et al. (Citation2017) stated that chickens raised in a free-range system generate lower fat deposition and better taste. Some consumers also believe that meat and other products from free-range chickens are healthier and preferable than those from exotic chickens. In addition, Tukong is popular in West Kalimantan due to its tolerance to common poultry diseases and fluctuations in feed quality and quantity, thus requiring minimal input. Despite its uniqueness, this breed is minimally explored and commercially exploited. Therefore, in this current review, we review information on the historical distribution, phenotypic and reproductive characteristics, as well as the meat and egg quality of the Tukong chicken.
History and distribution area of Tukong Chicken
According to the information from the community and traditional village elders (Tumenggung), Tukong is a descendant of the Tabulangking chicken, a jungle fowl from West Kalimantan (Gufroni and Ibrahim Citation2005). The Tabulangking resembles quail but is larger in size, mainly yellow in feather colour, has a relatively small head, and has no tail bud (Tungging). The Tabulangking is a crossbreed derived from exotic and indigenous chickens. Genealogically, the indigenous chicken breeds in Indonesia are descended from the red jungle fowl chicken (Sulandari et al. Citation2008; Sulandari and Zein Citation2009). According to Fumihito et al. (Citation1994) and Hillel et al. (Citation2003), the current domesticated chickens are descendants of the Indochinese red jungle fowl (Gallus gallus). Chicken domestication came from a single ancestor (monophyletic origin), with domestication centres in China, India, and Southeast Asia (Ulfah et al. Citation2017; Zein and Sulandari Citation2009).
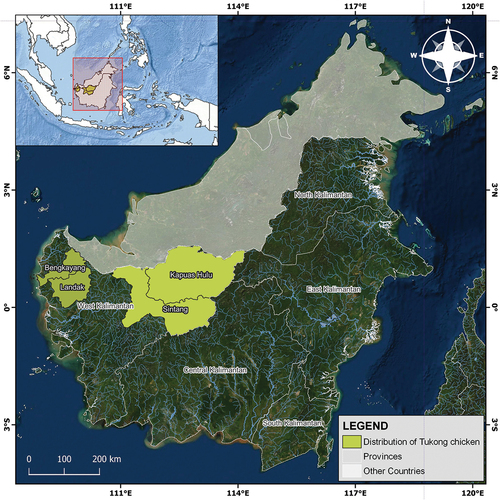
The Tukong breed is widely distributed in the Landak, Bengkayang, Sintang, and Kapuas Hulu districts of West Kalimantan Province (). To date, the current population data for this breed have not been well documented due to lack of information and survey from the breeding office. This breed is raised by communities for self-consumption purposes. The West Kalimantan region is dominated by Chinese and Dayak tribes, whose traditional events and religious festivals require an intact and perfect animal (Gufroni and Ibrahim Citation2005). The Tukong is not used for both activities because it is considered deformed due to the lack of a torso. A picture of this bird with its breed is presented in .
The description of Tukong chicken
Phenotypic appearance
As shown in , the feathers of Tukong chickens vary from greenish-black, reddish-black, bluish-black, brown, and white (Gufroni and Ibrahim Citation2005). The comb is generally a red pea-shaped comb (small jagged flowers) (). In Kampung chicken, Sartika and Iskandar (Citation2007) reported five feather colour variations; black, wild type, Columbian, white, and striated (barred) colours. The skin colour consists of white/yellow, black, or greenish (Zein and Sulandari Citation2009). Single (pprr), peas (P-rr), rose (ppR-), or walnut (P-R-) of comb-type was observed in indigenous chickens of Indonesia (Maharani et al. Citation2019, Citation2021).
Figure 3. Feather colour variation of Tukong chicken (a) private collection, (b) Gufroni and Ibrahim (Citation2005), (c) Tribudi et al. (Citation2020).

Figure 4. Comb-type of Tukong chicken: a = male Tukong; b-d = female Tukong (Tribudi et al. Citation2020).

Tukong chicken mostly has an orange eye colour and two shank colour variations: black and yellow. Eye colour is influenced by the presence of carotenoids and melanin in the blood, causing changes in the surface of the iris (Corti and Vogelaar Citation2010). For shank colour, a wide range from grey-green, black, white, green, black-white, to black-yellow green, has been reported in indigenous chickens of Indonesia (Maharani et al. Citation2021). Variations in shank colour are primarily affected by the nutrition of feed sources containing carotene (Negassa et al. Citation2014).
The unique feature of Tukong chicken is the absence of the tail bud (rumpless). Dunn and Landauer (Citation1936) stated that chickens’ rumpless condition is caused by the absence of the caudal vertebra and uropygial glands. The rumpless condition was also found in several chicken breeds, such as North American Araucana chicken (Noorai et al. Citation2019), Chinese Hongshan chicken (Wang et al. Citation2017) and Chinese Piao chicken (Wang et al. Citation2021). The rumpless condition may be caused by the Rp. The Rp gene is found on chromosome 2, proximal to the Iroquois homeobox (IRX1 and IRX2) genes. It is inherited in an autosomal dominant manner so that individuals without tails have a Rprp+ or RpRp genotype (Noorai et al. Citation2012; Freese et al. Citation2014). In addition to the Rp gene, Zwilling (Citation1942) found that mutations in the Brachyury (T) gene caused dominant rumplessness and were shown to be characterised by a reduction in the number of coccygeal vertebrae. In Chinese Hongshan chicken, there was no reduction in the number of coccygeal vertebrae. However, it was indicated that the rumplessness is caused by abnormal feather development, a Z-linked dominant character. A pseudo-gene inositol polyphosphate 5-phosphatase OCRL-1 (LOC431648 located in 71.8-72Mb of Z chromosome) is significantly involved in Wnt/β-catenin signalling pathway to regulate feather development (Wang et al. Citation2017). Genome-wide comparative analyses in Chinese Piao chicken revealed several genomic regions contain some essential genes that might be implicated in axis elongation, such as Irx4, Il18, Hspb2, and Cryab. These gene signalling pathways like NF-kB, calcium, or apoptosis and their activity affected the changes in mesenchymal stem cells in the tail bud resulting in a rumpless variant (Wang et al. Citation2021).
The Tukong chickens have a slow feather growth (Tribudi et al. Citation2020). For Tukong chicken, the complete feathers grow at 4–5 weeks (). This condition is also called late feathering (LF) with the symbol Kn caused by mutations in the K gene on the Z chromosome. Mutations in the form of insertions in endogenous viral gene 21 (EV21) members of avian leukosis virus subgroup E (ALVE) family also contribute to LF characteristics other than Kn (Tixier-Boichard et al. Citation1997). The nature of slow feather growth can benefit the livestock industry because the energy in the body will focus on growth (Galal and Younis Citation2006; Tribudi et al. Citation2020). Protein metabolism, particularly sulphur-containing amino acids (cysteine and methionine) becomes more efficient and can be compensated for meat production (Sartika et al. Citation2016). In addition, the slow feather growth makes this chicken more suitable to be developed in the tropical West Kalimantan region (temperature 27.5°C; relative humidity 83.75%; average rainfall 3745.3 mm).
The average body weight of rooster and hen Tukong chickens was 1.65 ± 0.82 kg and 1.42 ± 0.55 kg, respectively (Tribudi et al. Citation2020). The size of the wings and shank of this chicken is also shorter than other indigenous chickens. The short shank size may accommodate the body proportions that tend to widen in Tukong chickens (Gufroni and Ibrahim Citation2005).
Reproduction characteristics
The reproductive characteristics of Tukong chickens are provided in . The age at first laying eggs in Tukong chickens is higher than in the other indigenous chickens. Tukong egg weights (40.37–47 g) are comparable with other indigenous chicken breeds, such as Tolaki (41.56 g), Sentul (43.87 g), and Pelung (48.87 g) (Nataamijaya et al. Citation1994; Hidayat and Sopiyana Citation2010; Darwati et al. Citation2002). The low egg weight of Tukong might be influenced by the low body weight, causing a delay in the age of the first egg production and low egg weight. Body weight with egg weight are positively correlated so that an increase in the body weight of female Tukong chickens can increase the size of the eggs produced.
Table 1. Reproduction characteristics of Tukong chicken.
Tukong egg production ranges from 8 to 14 eggs per period (mean = 9.45 ± 1.22 eggs/hen) during the 6-month observation period (Tribudi et al. Citation2020). The egg production period in Tukong chickens varies considerably, both within one production period (clutch). In one clutch, Tukong chickens can lay eggs every day, every two or every 3 days, or even irregularly. Likewise, the length of days in one clutch or the time interval from one clutch to the next also varies and is irregular. Egg production from Tukong chickens also varies depending on the maintenance system, feed quality, and genetics (Tribudi et al. Citation2020).
The fertility and hatchability values are similar to Kampung Unggul Balitnak (KUB) chickens (81.40% and 69.54%; (Pratiwi and Sartika Citation2019) and Sentul chickens (77.12% and 71.40%; Mugiyono et al. Citation2015). Fertility and hatchability in chickens are influenced by breed, mating system, male-to-female ratio and feed and rearing management. The hatching weight of Tukong chicken (26.89 g) (Tribudi et al. Citation2020) was in contrast with the similar rumpled chicken, Nunukan chicken from East Kalimantan province, which reached 45.13 g (Junaedi and Husnaeni Citation2019). The hatching weight of Tukong chickens is also lower than black Kedu chickens at 28.98 g (Nataamijaya Citation2008) and Sentul chickens at 32.2 g (Hidayat and Sopiyana Citation2010). The low hatching weight in Tukong chickens is due to their small body weight and egg weight, which affects the hatching weight. Larger egg weights will produce larger chicks compared to low-weight eggs. Age at first egg, weight at maturity, and egg production at Tukong should be improved through selection.
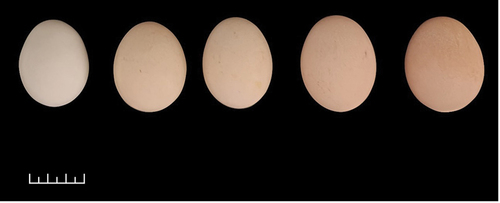
Egg’s colour variation
The eggshell colour of Tukong chickens varies from white to brown, as shown in . Differences in the colour of chicken egg shells from one another are influenced by many factors, such as chicken age, feed, and genetics (Mori et al. Citation2020). The brownish colour of the eggshell was due to the presence of the porphyrin pigment (Zhao et al. Citation2006). In line with this, nutrient intake is an essential factor in the formation of eggshell colour. The low secretion of porphyrin when colouring the eggshell will make the eggshell’s colour white.
Behaviour
Tukong chickens are known for their docile temperament, limited flight ability, not picky for feed and are actively looking for feed (Gufroni and Ibrahim Citation2005). The short wing length causes this limited flight ability. Furthermore, the absence of a tail is considered a factor in the limited ability to fly, where the tail functions to help maintain the chicken’s balance when flying. The tails of the vertebrae vary widely in form and function. In birds, the tail evolved and adapted specifically for flight and sexual selection (Rashid et al. Citation2020). These characteristics support the development of Tukong chickens as meat and egg-producing chickens. The low activity of Tukong chickens makes the metabolism of feed intake optimally utilised to produce meat and egg products.
Feed
In Kalimantan, Tukong chickens are extensively reared by rural households. Mostly, Tukong chickens roam during the day to find their feed and return to the coop in the evening. Generally, because they are kept traditionally, Tukong chickens are given additional feeds such as dried sweet potatoes, rice, bran, grasshoppers, other insects, grass, and household waste. The type and amount of feed given varies depend on the crops grown in the area and the seasons. Tukong chickens are known to like to eat grass so it is suspected that there are slight morphological and physiological differences in the digestive organs of Tukong chickens compared to other indigenous chickens (Gufroni and Ibrahim Citation2005). Lack of knowledge of the community or breeders regarding poultry management, veterinary support, and feeding practices leads to failing indigenous chicken improvement (Manyelo et al. Citation2020).
Health
Even though there were limited reports of disease resistance or health information specific to Tukong chicken, almost all indigenous chickens in Indonesia reported to have high disease tolerance. Gufroni and Ibrahim (Citation2005) reported that mortality in DOC and adult Tukong chicken were 25% and 20%, respectively. This result is considered lower than other indigenous chicken reported by (Juarini et al. Citation2005), which ranged from 34% to 42% (semi-extensive) and 50–56% (extensive) at age 0 to 6 weeks. Gufroni and Ibrahim (Citation2005) do not specifically mention the major cause of Tukong’s mortality. However, other results of local chicken’s mortality are caused by diseases and predation. Dunn and Landauer (Citation1936) stated that the absence or defective development of the oil gland in both types is likely to be associated with the excess mortality of rumpless chicken during growth. As previously stated, such birds’ plumage is incapable of shedding water, and they are likely to experience chill during wet weather.
Since Tukong consumes a lot of grasses, it may benefit from grass-associated vitamins, which serve as immune booster against diseases. Grasses can be considered as a source of energy, protein and vitamins and can also reduce feed intake to varying degrees. Free-range hens have a substantial supply of grass and other easily accessible foods. It has been reported that the most relevant role of grass for organic poultry is represented by the supply of several bioactive compounds (e.g. polyunsaturated fatty acids, vitamins and pigments) that have a direct effect on the quality of meat and eggs yet cannot be added in synthetic forms to organic diets. For these reasons, it is important to determine the nutritional suitability and supply of pastures to develop diets for free-range poultry and to investigate the possibility of transferring these compounds into poultry products (Mugnai et al. Citation2014).
Meat characteristics
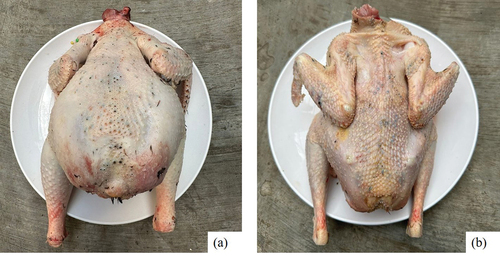
Gufroni and Ibrahim (Citation2005) reported that the dressing percentage of Tukong chicken was 79.50%. The carcass of Tukong chicken is shown in . The calm behaviour of the Tukong chickens, limited mobility, and ability to fly makes them spend less energy on activities. Thus, the results of food metabolism will be stored more for meat. In addition, the absence of tail buds and tail feathers also reduces the body parts of Tukong chickens that are not included in the carcass. The appearance of the Tukong chicken also shows a broader and thicker breast than the native chicken (Tribudi et al. Citation2020), and it can increase the composition of the carcass.
Figure 7. The carcass of Tukong chicken: a = male Tukong; b = female Tukong; tray diameter: 35 cm (private collection).
Tukong chicken meat is more delicious or savoury taste when compared to other indigenous chicken meat (Gufroni and Ibrahim Citation2005). It has been reported that the nutritional value and quality of meat is affected by a number of factors, such as age, body weight, growth performance and environmental conditions (Jung et al. Citation2011). Chicken meat also contains many endogenous bioactive compounds that, in the daily diet, may reduce the incidence of many diseases and provide some health benefits (Jayasena et al. Citation2014). Several studies have shown that Korean native chickens possess higher amounts of bioactive compounds than commercial broilers, which are influenced by genotype, muscle fibre, meat ratio, sex, age and cooking method (Jayasena et al. Citation2014, Citation2014; Jung et al. Citation2013). Likewise, the meat of native Japanese chicken is considered more palatable than that of conventional broiler chicken due to its high nutritional value (Rikimaru and Takahashi Citation2010).
Sustainability strategy
The main problem with Indonesian indigenous chicken germplasm is the threat of genetic degradation and erosion (FAO Citation2014). Livestock keepers in Indonesia believe that they will make more money through livestock keeping simply by switching from low-yielding native breeds to exotic breeds. However, this is not very effective for most small farmers, as most exotic breeds fail to thrive in their new environment. Therefore, for smallholder farmers, it may be more beneficial to raise indigenous chicken breeds than exotic breeds because they are adapted to local conditions and will yield some level of production even in poorer conditions, such as limited food and water supplies, prevalence of parasites and disease outbreaks. The breeding of indigenous breeds like Tukong chicken also contributes to the maintenance of local biodiversity and the preservation of the native genetic resources of livestock (Nataamijaya Citation2010).
Considering that Tukong chicken is indigenous chicken germplasm typical of West Kalimantan, which is not widely known and its population is limited, it is necessary to conserve it by increasing its population. Stakeholders such as local government, research institutions, universities, and farmer groups play essential roles in conservation efforts (Asmara et al. Citation2017), especially Tukong chickens so that national assets are maintained. Indigenous chicken development strategies can be carried out through genetic quality improvement (development of indigenous chicken nurseries, multiplication centres and through conduct of research on improving seed quality), environmental management enhancement (extensive restructuring to intensive and development of local feed alternatives), as well as socio-economic empowerment (increasing business scale and institutional development) (Suprijatna Citation2010).
Genetic improvement and conservation
At the development level, it is necessary to have a zoning policy for the identification of superior traits and development of Tukong chicken as a germplasm’ source. Development at the community level can be carried out in-situ by reviving and utilising indigenous knowledge that supports Tukong chicken conservation activities (Iskandar et al. Citation2005). Other than that, establishing a Farm Centre designated for Tukong chickens (Sartika et al. Citation2006) could help the stakeholders assign a complete identification of Tukong chicken, qualitative and quantitative traits, and genetic profile. Based on that data, the legal and legislative frameworks that support Tukong breed development through the Ministry of Agriculture could be initiated. Subsequently, when the population is established, a gene bank facility, which utilises modern technologies, such as primordial germ cell (PGC) and PGC collected for utilisation can be established (Tribudi and Kostaman Citation2020).
Nutritional and health enhancement
The productivity of indigenous chickens, including Tukong chickens, is still below its genetic potential. Therefore, it is necessary to create proper environmental conditions to get optimal performance so that genetic potential can be expressed optimally. For example, the levels of production can be increased through improved feeding, housing and healthcare conditions. One of these environmental conditions is feeding and health. The feed given must also meet the basic needs of the chicken. The composition of the diet consumed by free-range chicken is complex. Chicken can eat soil, pebbles, grass, weeds, crops seeds and insects in the yard. This makes it difficult to develop a complementary feeding strategy to respond to nutritional requirements. Ideally, the amount of supplement required must be based on the amount of nutrients foraged and the total requirement. However, there is limited information on forage consumption by free-range chicken over time season. A better understanding of foraging behaviour and the consumption of feed for free-range chickens will enable livestock keepers to develop an economic feeding system (Miao et al. Citation2005). Furthermore, although indigenous chickens are known to be more resistant to disease, additional immunity is needed through vaccination against prevalent diseases. Vaccines still need to be given for immunity against diseases that commonly infect chickens, including Gumboro, Newcastle disease (ND), and avian influenza (AI) (Mahendri et al. Citation2021).
Socio-economic empowerment
Tukong chicken has a relatively high proportion of carcass, so people prefer it for meat consumption. The Tukong chicken had a higher carcass percentage (68–72%) (Tribudi et al., unpublished) compared to Kampong chicken (62.25%) reported by (Tamzil et al. Citation2015). Advocacy and awareness of the benefits of eating Tukong chickens as well as public education should be intensified to the community in order to increase demands for Tukong chickens. This ensitization can be done through community associations or cooperatives. Suppose the appearance of the carcass is not attractive due to the condition of the rumpless. The product can be diversified into processed chicken meat, such as nuggets, sausages, or meatballs. If the demand for Tukong chickens increases, farmers will be eager to increase the population and breed Tukong chickens correspondingly.
Conclusion
Tukong is an indigenous chicken genetic resource from West Kalimantan. This chicken has experienced a population decline, and efforts are needed to save this genetic resource. The hallmark of the Tukong chicken is the absence of the base of the tail/rumpless. Judging from the phenotype’s appearance and several indicators of its production, this chicken has the potential to be developed as a meat-producing chicken. This chicken has a high proportion of carcasses, calm behaviour, and is adaptive to hot climates, especially in the West Kalimantan region. Of course, further research is needed to fully identify the characteristics of Tukong chickens, including determining genetic diversity and current population structure, as a basis for future selection, population improvement, and genetic improvement programmes. Strategies for developing Tukong chickens must be carried out in all aspects (genetic, environmental management and socio-economics) by involving all stakeholders (government, academics, and community).
Acknowledgement
The authors are grateful to the Ministry of Education, Culture, Research and Technology of the Republic of Indonesia (Kemendikbudristek) and Lembaga Pengelola Dana Pendidikan (LPDP) for providing the Indonesian Education Scholarship (BPI-Beasiswa Pendidikan Indonesia) and Universitas Brawijaya.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Asmara, I. Y., A. Anang, T. Widjastuti, and E. Sujana. 2017. “In-Situ Conservation Strategy to Safeguard Sentul Chickens in the Future.” KnE Life Sciences 2 (6): 110. doi:10.18502/kls.v2i6.1026.
- Corti, E., and E. Vogelaar. 2010. “Concerning Poultry: The Eye.” Aviaculture Europe. http://www.aviculture-europe.nl/nummers/10E06A11.pdf
- Darwati, S., B. Pangestu, and H. I. Rahayu. 2002. “Karakteristik Genetik Eksternal Ayam Merawang.” Prosiding Seminar Nasional Teknologi Peternakan Dan Veteriner, Bogor, 30 September - 1 Oktober, 271–273.
- Dunn, L. C., and W. Landauer. 1936. “Further Data on Genetic Modification of Rumplessness in the Fowl.” Journal of Genetics 33 (3): 401–405. doi:10.1007/BF02982895.
- FAO. 2014. “Synthesis Progress Report on the Implementation of the Global Plan of Action for Animal Genetic Resources.” http://www.fao.org/3/a-mm282e.pdf
- FAO. 2019. “The State of the World’s Biodiversity for Food and Agriculture.” FAO Commission on Genetic Resources for Food and Agriculture Assessments. http://www.fao.org/3/ca3129en/ca3129en.pdf
- Freese, N. H., B. A. Lam, M. Staton, A. Scott, S. C. Chapman, and M. Mallo. 2014. “A Novel Gain-Of-Function Mutation of the Proneural IRX1 and IRX2 Genes Disrupts Axis Elongation in the Araucana Rumpless Chicken.” PloS One 9 (11): e112364. doi:10.1371/journal.pone.0112364.
- Fumihito, A., T. Miyake, S. I. Sumi, M. Takada, S. Ohno, and N. Kondo. 1994. “One Subspecies of the Red Junglefowl (Gallus gallus gallus) Suffices as the Matriarchic Ancestor of All Domestic Breeds.” In Proceedings of the National Academy of Sciences of the United States of America, 91:12505–12509. doi:10.1073/pnas.91.26.12505.
- Galal, A., and H. Younis. 2006. “Minimizing Residual Feed Consumption by Introducing Dwarf and Naked Neck Genes in Laying Chicken.” Egyptian Poultry Science Journal 26 (2): 677–694.
- Gufroni, A. R., and T. M. Ibrahim. 2005. “Potensi Ayam Tukong Sebagai Ayam Lokal Di Kalimantan Barat.” Prosiding Lokakarya Nasional Inovasi Teknologi Pengembangan Ayam Lokal, Semarang, Agustus 25–26, 196–204.
- Hidayat, C., and Sopiyana, S. 2010. “Potensi Ayam Sentul Sebagai Plasma Nutfah Asli Ciamis Jawa Barat.” Wartazoa 20 (4): 190–205.
- Hillel, J., M. A. M. Groenen, M. Tixier-Boichard, A. B. Korol, L. David, V. M. Kirzhner, T. Burke, et al. 2003. “Biodiversity of 52 Chicken Populations Assessed by Microsatellite Typing of DNA Pools.” Genetics Selection Evolution 35 (6): 533–557. doi:10.1186/1297-9686-35-6-533.
- Iskandar, S., T. Susanti, S. Sopiyana, K. Suawarman, D. Sartika, F. Nenny, and E. Wahyu. 2005. “Identifikasi Dan Inventarisasi Induk-Induk Petelur Dan Pedaging Unggul Dalam Upaya Pembentukan Nucleus Ayam Sentul Unggul Di Mitra Kerjasama.” Prosiding Seminar Hasil-Hasil Penelitian Ternak Non Ruminansia, Bogor, September 12–13, Vol 2, 137–142.
- Jayasena, D. D., S. Jung, Y. S. Bae, S. H. Kim, S. K. Lee, J. H. Lee, and C. Jo. 2014. “Changes in Endogenous Bioactive Compounds of Korean Native Chicken Meat at Different Ages and During Cooking.” Poultry Science 93 (7): 1842–1849. doi:10.3382/ps.2013-03721.
- Jayasena, D. D., S. H. Kim, H. J. Lee, S. Jung, J. H. Lee, H. B. Park, and C. Jo. 2014. “Comparison of the Amounts of Taste-Related Compounds in Raw and Cooked Meats from Broilers and Korean Native Chickens.” Poultry Science 93 (12): 3163–3170. doi:10.3382/ps.2014-04241.
- Juarini, E., Sumanto, and D. Zainuddin. 2005. “Pengembangan Ayam Lokal Dan Permasalahannya Di Lapangan.” Prosiding Lokakarya Nasional Inovasi Teknologi Pengembangan Ayam Lokal, Semarang, Agustus 25–26, 280–293.
- Junaedi, J., and H. Husnaeni. 2019. “Hubungan Bobot Telur Tetas Dengan Egg Weight Loss Dan Bobot DOC Ayam Hasil Persilangan Pejantan Sentul Dengan Induk Ayam Nunukan.” Musamus Journal of Livestock Science 2 (1): 1–10. doi:10.35724/mjls.v2i1.2199.
- Jung, S., Y. S. Bae, H. J. Kim, D. D. Jayasena, J. H. Lee, H. B. Park, K. N. Heo, and C. Jo. 2013. “Carnosine, Anserine, Creatine, and Inosine 5 ′-Monophosphate Contents in Breast and Thigh Meats from 5 Lines of Korean Native Chicken.” Poultry Science 92 (12): 3275–3282. doi:10.3382/ps.2013-03441.
- Jung, Y., H. J. Jeon, S. Jung, J. H. Choe, J. H. Lee, K. N. Heo, B. S. Kang, and C. Jo. 2011. “Comparison of Quality Traits of Thigh Meat from Korean Native Chickens and Broilers.” Korean Journal for Food Science of Animal Resources 31 (5): 684–692. doi:10.5851/kosfa.2011.31.5.684.
- Kawabe, K., R. Worawut, S. Taura, T. Shimogiri, T. Nishida, and S. Okamoto. 2014. “Genetic Diversity of MtDna D-Loop Polymorphisms in Laotian Native Fowl Populations.” Asian-Australasian Journal of Animal Sciences 27 (1): 19–23. doi:10.5713/ajas.2013.13443.
- Li, Y., C. Luo, J. Wang, and F. Guo. 2017. “Effects of Different Raising Systems on Growth Performance, Carcass, and Meat Quality of Medium-Growing Chickens.” Journal of Applied Animal Research 45 (1): 326–330. doi:10.1080/09712119.2016.1190735.
- Maharani, D., F. Mustofa, A. P. Z. N. L. Sari, A. Fathoni, H. Sasongko, and D. N. H. Hariyono. 2021. “Phenotypic Characterization and Principal Component Analyses of Indigenous Chicken Breeds in Indonesia.” Veterinary World 14 (6): 1665–1676. doi:10.14202/vetworld.2021.1665-1676.
- Maharani, D., G. A. I. Wihandoyo, D. N. H. Adinda, L. Hariyono, and D. Nur Happy. 2019. “Phenotypic Characterization of Indonesian Native Chicken with Different Combs.” International Journal of Poultry Science 18 (3): 136–143. doi:10.3923/ijps.2019.136.143.
- Mahardhika, I. W. S., I. Habibah, A. B. I. Perdamaian, Trijoko, and B. S. Daryono. 2021. “Origin, Phenotypes, and Plumage Coloration of Golden Pelung Chicken Progenies (G. Gallus, Linn.1758).” Research Square 1–29. doi:10.21203/rs.3.rs-411024/v1.
- Mahendri, I. G. A. P., R. A. Saptati, I. Syarifah, and I. S. Nurhayati. 2021. “The Relationship Between Avian Influenza Disease Vaccination and Chicken Mortality in Sukabumi District, West Java Province, Indonesia.” IOP Conference Series: Earth and Environmental Science 788 (1): 012199. doi:10.1088/1755-1315/788/1/012199.
- Manyelo, T. G., L. Selaledi, Z. M. Hassan, and M. Mabelebele. 2020. “Local Chicken Breeds of Africa: Their Description, Uses and Conservation Methods.” Animals 10 (12): 1–18. doi:10.3390/ani10122257.
- Miao, Z. H., P. C. Glatz, and Y. J. Ru. 2005. “Free-Range Poultry Production - a Review.” Asian-Australasian Journal of Animal Sciences 18 (1): 113–132. doi:10.5713/ajas.2005.113.
- Mori, H., M. Takaya, K. Nishimura, and T. Goto. 2020. “Breed and Feed Affect Amino Acid Contents of Egg Yolk and Eggshell Color in Chickens.” Poultry Science 99 (1): 172–178. doi:10.3382/ps/pez557.
- Mugiyono, S., D. M. Saleh, and S. Sukardi. 2015. “Reproductive Performance of Various Breeds of Sentul Chicken.” Animal Production 17 (3): 169. doi:10.20884/1.jap.2015.17.3.512.
- Mugnai, C., E. N. Sossidou, A. D. Bosco, S. Ruggeri, S. Mattioli, and C. Castellini. 2014. “The Effects of Husbandry System on the Grass Intake and Egg Nutritive Characteristics of Laying Hens.” Journal of the Science of Food and Agriculture 94 (3): 459–467. doi:10.1002/jsfa.6269.
- Nataamijaya, A. G. 2008. “Karakteristik Dan Produktivitas Ayam Kedu Hitam.” Buletin Plasma Nutfah 14 (2): 85–89. doi:10.21082/blpn.v14n2.2008.
- Nataamijaya, A. G. 2010. “Pengembangan Potensi Ayam Lokal Untuk Menunjang Peningkatan Kesejahteraan Petani.” Jurnal Litbang Peternakan 29 (10): 131–138.
- Nataamijaya, A. G., K. Diwyanto, E. S. Haryono, and M. Kusni. 1994. “Karakteristik Morfologi Delapan Varietas Ayam Bukan Ras (Buras) Langka.” Prosiding Seminar Nasional Sains Dan Teknologi Peternakan, Bogor, January 25–26, 605–614.
- Negassa, D., A. Melesse, and S. Banerjee. 2014. “Phenotypic Characterization of Indigenous Chicken Populations in Southeastern Oromia Regional State of Ethiopia.” Animal Genetic Resources/Ressources Génétiques Animales/Recursos Genéticos Animales 55 (December 2017): 101–113.doi:10.1017/s2078633614000319.
- Noorai, R. E., N. H. Freese, L. M. Wright, S. C. Chapman, L. A. Clark, and B. Alsina. 2012. “Genome-Wide Association Mapping and Identification of Candidate Genes for the Rumpless and Ear-Tufted Traits of the Araucana Chicken.” PloS One 7 (7): e40974. doi:10.1371/journal.pone.0040974.
- Noorai, R. E., V. Shankar, N. H. Freese, C. M. Gregorski, S. C. Chapman, and P. Xu. 2019. “Discovery of Genomic Variations by Whole-Genome Resequencing of the North American Araucana Chicken.” PloS One 14 (12): 1–22. doi:10.1371/journal.pone.0225834.
- Pratiwi, N., and T. Sartika. 2019. “Fertilitas Dan Daya Tetas Ayam KUB Non Kaki Kuning Dan Kaki Kuning Di Balai Penelitian Ternak Ciawi.” Prosiding Seminar Nasional Teknologi Peternakan Dan Veteriner 547–551. doi:10.14334/pros.semnas.tpv-2019-p.547-551.
- Rashid, D. J., R. Bradley, A. M. Bailleul, K. Surya, H. N. Woodward, P. Wu, Y. H. Wu, et al. 2020. “Distal Spinal Nerve Development and Divergence of Avian Groups.” Scientific Reports 10 (1): 1–16. doi:10.1038/s41598-020-63264-5.
- Rikimaru, K., and H. Takahashi. 2010. “Evaluation of the Meat from Hinai-Jidori Chickens and Broilers: Analysis of General Biochemical Components, Free Amino Acids, Inosine 5′-Monophosphate, and Fatty Acids.” The Journal of Applied Poultry Research 19 (4): 327–333. doi:10.3382/japr.2010-00157.
- Sartika, T., and S. Iskandar. 2007. Mengenal Plasma Nutfah Ayam Indonesia Dan Pemanfaatannya, Edited by K. Diwyanto. Bogor: Balai Penelitian Ternak, Pusat Penelitian dan Pengembangan Peternakan, Badan Penelitian dan Pengembangan Pertanian.
- Sartika, T., S. Iskandar, and B. Tiesnamurti. 2016. Sumber Daya Genetik Ayam Lokal Indonesia Dan Prospek Pengembangannya. Jakarta: IAARD Press.
- Sartika, T., S. Sulandari, M. S. A. Zein, and S. Paryanti. 2006. “Ayam Nunukan: Karater Genetik, Fenotipe Dan Pemanfaatannya.” Wartazoa 16 (4): 216–222.
- Sulandari, S., and M. S. A. Zein. 2009. “Analisis D-Loop DNA Mitokondria Untuk Memposisikan Ayam Hutan Merah Dalam Domestikasi Ayam Di Indonesia.” Media Peternakan 32 (1): 31–39.
- Sulandari, S., M. S. A. Zein, and T. Sartika. 2008. “Molecular Characterization of Indonesian Indigenous Chickens Based on Mitochondrial DNA Displacement (D)-Loop Sequences.” HAYATI Journal of Biosciences 15 (4):145–154. Institut Pertanian Bogor. doi:10.4308/hjb.15.4.145.
- Suprijatna, E. 2010. “Strategi Pengembangan Ayam Lokal Berbasis Sumber Daya Lokal Dan Berwawasan Lingkungan.” Prosiding Seminar Nasional Unggas Lokal Ke IV Fakultas Peternakan Universitas Diponegoro, Semarang, October 7, 55–88.
- Tamzil, M. H., M. Ichsan, N. S. Jaya, and M. Taqiuddin. 2015. “Growth Rate, Carcass Weight and Percentage Weight of Carcass Parts of Laying Type Cockerels, Kampong Chicken and Arabic Chicken in Different Ages.” Pakistan Journal of Nutrition 14 (7): 377–382. doi:10.3923/pjn.2015.377.382.
- Tixier-Boichard, M., A. Boulliou-Robic, M. Morisson, G. Coquerelle, P. Horst, and B. Benkel. 1997. “A Deleted Retroviral Insertion at the Ev21-K Complex Locus in Indonesian Chickens.” Poultry Science 76 (5): 733–742. Poultry Science Association Inc. doi:10.1093/ps/76.5.733.
- Tribudi, Y. A., and T. Kostaman. 2020. “Panjang Gonad Dan Jumlah Primordial Germ Cell Ayam White Leghorn.” Majalah Ilmiah Peternakan 23 (3): 107. doi:10.24843/mip.2020.v23.i03.p02.
- Tribudi, Y. A., A. Tohardi, and A. L. Ryadi. 2020. “Karakteristik Produksi Ayam Tukong: Plasma Nutfah Ayam Lokal Di Kalimantan Barat.” Jurnal Ilmu Ternak Universitas Padjadjaran 20 (2): 108. doi:10.24198/jit.v20i2.30461.
- Ulfah, M., D. Perwitasari, M. Jakaria, A. Farajallah, and A. Farajallah. 2015. “Breed Determination for Indonesian Local Chickens Based on Matrilineal Evolution Analysis.” International Journal of Poultry Science 14 (11): 615–621. doi:10.3923/ijps.2015.615.621.
- Ulfah, M., D. Perwitasari, J. Jakaria, M. Muladno, and A. Farajallah. 2017. “Multiple Maternal Origins of Indonesian Crowing Chickens Revealed by Mitochondrial DNA Analysis.” Mitochondrial DNA Part A, DNA Mapping, Sequencing, and Analysis 28 (2): 254–262. doi:10.3109/19401736.2015.1118069.
- Wang, Y. M., S. Khederzadeh, S. R. Li, N. O. Otecko, D. M. Irwin, M. Thakur, X. D. Ren, M. S. Wang, D. D. Wu, and Y. P. Zhang. (2021). “Integrating Genomic and Transcriptomic Data to Reveal Genetic Mechanisms Underlying Piao Chicken Rumpless Trait.” Genomics, Proteomics & Bioinformatics 19 (5): 787–799. Beijing Institute of Genomics. doi:10.1016/j.gpb.2020.06.019.
- Wang, Q., J. Pi, A. Pan, J. Shen, and L. Qu. 2017. “A Novel Sex-Linked Mutant Affecting Tail Formation in Hongshan Chicken.” Scientific Reports 7 (1): 1–8. Springer US. 10.1038/s41598-017-10943-5.
- Zein, M. S. A., and S. Sulandari. 2009. “Investigasi Asal Usul Ayam Indonesia Menggunakan Sekuens Hypervariable-1 D-Loop DNA Mitokondria.” Jurnal Veteriner 10 (1): 41–49.
- Zhao, R., G. Y. Xu, Z. Z. Liu, J. Y. Li, and N. Yang. 2006. “A Study on Eggshell Pigmentation: Biliverdin in Blue-Shelled Chickens.” Poultry Science 85 (3): 546–549. doi:10.1093/ps/85.3.546.
- Zwilling, E. 1942. “The Development of Dominant Rumplessness in Chick Embryos.” Genetics 27 (6): 641–656. doi:10.1093/genetics/27.6.641.