Abstract
AIM: To evaluate the efficacy and safety of the novel anthelmintic combination, derquantel-abamectin, against gastrointestinal nematode populations in sheep, under field-use conditions.
METHODS: Controlled faecal egg count reduction tests (FECRT) were conducted in New Zealand in 14 trials, covering a range of geographic locations, farming enterprises, breeds, nematode populations, and anthelmintic-resistance profiles. Enrolled animals were naturally infected with mixed populations of gastrointestinal nematodes. All trials included a group treated with derquantel-abamectin, and a negative control group. Nine trials included additional groups each treated with a single- or dual-active oral reference anthelmintic, selected from albendazole, levamisole, albendazole-levamisole, ivermectin, abamectin and moxidectin. A total of 838 animals were enrolled across all trials, and were randomly allocated to treatment groups within blocks defined by faecal nematode egg counts (FEC) pretreatment. On Day 0 derquantel-abamectin was administered orally at 1 ml/5 kg bodyweight (2 mg/kg derquantel, 0.2 mg/kg abamectin), and each reference anthelmintic was given at the recommended label dose. Faecal samples were collected on Day 14 (± 1 day), to determine the percentage reduction in mean FEC for each anthelmintic tested. Larval differentiation was also performed post-treatment, to estimate efficacy at the genus level. Animals were weighed on or before Day 0, and on Day 14 (± 1 day) in 13 trials.
RESULTS: The efficacy of derquantel-abamectin against mixed strongyle populations was ≥99.2%, based on the percentage reduction in geometric mean FEC. Nematodirus sp. was present in six trials at a level sufficient for efficacy calculations to be conducted; in all cases, the efficacy of derquantel-abamectin was 100%. In those trials where the efficacy of at least one reference anthelmintic was <95% against strongyles and/or Nematodirus sp., derquantel-abamectin was 100% effective. In five trials, the mean gain in bodyweight was significantly greater in the derquantel-abamectin group than the negative controls.
CONCLUSIONS AND CLINICAL RELEVANCE: When administered orally at 1 ml/5 kg bodyweight, derquantel-abamectin is highly effective for the treatment of gastrointestinal nematodes in sheep, including populations of strongyles and Nematodirus sp. with resistance to one or more single- or dualactive anthelmintics. Derquantel-abamectin presents sheep producers with a unique opportunity to introduce a new class of anthelmintic to their nematode control programmes, with the added benefits offered by a combination anthelmintic.
| FEC | = |
Faecal nematode egg count(s) |
| FECRT | = |
Faecal egg count reduction test(s) |
| L4 | = |
Fourth-stage larvae |
| WAAVP | = |
World Association for the Advancement of Veterinary Parasitology |
Introduction
Gastrointestinal parasitism, and emerging resistance to single-, dual- and triple-active anthelmintic products, continues to represent a major production cost to sheep farmers throughout the world. Treatment with effective anthelmintics continues to be the cornerstone of internal parasite control, when used strategically in conjunction with other nematode-management practices.
Several reviews of the status of anthelmintic resistance in small ruminants have been published (Besier and Love Citation2003; Kaplan Citation2004; Jabbar et al. Citation2006). In New Zealand, resistance in gastrointestinal nematodes to the macrocyclic lactones, as well as emerging resistance to dual- and triple-combination anthelmintics, has been reported (Wrigley et al. Citation2006; Hughes et al. Citation2007; Sutherland et al. 2008). A nationwide survey of sheep farms conducted in 2005 showed widespread resistance to commercially available anthelmintics (Waghorn et al. Citation2006), while Leathwick (Citation2004) conducted an analysis that estimated the total discounted cost of anthelmintic resistance accumulated over 30 years (from 2002) would exceed NZ$1.3 billion. Similar reports of anthelmintic resistance have been published in Australia (Wooster et al. Citation2001; Love et al. Citation2003), South Africa (Van Wyk et al. Citation1989, Citation1999), and the United Kingdom (UK) (Yue et al. Citation2003; Bartley et al. Citation2005; Sargison et al. Citation2007). A trial conducted in the southern sheepproducing zone of Western Australia, in weaned Merinos, estimated that production losses associated with using an ineffective anthelmintic compared with a fully effective one would be >A$2/animal in wool returns and >A$4/animal in animal value over a 12-month period (Besier et al. Citation1996). In the UK, resistance to all available broad-spectrum anthelmintics has resulted in the culling of flocks on individual farms (Sargison et al. Citation2005; Blake and Coles Citation2007).
It is clear there is an urgent need for new anthelmintics to be introduced for sheep, particularly in those countries where products currently available are beginning to fail (Besier Citation2007). From the introduction of ivermectin in the early 1980s, until the recent discovery of the amino-acetonitrile derivatives and identification of monepantel as a drug-development candidate (Ducray et al. Citation2008; Kaminsky et al. Citation2008; Mason et al. Citation2009), over a quarter of a century had elapsed before a chemical class with a new mode of action was developed for use in livestock.
The discovery of paraherquamide and its semi-synthetic derivative, derquantel (2-desoxoparaherquamide), both members of the spiroindole class of anthelmintics, has been reported previously (Shoop et al. Citation1990; Lee et al. Citation2001, Citation2002; Johnson et al. Citation2004). The efficacy of derquantel against gastrointestinal nematodes of sheep was assessed early in its development, and in subsequent dose-determination studies; in the dose range tested (0.5–8.0 mg/kg), derquantel was found to be a mid-spectrum anthelmintic.
Derquantel has been developed as an oral anthelmintic for sheep in combination with abamectin, to provide broad-spectrum utility, efficacy against strains of nematodes resistant to existing anthelmintics, and a means of protecting the new class from the rapid emergence of anthelmintic resistance. Combination anthelmintics have been shown to be effective against populations of gastrointestinal nematodes that have developed resistance to single-active anthelmintics, thus extending the useful life of existing classes (McKenna Citation1990; Anderson et al. Citation1991a Citationb). The potential for combinations to delay or slow the development of resistance to their individual components has also been proposed (Anderson et al. Citation1988; Smith Citation1990; Barnes et al. Citation1995; Dobson et al. Citation2001; Leathwick et al. Citation2009).
At the dose rate selected for the combination product, derquantel alone was found to have excellent anthelmintic activity (>95% reduction in mean worm count) against adults and fourth-stage larvae (L4) of Trichostrongylus and Nematodirus spp., and the adult stage of Haemonchus contortus. It was less than 95% effective against Teladorsagia (=Ostertagia) circumcincta (adults and L4), L4 of H. contortus, and some large intestinal nematodes (PR Little and SJ Maeder, unpubl. data). Here, we report the field efficacy and safety of the proposed commercial formulation of derquantel-abamectin (10 mg/ml derquantel, 1 mg/ml abamectin), in a series of trials conducted on 14 farms throughout New Zealand.
Materials and methods
Experimental design
Controlled FECRT were conducted during 2006–2009 in 14 trials, to evaluate the anthelmintic efficacy of the proposed commercial formulation of derquantel-abamectin (Startect; Pfizer New Zealand Ltd, Auckland, NZ) when administered orally to sheep. They were conducted against naturally acquired, mixed infestations of gastrointestinal nematodes, on farms that were considered representative of sheep enterprises in the region, and managed as a commercial operation. Trial sites were selected to capture a range of geographic locations, climatic conditions, farming operations, breeds, sexes and ages, and to ensure representation of the economically important species of gastrointestinal nematodes. All trials were approved by either the AgResearch Grasslands Animal Ethics Committee, Palmerston North, New Zealand, or the Kaiawhina Animal Ethics Committee, Palmerston North, New Zealand.
The design of the study and technical procedures were similar across all farms, and consistent with the recommendations of the World Association for the Advancement of Veterinary Parasitology (WAAVP) for evaluating the efficacy of anthelmintics in ruminants (Wood et al. Citation1995). For each type of nematode egg (strongyle and/or Nematodirus sp.), efficacy of the product under investigation and each reference anthelmintic was given by the percentage reduction in geometric mean FEC compared with a negative control group. Percentage reductions in arithmetic mean FEC were also determined for completeness. In all trials, the interval between treatment (Day 0) and collection of faecal samples post-treatment was 14 (± 1) days. Pooled larval cultures were assessed pre- and post-treatment, to identify the strongyle genera present.
Table 1. Details of individual trials conducted to evaluate the field efficacy of the novel combination anthelmintic derquantel-abamectin in sheep, with range of pre-treatment strongyle faecal nematode egg counts (FEC), target group size (n), and reference anthelmintics used.
A summary of the location, animal details, pre-treatment strongyle FEC, group size and reference anthelmintics used in each trial is presented in Table . Each was a single-site, negatively controlled efficacy trial, using a randomised block design, with the individual animal as the experimental unit. Negative control animals either received tap water as a placebo or remained untreated.
Trials 1–5 were conducted to support registration of the derquantel-abamectin combination product, and did not include reference anthelmintics; a prior history of anthelmintic resistance was not a requirement for selection of these farms. Trials 6–14 were conducted to generate additional efficacy data on sheep farms with a history of resistance to single-active and/or combination anthelmintics available commercially. The selection of reference anthelmintics for these trials was guided by the results of previous anthelmintic-resistance tests on each farm, and were selected from albendazole (Albendazole Sheep; Ancare New Zealand Ltd, Auckland, NZ), levamisole (Levicare; Ancare New Zealand Ltd), combined albendazole-levamisole (Arrest; Ancare New Zealand Ltd), ivermectin (Ivomec Liquid for Sheep and Goats; Merial New Zealand Ltd, Manukau City, NZ), abamectin (Genesis Oral Drench; Ancare New Zealand Ltd), and moxidectin (Vetdectin Oral Drench for Sheep; Fort Dodge Animal Health Ltd, Auckland, NZ).
Experimental animals
The sheep used were aged 2–12 months, weighed 18.5–53.0 kg at the time of treatment, and represented a range of breeds. A single sex (female) was used in nine trials, and mixed sex (female/castrated male) in the other five (see Table ). A total of 838 animals were enrolled across the 14 individual trials.
On each trial site, individual mobs were screened in the weeks leading up to commencement of the trial, to determine the parasite burden and range of species present. Source flocks with a mean strongyle FEC of ≥400 epg, and with at least two nematode genera present, were required. On some farms the study animals had significantly higher nematode burdens than this, with several genera represented. Potential source flocks were only considered on the basis that they had not been treated in the previous 60 days with a persistent macrocyclic lactone, or the previous 150 days with a sustained-release anthelmintic.
Faecal samples were collected 2–5 days prior to treatment, for the determination of individual FEC, as well as for differentiation of larvae from pooled coproculture. In each trial, up to 50% more animals than the target number were sampled, to ensure that all enrolled animals had an established nematode burden. Individual animals were included on the basis of good general health and a strongyle FEC of ≥100 epg; the animals with the highest egg counts were selected for each trial. In certain trials, animals with a very high FEC were excluded on welfare grounds, due to the potential for clinical parasitism in the event that those animals were allocated to the negative control group.
Allocation to experimental groups
The selected animals were sorted and blocked by pre-treatment strongyle FEC and, when possible, Nematodirus sp. FEC, and randomly allocated to experimental groups within each block. As a result of this procedure, each group had a similar mean and range of FEC prior to treatment.
Administration of test and reference anthelmintics
Animals were weighed for calculation of the dose on Day 0 (the day of treatment), or up to 5 days prior. In Trials 1–5, animals were treated with either derquantel-abamectin or tap water placebo; the dose for each animal was calculated on individual bodyweight at the rate of 1 ml/5 kg (nominal dose rates of 2 mg/kg derquantel and 0.2 mg/kg abamectin). In Trials 6–14, the doses of derquantel-abamectin and each reference anthelmintic were based on the heaviest animal enrolled in the trial; in Trial 7, the animals were split into two lines in order to avoid excessive overdosing, and as such the doses in this trial were based on the heaviest animal in each line. Based on the stated concentration (s) and recommended label dose for each reference anthelmintic, the nominal (minimum) dose rate of each active drug was 4.75 mg/kg albendazole, 7.5 mg/kg levamisole, 0.2 mg/kg ivermectin, 0.2 mg/kg abamectin, and 0.2 mg/kg moxidectin. The negative control animals remained untreated in Trials 6–14. In all trials, individual doses were administered using a 10-ml or 20-ml disposable plastic syringe. Except in Trial 1, the presence or absence of coughing immediately following treatment was assessed and recorded.
Clinical observations and bodyweight
Clinical observations were performed by a veterinarian, who was blinded to allocation to treatment groups, from the commencement of treatment until at least 30 minutes after the final animal was treated. In Trials 1–5, additional clinical observations were made at 2 and 6 hours following treatment. After completion of clinical observations on Day 0, the study animals were returned to pasture and run as a single group until Day 14 (± 1 day). During this period, the animals were observed in the paddock by the farm manager on the day following treatment, then at least three times a week. The animals were weighed on the final day of the trial, except in Trial 7.
Parasitological techniques
Faecal samples were collected per rectum pre- and post-treatment, and transported to a commercial veterinary laboratory (Gribbles Veterinary Pathology, Palmerston North or Dunedin), for individual FEC and pooled larval culture. In several trials it was not possible to obtain a faecal sample on the final day of the trial from every enrolled animal; this resulted in no more than one animal from any experimental group being excluded from the efficacy calculations. FEC were performed according to standard laboratory procedures, using a modified McMaster technique, and reported as epg; pre-treatment samples were counted at a sensitivity of 1:100 epg, while post-treatment samples were counted at either 1:50 epg (eight trials) or 1:100 epg (six trials). To reduce observational bias, post-treatment faecal samples were not sorted into their respective treatment groups prior to counting, and laboratory personnel were blinded to the allocation to treatment groups. In Trial 5, a repeat FEC was performed due to a suspected mix-up or identification error of samples; this repeat count was performed 6 days after collection of the samples, using new subsamples from stored faeces, and laboratory personnel remained blinded to the allocation to treatment groups.
Once FEC were completed, faecal samples collected post-treatment were pooled by treatment group for larval culture and identification, according to standard laboratory procedures. Differentiation of larvae for strongyle genera is reported as the percentage of each genus identified. In line with standard practice, Chabertia and Oesophagostomum spp. larvae were not differentiated, thus percentages of larvae of those genera are reported as a single combined figure.
Table 2. Range and geometric mean (GM) strongyle faecal nematode egg counts, and percentage reductions compared with negative controls (Control), for sheep treated with derquantel-abamectin (DQL-ABA) and reference anthelmintics, 14 (± 1) days post-treatment in 14 trials.
Statistical analysis and efficacy calculations
As per WAAVP guidelines (Wood et al. Citation1995), the primary outcome measure was the percentage reduction in the geometric mean of individual FEC, compared with a negative control group, for each type of nematode egg identified, i.e. strongyle or Nematodirus sp. A log-transformation [ln(x+1)] was applied to the FEC data prior to analysis, and the transformed values were analysed using a GLM including the fixed effect of treatment group and the random effect of block. Geometric means were obtained using back transformation, and treatment differences were assessed at the 5% level of significance (two-tailed). Arithmetic means for each treatment group were also determined using a corresponding analysis of untransformed data.
Provided there was overall evidence of a treatment effect (p<0.05), each treated group was compared with the negative control group, to determine the statistical significance of treatment differences, with no further adjustments for multiple comparisons, and to estimate treatment efficacy. Statistical comparisons between the derquantel-abamectin group and the reference groups have not been presented, as the reference anthelmintics were used in these trials solely to establish or confirm the resistance profile of the nematode population present on each farm.
Geometric and arithmetic means were used to estimate efficacy for each of the treated groups (derquantel-abamectin, and each reference anthelmintic where relevant), using the following formula:
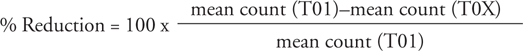
where T01 represents the negative control group, and TOX the treated group of interest
In the case of Nematodirus sp., efficacy calculations were not performed where the number of egg-positive animals in the negative control group at Day 14 (± 1 day) was fewer than six.
In Trials 6–14, larval differentiation figures post-treatment were used to estimate the percentage efficacy of derquantel-abamectin, as well as the reference anthelmintics, against each strongyle genus identified. The arithmetic mean count for each genus was determined by multiplying the arithmetic mean strongyle FEC by the larval culture percentage, for each experimental group. Efficacy was then calculated using the arithmetic mean genus counts using the formula above; geometric means were not calculated due to larval differentiation being based on a pooled culture rather than culture of individual faecal samples. Where the derived arithmetic mean in the negative control group was <50 epg, efficacy calculations were not considered a valid estimate of the true efficacy against that genus (McKenna Citation1996; Miller et al. Citation2006), and are therefore not reported.
The presence of resistance to any one of the broad-spectrum reference anthelmintics used was defined as <95% reduction in mean FEC (Presidente Citation1985; Coles et al. Citation2006).
For those trials where the animals were weighed twice (all except Trial 7), the change in bodyweight was analysed using a GLM, with the fixed effect of treatment group, the random effect of block, and the bodyweight pre-treatment fitted as a covariate. LSM changes in bodyweight are reported for each experimental group. Where there was overall evidence of a treatment effect (p<0.05), pair-wise differences significant at the 5% level are reported. For trials with more than two experimental groups, Tukey's method was used to adjust for multiple comparisons.
Statistical analyses were performed using SAS for Windows v9.1 (SAS Institute Inc, Cary NC, USA).
Results
Summaries of the FEC data post-treatment with treatment efficacies (based on geometric and arithmetic means) for strongyles are presented in Table . Nematodirus sp. was present at an adequate level to enable efficacy calculations to be conducted in six trials; these results are presented in Table .
Based on percentage reductions in both geometric and arithmetic mean FEC, the efficacy of derquantel-abamectin against strongyles was ≥99.2%, except in Trial 5, in which the geometric and arithmetic mean efficacies were 99.2% and 93.3%, respectively. In that trial, results from a repeat egg count indicated a geometric mean efficacy of 99.9% and an arithmetic mean efficacy 99.2%. Treatment efficacies of <95%, for at least one reference anthelmintic, were found in three trials (based on geometric means) and six trials (based on arithmetic means). In the six trials in which Nematodirus sp. was present at an adequate level, the efficacy of derquantel-abamectin was 100%; in three of those trials, efficacy of <95% was found for at least one reference anthelmintic.
Table 3. Range and geometric mean (GM) Nematodirus sp. faecal egg counts, and percentage reductions compared with negative controls (Control), for sheep treated with derquantel-abamectin (DQL-ABA) and reference anthelmintics, 14 (± 1) days post-treatment in six trials.
Table 4. Percentage larvae at the genus level, based on culture of pooled faeces, arithmetic mean (AM) genus faecal nematode egg count (FEC) in the negative control group, and percentage reduction in FEC for sheep treated with derquantel-abamectin (DQL-ABA) and reference anthelmintics, 14 (± 1) days post-treatment, in eight trials.
The percentage larval differentiation post-treatment, arithmetic mean FEC (by genus) for the control group, and treatment efficacies for Trials 6–8 and 10–14 are summarised in Table , in which the reference anthelmintics were selected from albendazole, levamisole, albendazole-levamisole and ivermectin. Table presents the data from Trial 9, in which ivermectin, abamectin and moxidectin were the reference anthelmintics used.
The change in mean bodyweight for each experimental group for 13 trials is presented in Table . In five trials, the change in bodyweight was significantly greater in the derquantel-abamectin group than for the negative control group. In three trials, one or more groups treated with a reference anthelmintic also had significantly greater increases in mean bodyweight compared with the negative control group.
Mild, transient coughing occurred immediately following treatment in 123/209 animals treated with derquantel-abamectin, 13/113 treated with ivermectin, 2/15 treated with abamectin, and 1/15 treated with moxidectin. No coughing occurred in animals treated with albendazole (n=43), levamisole (n=73), or albendazole-levamisole (n=113). No other adverse events occurred that could be attributed to treatment with the test product.
Table 5. Percentage larvae at the genus level, based on culture of pooled faeces, arithmetic mean (AM) genus faecal nematode egg count (FEC) in the negative control group, and percentage reduction in FEC for sheep treated with derquantel-abamectin (DQL-ABA) and reference anthelmintics, 14 (± 1) days post-treatment, in one trial.
Table 6. Mean change, and percentage change, in bodyweight from pre-treatment (Day -5 to Day 0) to Day 14 (± 1 day) for sheep treated with derquantel-abamectin (DQL-ABA) or reference anthelmintics, or negative controls (Control) in 13 trials evaluating the field efficacy and safety of DQL-ABA. In Trial 7, the sheep were not weighed on Day 14.
Discussion
In 13/14 trials, the efficacy of derquantel-abamectin against populations of strongyles was ≥99.9% based on geometric means and ≥99.2% based on arithmetic means. In the other trial, efficacy was 99.2% and 93.3%, respectively; this difference was largely due to an outlier in the FEC data, with one animal in the treated group having a post-treatment strongyle FEC of 500 epg. Based on a repeat count of all post-treatment faecal samples in this trial, the geometric mean efficacy was 99.9% and the arithmetic mean efficacy 99.2%.
Defining anthelmintic resistance as percentage reduction in mean FEC of <95%, resistance in the strongyle population to at least one reference anthelmintic was confirmed in three trials (based on geometric means) and six trials (based on arithmetic means). In each of these trials, the efficacy of derquantel-abamectin was 100%. In the population of Nematodirus sp., resistance to at least one reference anthelmintic was confirmed in three trials, with derquantel-abamectin again being 100% effective in each instance.
There is debate as to whether the percentage reduction in geometric or arithmetic mean FEC provides the more appropriate measure of efficacy in the FECRT (Dash et al. Citation1988; McKenna Citation1997a; Smothers et al. Citation1999; Dobson et al. Citation2009). In the trials reported here, percentage reductions based on arithmetic means were less than those based on geometric means (except when 100%), consistent with the view that the use of arithmetic means may provide a more stringent test of anthelmintic efficacy (Vercruysse et al. Citation2001). In accordance with the anthelmintic efficacy guidelines published by the WAAVP and the International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products (Wood et al. Citation1995; Vercruysse et al. Citation2001), we used the geometric mean FEC as the primary measure of anthelmintic efficacy against strongyles and Nematodirus sp.; arithmetic mean efficacies are also reported for completeness.
In Trials 6–14, where efficacy calculations at the genus level were considered valid, i.e. a mean genus FEC of ≥50 epg in the negative control group, the efficacy of derquantel-abamectin was 100% for Haemonchus (four trials), 100% for Teladorsagia (four trials), ≥98.9% for Trichostrongylus (eight trials), ≥98.6% for Cooperia (six trials), and ≥99.6% for Oesophagostomum/Chabertia (seven trials) spp. Of particular interest are the data generated that indicate established or emerging anthelmintic resistance in one or more genera, where that resistance was not always detectible in the strongyle population as a whole. Although the calculation of means at the genus level contains some inherent inaccuracies, due to a margin of error in both the mean strongyle FEC and percentage larval culture post-treatment, valuable information on emerging anthelmintic resistance in individual strongyle genera may be obtained using larval culture post-treatment (Presidente Citation1985; McKenna Citation1997b). In Trial 9, for example, the efficacy of ivermectin, abamectin and moxidectin against Teladorsagia sp. was 0%, 65% and 32%, respectively, compared with efficacy against the undifferentiated strongyle population of 86%, 96% and 93% (based on arithmetic means). The efficacy of derquantel-abamectin against Teladorsagia sp. in that trial was 100%, underscoring the role of derquantel in the combination anthelmintic.
While just over half of the animals treated with derquantelabamectin coughed immediately following treatment, this was of a mild and transient nature, with no recurrence or adverse sequelae. There were no reports of loss of the anthelmintic associated with the episodes of coughing. No other adverse events occurred that could be attributed to the test product.
In conclusion, these results demonstrate a high therapeutic efficacy of the combined oral formulation of derquantel-abamectin against naturally acquired gastrointestinal nematode populations in sheep, under a range of farming conditions in New Zealand. The proposed commercialisation of derquantel-abamectin offers sheep producers a unique opportunity to use a novel anthelmintic from a new chemical class as part of a highly effective combination anthelmintic, while resistance alleles are still rare, this being identified as one of the conditions required for combinations to be effective in delaying the emergence of resistance (Dobson et al. Citation2001; Leathwick et al. Citation2009). In association with sustainable anthelmintic treatment practices and maintaining a proportion of susceptible nematodes in refugia (either as free-living stages or adult nematodes in untreated sheep), the prudent use of highly effective combination anthelmintics may be a key element of internal parasite control in the future.
Acknowledgements
The authors would like to thank the trial investigators and associated staff for their expertise and professionalism in conducting these trials, in particular Drs Richard Lee, John Smart, Andrew Roe, David Seifert, Ginny Dodunski, Mark Colson, Stuart Bruere, Jeremy Lind and Mr Dan Robinson; as well as Phil McKenna, Melanie Taylor and associated staff at Gribbles Veterinary Pathology. We also wish to thank Tony Simon and Richard Clemence for reviewing the manuscript and for their constructive feedback and suggestions. All trials reported in this paper were funded by Pfizer Australia Pty Ltd, West Ryde, NSW, Australia.
References
- Anderson , N , Martin , PJ and Jarrett , RG . 1988 . Mixtures of anthelmintics: a strategy against resistance . Australian Veterinary Journal , 65 : 62 – 64 .
- Anderson , N , Martin , PJ and Jarrett , RG . 1991 a . The efficacy of mixtures of albendazole sulphoxide and levamisole against sheep nematodes resistant to benzimidazole and levamisole . Australian Veterinary Journal , 68 : 127 – 132 .
- Anderson , N , Martin , PJ and Jarrett , RG . 1991 b . Field evaluation of a mixture of albendazole sulphoxide and levamisole against Ostertagia and Trichostrongylus spp in sheep . Australian Veterinary Journal , 68 : 133 – 136 .
- Barnes , EH , Dobson , RJ and Barger , IA . 1995 . Worm control and anthelmintic resistance: adventures with a model . Parasitology Today , 11 : 56 – 63 .
- Bartley , DJ , Jackson , E , Sargison , N and Jackson , F . 2005 . Further characterisation of a triple resistant field isolate of Teladorsagia from a Scottish lowland sheep farm . Veterinary Parasitology , 134 : 261 – 266 .
- Besier , RB . 2007 . New anthelmintics for livestock: the time is right . Trends in Parasitology , 23 : 21 – 24 .
- Besier , RB and Love , SCJ . 2003 . Anthelmintic resistance in sheep nematodes in Australia: the need for new approaches . Australian Journal of Experimental Agriculture , 43 : 1383 – 1391 .
- Besier , RB , Lyon , J and McQuade , N . 1996 . Drench resistance - a large economic cost . Journal of Agriculture Western Australia , 37 : 60 – 63 .
- Blake , N and Coles , G . 2007 . Flock cull due to anthelmintic-resistant nematodes . Veterinary Record , 161 : 36 – 36 .
- Coles , GC , Jackson , F , Pomroy , WE , Prichard , RK , von , Samson-Himmelstjerna G , Silvestre , A , Taylor , MA and Vercruysse , J . 2006 . The detection of anthelmintic resistance in nematodes of veterinary importance . Veterinary Parasitology , 136 : 167 – 185 .
- Dash , KM , Hall , E and Barger , IA . 1988 . The role of arithmetic and geometric mean worm egg counts in faecal egg count reduction tests and in monitoring strategic drenching programs in sheep . Australian Veterinary Journal , 65 : 66 – 68 .
- Dobson , RJ , Besier , RB , Barnes , EH , Love , SCJ , Vizard , A , Bell , K and Le , Jarnbre LF . 2001 . Principles for the use of macrocyclic lactones to minimise selection for resistance . Australian Veterinary Journal , 79 : 756 – 761 .
- Dobson , RJ , Sangster , NC , Besier , RB and Woodgate , RG . 2009 . Geometric means provide a biased efficacy result when conducting a faecal egg count reduction test (FECRT) . Veterinary Parasitology , 161 : 162 – 167 .
- Ducray , P , Gauvry , N , Pautrat , F , Goebel , T , Fruechtel , J , Desaules , Y , Schorderet , Weber S , Bouvier , J , Wagner , T , Froelich , O and Kaminsky , R . 2008 . Discovery of amino-acetonitrile derivatives, a new class of synthetic anthelmintic compounds . Bioorganic Medicinal Chemistry Letters , 18 : 2935 – 2938 .
- Hughes , PL , Dowling , AF and Callinan , APL . 2007 . Resistance to macrocyclic lactone anthelmintics and associated risk factors on sheep farms in the lower North Island of New Zealand . New Zealand Veterinary Journal , 55 : 177 – 183 .
- Jabbar , A , lqbal , Z , Kerboeuf , D , Muhammad , G , Khan , MN and Afaq , M . 2006 . Anthelmintic resistance: the state of play revisited . Life Sciences , 79 : 2413 – 2431 .
- Johnson , SS , Coscarelli , EM , Davis , JP , Zaya , RM , Day , JS , Barsuhn , CL , Martin , RA , Vidmar , TJ , Lee , BH , Conder , GA , Geary , TG , Ho , NFH and Thompson , DP . 2004 . Interrelationships among physiochemical properties, absorption and anthelmintic activities of 2-desoxoparaherquamide and selected analogs . Journal of Veterinary Pharmacology and Therapeutics , 27 : 169 – 181 .
- Kaminsky , R , Ducray , P , Jung , M , Clover , R , Rufener , L , Bouvier , J , Schorderet , Weber S , Wenger , A , Wieland-Berghausen , S , Goebel , T , Gauvry , N , Pautrat , F , Skripsky , T , Froelich , O , Komoin-Oka , C , Westlund , B , Sluder , A and Mäser , P . 2008 . Anew class of anthelmintics effective against drug-resistant nematodes . Nature , 452 : 176 – 180 .
- Kaplan , RM . 2004 . Drug resistance in nematodes of veterinary importance: a status report . Trends in Parasitology , 20 : 477 – 481 .
- Leathwick , DM . 2004 . The future cost of anthelmintic resistance to the New Zealand sheep industry . New Zealand Journal of Zoology , 31 : 91 – 91 .
- Leathwick , DM , Hosking , BC , Bisset , SA and McKay , CH . 2009 . Managing anthelmintic resistance: Is it feasible in New Zealand to delay the emergence of resistance to a new anthelmintic class? . New Zealand Veterinary Journal , 57 : 181 – 192 .
- Lee , BH , Clothier , MF and Johnson , SS . 2001 . Semi-synthesis of 2-deoxo- and 3-epiparaherquamide A . Bioorganic Medicinal Chemistry Letters , 11 : 553 – 554 .
- Lee BH Clothier MF Dutton FE Nelson SJ Johnson SS et al. Marcfortine and paraherquamide class of anthelmintics: discovery of PNU-141962 Current Topics in Medicinal Chemistry 2002 2 779 793
- Love , SCJ , Nelson , FJA , Biddle , AJ and McKinnon , R . 2003 . Moxidectin-resistant Haemonchus contortus in sheep in northern New South Wales . Australian Veterinary Journal , 81 : 359 – 360 .
- Mason , PC , Hosking , BC , Nottingham , RM , Cole , DJW , Seewald , W , McKay , CH , Griffiths , TM , Kaye-Smith , BG and Chamberlain , B . 2009 . A large-scale clinical field study to evaluate the efficacy and safety of an oral formulation of the amino-acetonitrile derivative (AAD), monepantel, in sheep in New Zealand . New Zealand Veterinary Journal , 57 : 3 – 9 .
- McKenna , PB . 1990 . The use of benzimidazole-levamisole mixtures for the control and prevention of anthelmintic resistance in sheep nematodes: an assessment of their likely effects . New Zealand Veterinary Journal , 38 : 45 – 49 .
- McKenna , PB . 1996 . Potential limitations of the undifferentiated faecal egg count reduction test for the detection of anthelmintic resistance in sheep . New Zealand Veterinary Journal , 44 : 73 – 75 .
- McKenna , PB . 1997 a . Use of arithmetic and geometric means in the calculation of anthelmintic efficacy . Veterinary Record , 141 : 472 – 473 .
- McKenna , PB . 1997 b . Further potential limitations of the undifferentiated faecal egg count reduction test for the detection of anthelmintic resistance in sheep . New Zealand Veterinary Journal , 45 : 244 – 246 .
- Miller , CM , Waghorn , TS , Leathwick , DM and Gilmour , ML . 2006 . How repeatable is a faecal egg count reduction test? . New Zealand Veterinary Journal , 54 : 323 – 328 .
- Presidente , PJA . 1985 . “ Methods for detection of resistance to anthelmintics ” . In Resistance in Nematodes to Anthelmintic Drugs , Edited by: Anderson , N and Waller , PJ . 13 – 27 . Glebe, NSW, , Australia : CSIRO Division of Animal Health and Australian Wool Corporation . In:
- Sargison , ND , Jackson , F , Bartley , DJ and Moir , ACP . 2005 . Failure of moxidectin to control benzimidazole- levamisole- and ivermectin-resistant Teladorsagia circumcincta in a sheep flock . Veterinary Record , 156 : 105 – 109 .
- Sargison , ND , Jackson , F , Bartley , DJ , Wilson , DJ , Stenhouse , LJ and Penny , CD . 2007 . Observations on the emergence of multiple anthelmintic resistance in sheep flocks in the south-east of Scotland . Veterinary Parasitology , 145 : 65 – 76 .
- Shoop , WL , Egerton , JR , Eary , CH and Suhayda , D . 1990 . Anthelmintic activity of paraherquamide in sheep . Journal of Parasitology , 76 : 349 – 351 .
- Smith , G . 1990 . A mathematical model for the evolution of anthelmintic resistance in a direct life cycle nematode parasite . International Journal for Parasitology , 20 : 913 – 921 .
- Smothers , CD , Sun , F and Dayton , AD . 1999 . Comparison of arithmetic and geometric means as measures of a central tendency in cattle nematode populations . Veterinary Parasitology , 81 : 211 – 224 .
- Sutherland , IA , Damsteegt , A , Miller , CM and Leathwick , DM . 2008 . Multiple species of nematodes resistant to ivermectin and a benzimidazole-levamisole combination on a sheep farm in New Zealand . New Zealand Veterinary Journal , 56 : 67 – 70 .
- Van Wyk , JA , Malan , FS , Gerber , HM and Alves , RMR . 1989 . The problem of escalating resistance of Haemonchus contortus to the modern anthelmintics in South Africa . Onderstepoort Journal of Veterinary Research , 56 : 41 – 49 .
- Van Wyk , JA , Stenson , MO , van der Merwe , JS , Vorster , RJ and Viljoen , PG . 1999 . Anthelmintic resistance in South Africa: surveys indicate an extremely serious situation in sheep and goat farming . Onderstepoort Journal of Veterinary Research , 66 : 273 – 284 .
- Vercruysse , J , Holdsworth , P , Letonja , T , Barth , D , Conder , G , Hamamoto , K and Okano , K . 2001 . International harmonisation of anthelmintic efficacy guidelines . Veterinary Parasitology , 96 : 171 – 193 .
- Waghorn , TS , Leathwick , DM , Rhodes , AP , Lawrence , KE , Jackson , R , Pomroy , WE , West , DM and Moffat , JR . 2006 . Prevalence of anthelmintic resistance on sheep farms in New Zealand . New Zealand Veterinary Journal , 54 : 271 – 277 .
- Wood , IB , Amaral , NK , Bairden , K , Duncan , JL , Kassai , T , Malone , JB Jr , Pankavich , JA , Reinecke , RK , Slocombe , O , Taylor , SM and Vercruysse , J . 1995 . World Association for the Advancement of Veterinary Parasitology (WAAVP) second edition of guidelines for evaluating the efficacy of anthelmintics in ruminants (bovine, ovine, caprine) . Veterinary Parasitology , 58 : 181 – 213 .
- Wooster , MJ , Woodgate , RB and Chick , BF . 2001 . Reduced efficacy of ivermectin, abamectin and moxidectin against field isolates of Haemonchus contortus . Australian Veterinary Journal , 79 : 840 – 842 .
- Wrigley , J , McArthur , M , McKenna , PB and Mariadass , B . 2006 . Resistance to a triple combination of broad-spectrum anthelmintics in naturally-acquired Ostertagia circumcincta infections in sheep . New Zealand Veterinary Journal , 54 : 47 – 49 .
- Yue , C , Coles , GC and Blake , N . 2003 . Multiresistant nematodes on a Devon farm . Veterinary Record , 153 : 604 – 604 .