Abstract
The introduced Australian brushtail possum (Trichosurus vulpecula) is a maintenance host for bovine tuberculosis (TB) in New Zealand and plays a central role in the TB problem in this country. The TB-possum problem emerged in the late 1960s, and intensive lethal control of possums is now used to reduce densities to low levels over 8 million ha of the country. This review summarises what is currently known about the pathogenesis and epidemiology of TB in possums, and how the disease responds to possum control. TB in possums is a highly lethal disease, with most possums likely to die within 6 months of becoming infected. The mechanisms of transmission between possums remain unclear, but appear to require some form of close contact or proximity. At large geographic scales, TB prevalence in possum populations is usually low (1–5%), but local prevalence can sometimes reach 60%. Intensive, systematic and uniform population control has been highly effective in breaking the TB cycle in possum populations, and where that control has been sustained for many years the prevalence of TB is now zero or near zero. Although some uncertainties remain, local eradication of TB from possums appears to be straightforward, given that TB managers now have the ability to reduce possum numbers to near zero levels and to maintain them at those levels for extended periods where required. We conclude that, although far from complete, the current understanding of TB-possum epidemiology, and the current management strategies and tactics, are sufficient to achieve local, regional, and even national disease eradication from possums in New Zealand.
Introduction
The introduced Australian brushtail possum (Trichosurus vulpecula) is a true maintenance host for Mycobacterium bovis in New Zealand (Morris and Pfeiffer Citation1995; Corner Citation2006) and as such plays a central role in the tuberculosis (TB) problem in this country. The few remaining unmanaged M. bovis-infected possum populations are, arguably, now the only host populations in New Zealand able to indefinitely and independently sustain intra-species transmission of TB. This is because in cattle, the classic maintenance hosts of bovine TB, systematic and intensive testing coupled with culling of infected animals prevents long-term cycling of the disease within herds (Buddle et al. Citation2015); while other wildlife hosts are rarely, if ever, capable of independently maintaining the disease (Nugent Citation2011). Intensive lethal control of possum populations has likewise “broken the TB cycle” in most affected areas of the country, and since 2011, eradication of TB from possums has been the management objective for 2.5 million ha or a quarter of the affected area (Anonymous Citation2009).
The TB-possum problem is relatively recent, only emerging in the late 1960s, even though possums, cattle, and M. bovis were all imported into New Zealand in the 1800s (Morris et al. Citation1994; Morris and Pfeiffer Citation1995). TB is highly lethal in possums but has not greatly suppressed possum densities at more than localised scales (Barlow Citation1991a; Arthur et al. Citation2004). Since the 1960s, Vector Risk Areas (VRA), which are areas designated as being potentially occupied by M. bovis-infected possums, have increased in size to a total of about 10.5 million ha, or 38% of New Zealand (Anonymous Citation2009). Intensive vector control (the term widely used for management of TB in wildlife) was initiated in the early 1990s and currently costs ∼$NZ50 million per annum. Vector control is considered to have underpinned major reductions (>95%) in the number of infected cattle and deer herds but has not yet resulted in any discrete area of >100,000 ha being declared TB free (Anonymous Citation2012). Vector control is, therefore, likely to be required for several decades more, creating a need for ever-more cost-effective ways of preventing TB transmission between possum populations and from possums to other wildlife or livestock hosts. That creates a need for on-going improvement in understanding of TB dynamics within possum populations, in order to enable more accurate and reliable prediction of TB management outcomes.
Here, we provide a synthesis of what is currently known about the pathogenesis and epidemiology of TB in possums, and how the disease responds to possum population control. We also aim to identify where improved understanding is most urgently required to enable more efficient local and regional suppression or eradication of the disease.
Ecology and epidemiology of TB in possums
Biology and ecology of possums
Biology
Brushtail possums are 2–3 kg, nocturnal, arboreal marsupials, native to Australia. Although introduced to New Zealand in 1858 to establish a fur trade (Cowan Citation2005) they came to be a major conservation and/or agricultural pest (King Citation2005). The introduction, biology, and management of possums have been documented in detail (Montague Citation2000). Possums now occupy most of New Zealand, with the national carrying capacity estimated at ∼48 million. However, the actual population is only ∼30 million as a result of population control measures and fur harvesting (Warburton et al. Citation2009), suggesting a national average density of ∼1.1 possums/ha. However local carrying capacity densities range from <1/ha in semi-arid scrubland (Rouco et al. Citation2012) or low-diversity forests (Clout and Gaze Citation1984) to over 20/ha in more diverse forests (Efford Citation2000).
Females usually produce a single offspring in the autumn from 1 to 2 years of age onward, and young may also be produced in the spring (Fletcher and Selwood Citation2000). Young are dependent for approximately 6 months, and associate with their mothers for up to 9 months (Fletcher and Selwood Citation2000). Estimates of the intrinsic exponential rate of maximum population increase varies widely, but a mid-point estimate of ∼0.35 per year (Hickling and Pekelharing Citation1989) is often used for modelling purposes (Morgan et al. Citation2006), although Nugent et al. (Citation2010) have suggested that this figure is likely to be too conservative. Females live longer than males, and can survive for 12 years or more. Survival rates vary widely between years, but are typically 60–70% per annum in yearlings, about 90% per annum for 2–5-year old females, 80% per annum for similarly aged males, reducing to below 50% per annum in possums that are >10 years of age (Efford Citation2000).
Ecology
Possums are predominantly arboreal folivores, but will feed opportunistically on other foods, and will occasionally eat meat (Nugent et al. Citation2000) including mammalian carrion (Ragg et al. Citation2000) and, occasionally, possum carcasses (Nugent Citation2005). During the day they den in dark, protected places such as hollow trees, with a single possum using 10–15 different dens per year (Green and Coleman Citation1987). Den use was once considered to be important in TB epidemiology, to the extent that one early TB model (PossPop) was centred largely on modelling den location and use (Pfeiffer Citation1994; Pfeiffer et al. Citation1994a,b). This presumably reflected a belief that, because TB was a respiratory disease, most transmission was likely to be by a respiratory route, and that the damp confined air space in shared dens would be extremely conducive to aerosol transmission. However, although sequential den use by different possums is common, simultaneous den sharing is not (Cowan Citation1989; Cowan and Clout Citation2000; Ji et al. Citation2003). Furthermore, subsequent research has neither supported aerosol transmission as the primary route of infection (Cooke Citation2000), nor demonstrated that dens play a major role in facilitating transmission (Caley Citation1996). Home ranges overlap between the sexes, varying from 0.7 to 5 ha in continuous forest, but sometimes exceeding 100 ha in patchy habitat (Cowan and Clout Citation2000; Rouco et al. Citation2012). About 20–30% of juveniles disperse (mostly males) mainly when 1–2 years old, moving an average of ∼5 km, but distances of >40 km have been occasionally recorded (Cowan and Clout Citation2000).
TB in possums
Origins
Tuberculosis was first identified in captive possums in Europe, possibly as early as 1895 (Moore Citation1903), however, it has never been observed in wild possums in Australia, even though they would have been sympatric with M. bovis-infected cattle for a long time (Lehane Citation1996). Possums will also have been sympatric with M. bovis-infected cattle in New Zealand since the 1800s, but the first New Zealand case of tuberculosis in a possum was not recorded until 1967 (Ekdahl et al. Citation1970). Given the high visibility of TB lesions in possums (see below), and the millions of possums skinned for fur before 1967 (Parkes et al. Citation1996), TB is unlikely to have gone undetected if it had been present in the species long before 1967. Between 1967 and 1981, possums with TB were recorded in many different localities (Julian Citation1981). By then, 33 different strain types had been identified among 83 isolates of M. bovis originating from possums, and isolates of the same type were usually found in the same geographic area (Collins et al. Citation1986). This implies that TB did not spill over from other infected species to possums until the 1960s, but at this time spillover events occurred many times.
Morris and Pfeiffer (Citation1995) suggested that TB did not spill over into possums directly from cattle, but indirectly via wild deer. Transmission between deer and cattle has been reported in Michigan, United States of America (Schmitt et al. Citation2002), and experiments have demonstrated that this could occur via shared feedstuffs (Palmer et al. Citation2004). That cattle-deer-possum spillover hypothesis has since been extended with the suggestion that transmission from deer to possums only occurred after about 1960 (Nugent Citation2011), because the advent of commercial deer hunting at that time resulted in the heads and alimentary tracts of large numbers of killed deer being left in the field, providing scavenging possums with easy access to the retropharyngeal and mesenteric lymph nodes, which are common sites for tuberculous lesions in deer (Lugton et al. Citation1998; Nugent Citation2005).
Examples of deer-to-possum transmission include the establishment of a de novo TB focus in a possum population in the North Island with a South Island M. bovis strain type (Mackereth Citation1993), and establishment of West Coast and Otago M. bovis strains in Mackenzie Basin possums soon after deer farms there were stocked with wild deer (de Lisle et al. Citation1995). Importantly, the risk of deer-to-possum spillover constrains how quickly TB can be eradicated from wildlife in New Zealand, because, like cattle and humans, some deer appear able to survive in an infected state for many years (Nugent Citation2005). Modelling suggests the risk of spillback from deer to possums can persist for over a decade after possum control is initiated in an area (Barron et al. Citation2013), suggesting that where deer are present possum control must be maintained for longer than 10 years to ensure TB eradication.
Diagnostics
The diagnostic techniques developed for TB detection and characterisation in New Zealand livestock and wildlife are summarised elsewhere (e.g. Anderson et al. Citation2015). The gold standard for diagnosis of TB in possums has been mycobacterial culture of M. bovis, although a presumptive diagnosis can be based on the characteristic histopathology and presence of acid-fast bacilli. However, for cost reasons, culture has historically been used in operational surveys only to confirm whether TB-like lesions detected during necropsy were caused by M. bovis. Field surveillance of possums has therefore traditionally relied almost exclusively on cross-sectional necropsy surveys of killed possums; however, as perhaps 20–30% of M bovis-infected possum have no visible lesions (see below), such surveys probably have a detection sensitivity of only 70–80%.
Culture of pooled samples of frequently infected tissue types from possums without lesions is occasionally used in research projects and, increasingly, in surveys conducted to determine whether TB is absent from possums (de Lisle et al. Citation2009). In places where possum numbers are low, it is more cost-effective to increase detection sensitivity through culture rather than relying on visual detection of gross tuberculous lesions in additional possums. Ante mortem diagnosis of M. bovis infection in possums has only been used in research, because diagnostic sensitivity is only moderate or low (e.g. 40–55% for serology; Buddle et al. Citation1995; Lyashchenko et al. Citation2008).
Pathology
Tuberculous possums develop thinly walled lesions that are seldom fibrotic and which typically contain high numbers of bacilli (Cooke et al. Citation1995). Wild possums that are naturally infected with M. bovis frequently present with single or low numbers of gross lesions; however, in cachectic, terminally ill possums, lesions are widely disseminated affecting many tissues (Jackson et al. Citation1995a; Cooke Citation2000). In infected wild possums, about three quarters have gross lesions in the lungs and/or associated lymph nodes, with a similar proportion having lesions in the superficial (inguinal and axillary) lymph nodes (Jackson et al. Citation1995a). However, lesion distribution is markedly different in apparently early stage infection (Cooke Citation2000). An intensive microscopic study of 17 naturally infected tuberculous possums in which a single lesion (micro- or macroscopic) was seen, found that 82% had evidence of infection in the peripheral axillary or inguinal lymph nodes, 12% had evidence of lung infection, and 6% had evidence of mesenteric infection (M.M. CookeFootnote1, pers. comm.) The exquisite susceptibility of possums to TB is evident from the very rapid course of infection observed in artificially infected possums that can die within 8–10 weeks after aerosol or intra-tracheal inoculation challenge with as few as 20–125 colony-forming units of M. bovis (Buddle et al. Citation1994; Pfeffer et al. Citation1994a,b). These low-dose artificial infections produced extensive lesions in the lungs and pulmonary-associated lymph nodes, with bacterial counts of up to 107 colony forming units/g of lung tissue. In the terminal stages, small macroscopic lesions can also be found in the spleen, liver or kidneys, along with microscopic lesions throughout the lymphatics (Cooke et al. Citation1999). Histologically, at 2 weeks post-infection lung lesions consist of aggregates of macrophages and lymphocytes around small blood vessels or in alveolar spaces. However, by 4 weeks post-infection expansive lesions with evidence of necrosis and/or caseation and large numbers of acid-fast bacilli can be seen, while multinucleate giant cells, fibroplasia and mineralisation are very rarely recorded. Cellular immune responses as indicated by lymphocyte proliferation assay were first observed 3 weeks after artificial infection (Cooke et al. Citation1999).
Tuberculosis in possums is usually fatal, but complete resolution of infection did occur in two of 90 possums with confirmed M. bovis infections (Corner and Norton Citation2003). Possums are presumed to die of respiratory failure as a result of pulmonary TB with extensive caseation and necrosis. In 20 terminally ill possums, all of six lung lobes in each possum had microscopic lesions and almost all had macroscopic lesions. In contrast, only 60–95% and 15–35% of the individual superficial lymph nodes had micro- and macroscopic lesions, respectively (Cooke Citation2000). As a result, TB in possums is widely characterised as a predominantly respiratory disease (Corner Citation2006).
Lethality
Tuberculous possums can survive for several years (Lugton Citation1997; Corner and Norton Citation2003), but most appear to die within a few months of infection. Naturally infected wild possums, in which TB was detected by palpation of superficial lesions, survived for an average of 4.7 months and a maximum of 14 months in one study (Ramsey and Cowan Citation2003), and for 3.4 months in another (Norton et al. Citation2005). These estimates probably overstate survival because palpation would not detect possums with lung infection alone, and studies have suggested that possums with lung infections die quickly, both in captivity (Buddle et al. Citation1994) and in the wild (Ramsey et al. Citation2009).
If cross-sectional necropsy studies can be assumed to be representative of disease progression, then the ratio of M. bovis-infected possums in the pre-clinical and clinical phases (with and without visible lesions, respectively) should approximate the relative duration of these two phases. In surveys of four wild populations in which all commonly infected tissue sites were cultured, 44% of those culture-positive for M. bovis had no visible lesions (de Lisle et al. Citation2009). Another study suggests that only 11% had no visible lesions (Cooke Citation2000), while in a third study about one-third had no lesions (Lugton Citation1997). Averaging across these studies suggests the pre-clinical phase (i.e. M. bovis infection prior to the development of macroscopic lesions) is probably less than half the duration of the approximately 4-month clinical phase. In line with that, possums experimentally injected in the paws with low doses of M. bovis usually develop detectable lesions by 7 weeks post-infection, but not before (Nugent et al. Citation2013). Overall, most possums appear to die within 4–6 months of becoming infected with M. bovis, with survival of those bearing infection in the lungs likely to be even shorter.
Transmission route
The primary route by which possums become infected remains unclear, but it seems mostly to involve direct transmission between possums rather than indirect transmission via environmental contamination. M. bovis can survive for extended periods in dark enclosed places (such as dens), although the bacterium is short-lived in open well-lit environments (Jackson et al. Citation1995c). However, re-emergence of TB after localised possum eradication at Castlepoint, a town in the lower North Island, was likely to be due to reintroduction of infection in mature and immature diseased possums, and not the survival of M. bovis in the environment (Corner et al. Citation2003c).
There has been a longstanding presumption that respiratory transmission is important in possums, based mainly on the inference that the massively infected lungs in terminally ill possums are likely to generate infective aerosol droplets. Evidence that possums occasionally share dens (see above), and therefore a confined airspace, adds plausibility to this presumption, and under experimental conditions transmission has been shown to occur not only between possums in direct contact (Bolliger and Bolliger Citation1948; O'Hara et al. Citation1976; Corner and Presidente Citation1981), but also between possums in adjacent cages up to 180 cm apart (O'Hara et al. Citation1976). Inhalation has also been favoured because as few as 1–10 bacilli (estimated numbers) can produce infection in the lungs (Jackson et al. Citation1995b; Morris and Pfeiffer Citation1995).
However, other evidence argues against most transmission being via a respiratory route. First, transmission between live adults appears surprisingly difficult to achieve. For example, only 9% of uninfected possums that were each housed with an experimentally infected possum in a small cage, indoors for 3 weeks became infected. Furthermore, none of three possums that were held for 8 weeks with 19 infected possums in a small outdoor pen became infected (Corner et al. Citation2002b), despite densities in the pens being far higher than would be encountered in the wild. In fact, transmission was achieved in those outdoor pens only when socially dominant possums were infected. In addition, efforts to increase the force of M. bovis infection in field experiments by using artificial infection via respiratory or conjunctival routes have been largely unsuccessful (Corner et al. Citation2002a; Tompkins et al. Citation2007). Further, the distribution of lesions in naturally infected wild possums is not well replicated in possums artificially infected via the respiratory tract. Possums have been successfully infected by researchers using a variety of methods, including: intraperitoneal and intramuscular injection, or feeding them infected material (Bolliger and Bolliger Citation1948; Corner and Presidente Citation1980); subcutaneous inoculation and intranasal instillation (O'Hara et al. Citation1976); and intra-tracheal and aerosol inoculation (Buddle et al. Citation1994; Aldwell et al. Citation2003). However, as noted above (see also Pfeffer et al. Citation1994a,b; Cooke et al. Citation1999; Wedlock et al. Citation2005), respiratory challenge invariably results in rapid progression to death. Even microaerosols bearing as few as 20 bacilli (estimated numbers) are able to establish overwhelming lung infection, but with no macroscopic enlargement of peripheral lymph nodes (Aldwell et al. Citation2003).
Experimental intra-conjunctival infection results in slower more natural disease progression and greater involvement of peripheral lymph nodes, but predominantly those of the head (Corner et al. Citation2003a) rather than of the limbs' as appears to be characteristic of natural infection (Cooke Citation2000). This observation led Cooke (Citation2000) to suggest that the predominant route of natural transmission is likely to be percutaneous via the limbs. In line with that suggestion, a low-dose, inter-digital injection, artificial infection model (Nugent et al. Citation2013) has been reported to reliably produce lesions in the limb-associated lymph nodes, often with little evidence of early development of the lung lesions that are likely to result in rapid progression to death.
Overall, we conclude that transmission between possums is likely to occur by a variety of routes, sometimes respiratory, occasionally by ingestion of infected material (or milk), but probably most commonly by percutaneous infection of the limbs with small numbers of bacilli. A difficulty with this conclusion is the apparent rarity of entry-site lesions in the skin, but in humans lesions do not always develop at the entry site (Cooke et al. Citation2002).
Excretion and infectiousness
Excretion of M. bovis via urine or faeces appears to be rare in possums with TB lesions, whereas respiratory excretion (aerosol droplets and sputum) and external excretion (exudate from draining sinuses in peripheral lymph nodes lesions) are much more common, although not universal (Jackson et al. Citation1995b). Excretion from peripheral lymph nodes lesions is probably intermittent, as lesions are initially contained, but later burst, drain and may then heal over (Cooke et al. Citation1995). The mammary glands can occasionally be infected (Jackson et al. Citation1995b), so excretion in milk is also possible.
The period during which M. bovis-infected possums are actively infectious to other possums has not yet been well characterised. The current mainstream epidemiological model for possum TB (Ramsey and Efford Citation2010) assumes, unrealistically, that possums become infectious to other possums from the moment of initial infection up until death. More realistically, respiratory transmission is probably unlikely until large lesions are visible in the lungs (i.e. some 1.5 months after initial infection). Likewise, percutaneous transmission mediated via exudate from externally draining lesions appears unlikely to occur until peripheral lymph-node lesions have burst, which is usually 2–3 months or more after initial infection (Nugent et al. Citation2013). Dead infected possums could also be a source of infection for other species; see below.
Transmission mechanism
The pathways by which transmission occurs are also unclear. Respiratory transmission could occur during den sharing or at other times of close contact such as mating or fighting. However, close contact (to within about a metre) between wild possums outside the breeding season is brief and infrequent. Even during the breeding season the mean duration of contact was only 18–26 seconds per day, with only 2% of the interactions lasting for 5–15 minutes (Ji et al. Citation2005). In extreme high-density captive populations, den sharing and social dominance are the main risk factors for infection (Corner et al. Citation2002b); however, den sharing is rare in the wild (Caley et al. Citation1998).
Contact is obviously far more frequent between mothers and dependent young, and pseudovertical transmission from mother to dependent offspring has long been regarded as a potentially important transmission pathway (Pfeiffer Citation1994). However, the average survival time of <6 months for an M. bovis-infected possum (see above) suggests that it is unlikely to be so, given the 6–9-month period of a young possum's dependency and close association with its mother. Under this scenario, mothers infected at or before the time they gave birth would usually die before the young reached independence, while the offspring would likely experience a high force of infection by multiple mechanisms, including inhalation, resulting in severe infection and rapid death within a few months, before they disperse or reach social dominance.
Single-lesion cases of infection occurring only in the alimentary tract suggest that infection occasionally occurs via the oral route. However, transmission through ingestion of infected vegetation is considered unlikely, because M. bovis does not survive long on pasture (Jackson et al. Citation1995c) and a large infective dose would be required for alimentary tract infection. Moreover, TB infection is rare in other susceptible and frequently sympatric herbivores such as rabbits and sheep (Morris and Pfeiffer Citation1995). Licking or even feeding on infected carrion, including possum carcasses, appears to be a more plausible mechanism of oral-route transmission (Ragg et al. Citation2000; Nugent Citation2005), as M. bovis in infected animal tissues can remain viable for several weeks in winter (Barron et al. Citation2011). Furthermore, it has been shown experimentally that a small proportion of wild possums will lick or ingest sufficient material from deer lymph nodes injected with a dye for them to become marked by the dye (Nugent and Whitford Citation2007), suggesting intimate oral contact with high-risk tissue sites in carrion. That study, and related work, also showed that avian scavengers (weka (Gallirallus australis) and harrier hawks (Circus approximans)) fed in a way that was likely to spread the contents of lesions in carcasses of M. bovis-infected animals over a wide area around the carcass, particularly for possum carcasses, such that this could further increase the likelihood that other possums would come into contact with infectious material.
Percutaneous infection could occur via scratches and abrasions incurred during grooming, fighting, or mating (Cooke Citation2000), or during attacks on dead or dying possums (Nugent Citation2005). Gender-related behaviour appears to be important as gonadectomy increased the transmission rate in females and reduced that in males (Ramsey et al. Citation2006). The reduction in transmission to neutered males was correlated with a reduction in home range size and less fighting between males at mating, and therefore, presumably, a reduction in the number of possum-to-possum contacts and/or the extent of injuries incurred (Ramsey Citation2007). However, if aggressive behaviour during the main mating season in autumn is indeed important for M. bovis transmission, a consequent May-June peak in the seasonal incidence of clinically detectable TB could be expected (following the typical 2-month pre-clinical phase); however, there was no evidence of such a peak in data recorded from longitudinal studies at Castlepoint, Wairarapa, North Island (Lugton Citation1997).
The apparent predominance of limb-associated peripheral lesions in early stage TB pathology in wild possums, the often higher TB prevalences recorded in males, the observed changes in TB prevalence after neutering, and the lack of evidence of consistent seasonality in TB incidence, together suggest that the mechanism of M. bovis infection usually involves direct transmission between possums in close proximity or actual contact. This is most likely to be a result of a year-round behavioural trait that is somehow linked to dominance and/or mate-seeking ranging behaviour. However, indirect transmission due to environmental contact with exudate originating from possums with draining lesions cannot be entirely ruled out.
The presumption that the transmission rate between possums is likely to reflect some form of close contact or interaction has prompted investigation of contact patterns. Social network analysis indicates that possum contact rates are non-random, potentially facilitating higher overall TB transmission rates, as a result of the potential for particularly high rates of transmission to and from the most socially interactive individuals, than would otherwise be the case (Porphyre et al. Citation2008). Contact rates are probably not linearly related to density, although the direction of non-linearity is unclear. On one hand, after experimental lethal possum control was imposed, the frequency of den sharing, and presumably therefore the amount of close contact resulting from that, was shown to decrease disproportionately faster than predicted from density alone (Caley et al. Citation1998). Conversely, the rate of close-proximity contact between males and females during the breeding season tends to remain high even when density is reduced (Ramsey et al. Citation2002). The former form of non-linearity, i.e. a more rapid decrease in contact frequency, would result in TB eradication being easier to accomplish than would be possible with the latter.
Transmission to other species
Ingestion of M. bovis-infected tissue from the carcasses of infected possums is presumed to be the main route of transmission to scavengers such as ferrets and pigs, based on the predominance of head and alimentary tract infection in those species (Coleman and Cooke Citation2001). Transmission to cattle and deer is considered likely to result from them being attracted to and closely investigating, sniffing and licking dead or terminally ill infected possums (Paterson and Morris Citation1995; Sauter and Morris Citation1995). The frequency with which infected possums pass infection onto livestock has not been measured directly. However, Nugent et al. (Citation2006) analysed national livestock testing records for the 1995–2003 period, and identified 890 temporally isolated and short-lived (<1 year) TB outbreaks in livestock herds that they considered likely to reflect transmission events from a single possum. Overall 78% of these herds had a single TB case, 95% had three or fewer, and 99% had eight or fewer. Based on that frequency distribution, it was inferred that 70–80% of occurrences of M. bovis-infected possums on such farms had not resulted in any transmission of TB to livestock.
Epidemiology
Prevalence
There has been only one major in-depth longitudinal study of TB dynamics in an unmanaged possum population; this was conducted in the 1990s by a Massey University research team studying wild possums at Castlepoint, Wairarapa (Pfeiffer Citation1994; Jackson Citation1995; Lugton Citation1997). Most other epidemiological investigations of unmanaged populations have involved either one-off surveys (e.g. Pfeiffer et al. Citation1995; Nugent Citation2005) or annual or periodic resurveys (e.g. Coleman et al. Citation1994; Caley et al. Citation1999, Citation2001).
Where TB is long-established, the prevalence of macroscopic lesions recorded in landscape-scale necropsy surveys of possums is usually <5% and often only 1–2% (Coleman and Caley Citation2000), although it can exceed 60% in some locations (Coleman et al. Citation1999b). Prevalence is usually much higher (9–32%) within the local clusters of infection (so called hot spots) that characterise the distribution pattern of infection in possums (Hickling Citation1995). Prevalence is often, but not always, substantially higher in males than in females, particularly in immature possums (Coleman Citation1988; Jackson et al. Citation1995a; Ramsey et al. Citation2006).
Although tuberculosis is perceived to be a disease of crowding, there is no clear relationship between local possum density at the scale of tens of hectares, which is the scale at which interaction between neighbouring possums usually occurs, and TB prevalence (Barlow Citation1991b). At much larger scales, however, prevalence is related to possum density, at least in the central North Island ().
Figure 1. Relationship between possum abundance, represented by mean (and 95% CI) modified trap catch index (TCI), and mean prevalence of tuberculosis (TB) in possums from five study areas (>5,000 ha) in the North Island of New Zealand in the late 1990s. The modified TCI is likely to be substantially lower than the standard residual trap catch index measure of possum relative abundance, because far longer than normal trap lines were used and multi-kill cyanide baits were additionally placed between traps. Adapted from Nugent (Citation2005).
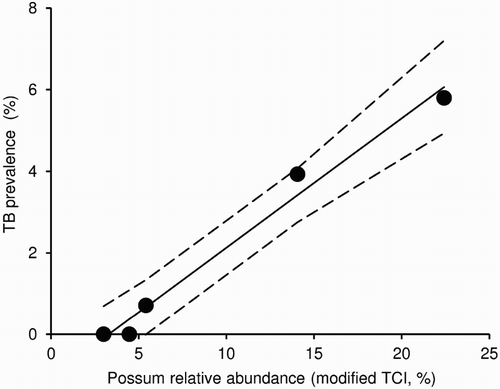
The absence of a positive density-prevalence relationship at possum home-range scales could be explained by a reduction in possum survival. However, monitoring of a local outbreak found no evidence of a measureable reduction in local density due to compensatory recruitment from unaffected neighbouring areas (Arthur et al. Citation2004). The implication is that outbreaks are highly localised and relatively short-lived, with the pool of susceptible animals within the outbreak area quickly being depleted (Arthur et al. Citation2004). That suggests two possible outcomes; either the outbreak dies out at the site, or, where the recruitment rate is high enough, the influx of new susceptible possums is higher than the rate of TB-induced mortality, enabling continuous presence of hot spots of infection in the same area. Barlow (Citation1991b) effectively modelled this scenario as one way of predicting the “extreme patchiness” observed for localised TB foci in possum populations.
On occasion, high prevalences of disease have been recorded at very low possum densities, e.g. a 19.4% prevalence recorded by Coleman and Fraser (Citation2005) in the vicinity of Omoto (Westland) where the possum residual trap-catch index was measured at 0.6%, which indicates a density of just 0.3 possums/ha. However, this may simply have reflected a marked reduction in local density at the end of a high-prevalence epidemic, as observed in sequential surveys at the nearby location of Flagstaff Flat (Coleman et al. Citation1999a).
Models
The dearth of robust empirical data on the epidemiological dynamics of TB in possums has led to the development of a plethora of mathematical and simulation models (Barlow Citation2000a; Caley Citation2006). Of these, the various models developed by Barlow are the most well-known and widely used. Subsequently, progressive efforts to more accurately emulate current epidemiological knowledge and beliefs has developed Barlow's (Citation2000a) non-spatial deterministic model into an individual-based spatially explicit simulation model known as the spatial possum model (Ramsey and Efford Citation2010). Ongoing development and refinement of this model has incorporated phenomena not previously considered, such as the simulation of re-aggregation of possums after they have become isolated from their neighbours due to control operations as a result of reduction to near-zero densities (Barron Citation2012), a control outcome that was rare historically but which is more common nowadays.
As well as being a tool used to explore the effects on TB persistence levels of different types and intensities of TB control measures, such as periodic or annual possum population reduction, vaccination and fertility control (Ramsey and Efford Citation2005; Ramsey et al. Citation2008), the spatial possum model is also being used to predict the likelihood that historical control has been sufficient to eliminate TB-infected possums from a given area. Those estimates are then “validated” using empirical surveillance before an area is declared to be free of TB (Hutchings et al. Citation2011; Anderson et al. Citation2013).
It is informative to re-evaluate historical models in light of new knowledge and current beliefs. For example, PossPop (Pfeiffer Citation1994) was predicated on the assumption that the availability of den sites limited population size. It effectively assumed that there was a large pool of latently infected individuals and that environmental conditions determined rates of progression to clinical disease and death – the “slum city” hypothesis (Caley Citation2006). However, as explained above, field and experimental infection studies indicate that TB is rapidly progressive in possums, with a short pre-clinical phase of <2 months. Posspop modelling also suggested pseudovertical transmission was important, both in maintaining the disease and in spreading it to new locations. However, while infection has been recorded in young dependent possums (Jackson et al. Citation1995b), evidence that the pre-clinical phase is short suggests that the probability of onward transmission from pseudovertically infected possums is probably low.
Another set of models (e.g. Roberts Citation1992) were only able to predict the low TB prevalences measured in large-area surveys by assuming that the threshold possum density above which TB was able to persist in possum populations was close to the carrying capacity density. The consequences of that assumption were the predictions that relatively little possum population control would be required to reduce TB levels, and that the disease would not recover rapidly (within 10 years) after large reductions in possum density. These predictions were considered to be unrealistic given the speed of recovery suggested by the immediate fall but subsequent increases within 4–5 years (after a single possum control operation) in the number of sympatric cattle that became infected (Barlow Citation1991a,b).
The problem of trying to emulate a high local prevalence but low overall prevalence, led Barlow (Citation1991b) to model spatially stable patchiness simply by allowing TB transmission to occur in only a few parts of the landscape. TB does sometimes persist for long periods in more or less the same place (Coleman et al. Citation1999a; Caley et al. Citation2001), but there is no strong evidence that this is universally so for all patches of infection. The current spatial possum model automatically predicts patchiness in infection (hotspots) because, logically, an infected possum can only infect its nearest neighbours. However, in uniform habitats, those clusters of infection are not predicted in the model as being geographically stable, rather they “migrate” as local populations are depleted, as suggested by the increase and subsequent disappearance of TB over 5 years at an unmanaged site (Arthur et al. Citation2004). However, the model predicts stable hot spots in locations where patches of high carrying capacity habitat attract immigrants at a faster rate than they die, which is possibly an explanation for TB persistence at places such as Flagstaff Flat (Coleman et al. Citation1999a).
Another contrast between the earlier deterministic models and the spatial possum model is the inability of the latter to easily emulate rapid in situ recovery of TB in possums after control. Barlow (Citation1991a,b) inferred that such rapid recovery was a real epidemiological characteristic of the disease in possum populations (one that therefore had to be emulated within a model). This inference was based on observations of rapid increases in TB-infected cattle following possum control measures. However, there may well be other explanations for those observations. The most likely being rapid re-establishment of infection through immigration of M. bovis-infected possums from uncontrolled areas, as observed after depopulation of the Castlepoint study area (Corner et al. Citation2003c). Thus it is possible, perhaps even probable, that predictions of slow recovery of TB in possums after control are correct, albeit in the cases of the Roberts (Citation1992) models for the wrong reasons.
An important feature of the Barlow models was the assumption that the transmission rate (β) and the threshold possum density for maintenance of disease are both proportional to the environmental carrying capacity (K) of the possums, rather than to the absolute density of the possums; i.e. the product βK (transmission rate x carrying capacity) is constant. For the models to be able to predict (as was the aim) a broadly similar steady state prevalence of 2–5% (example figures) in different habitats with vastly different carrying capacities, the assumption was made that transmission rate is high in low K habitats and vice versa. This could occur if home range size was inversely related to carrying capacity, such that in poor habitats the few possums present would range more widely and, therefore, contact just as many other possums as would possums in good habitats that have small ranges. This fundamental assumption has never been tested, but it is consistent with TB prevalences of 1–2% being typically observed in very different habitats across New Zealand; as has been observed, for example, in un-forested semiarid areas of the South Island high country where possum densities are low (Rouco et al. Citation2012) but their home ranges are large (Yockney et al. 2013), and also in mixed forest in the Orongorongo Valley in North Island where possum densities are high (Efford Citation2000) but their home ranges small (Cowan and Clout Citation2000). The spatial possum model, in contrast, emulates transmission in relation to density, and therefore requires home range size to be specified according to habitat (Barron Citation2012).
Accuracy of the models
The key parameters of the various possum models have been estimated largely by “tuning” the model to fit observed outcomes (Barlow Citation2000b). Recent sensitivity analysis of the spatial possum model (Barron Citation2012) indicates that its predictions are most sensitive to the disease-induced mortality rate (α), followed (at a much lower level) by the pseudo-vertical disease transmission rate (p) and then horizontal disease transmission rate (β). Only the first (α) is supported by empirical data, with indications that infected possums die faster than previously assumed resulting in a higher mortality rate (see lethality section above).
We also argue above that pseudovertical transmission is unlikely to be as important in onward disease transmission as was presumed, but never quantified, when it was believed that the disease often had a long pre-clinical phase. Using a higher value for mortality rate and a lower value for pseudo-vertical transmission rate in the models would result in predictions of faster eradication of TB after possum control than if the historical default values were used. Using those historically favoured default values, the spatial possum model predicts that 95% reductions in possum density applied to very large areas at 5 year intervals will almost always eradicate TB within 7 years and usually far sooner (Barron et al. Citation2013). TB managers consider this prediction overstates the ease of disease eradication from possums, because TB often continues to be detected in livestock or wild animals (although rarely in possums) after many years of apparently good possum control (P. LivingstoneFootnote2 pers. comm.). This mismatch between prediction and perception may reflect inadequacies in the models, or result from other causes. Greater persistence could, for example, be predicted by the models if contact rates were assumed to be non-linear; i.e. the contact rate and disease transmission do not decline in proportion to any reduction in density (Barlow Citation2000a,b), and there is some support for this (Ramsey et al. Citation2002). On the other hand, the apparent persistence in TB despite intensive possum control could simply reflect immigration from uncontrolled areas, spillback M. bovis infection from deer or other external hosts (as has been suggested for badgers by Hardstaff et al. Citation2012), or localised possum control failure (which could result in residual patches of high possum densities potentially capable of sustaining TB for a number of years; see below). We suggest that, on balance, the speed of eradication predicted using the current spatial possum model is more likely to be pessimistic than optimistic. That conclusion is supported by unpublished data indicating zero prevalence of TB recorded in possum populations in long-term managed areas (see ).
Table 1. Area surveyed, number of possums necropsied, sampling intensity (possums/ha), possums with tuberculosis (TB)-like lesions and lesions that were culture positive for Mycobacterium bovis, and the percentage prevalence of confirmed TB lesions (with upper 99%CI), in operational surveys conducted by TB free NZ during 2007–2012, in areas with different intensities of possum control (G Knowles, unpublished data).
Management of TB in possums
Possum control approach
Wildlife-disease management options
The “test-and-cull” approach used to reduce or eliminate TB from captive livestock has no practical applicability to free-ranging possums. Instead, tuberculosis managers aim to reduce the density of susceptible hosts to below the disease persistence threshold level at which each infected animal is able, on average, to infect one or more other susceptible hosts (Anderson and May Citation1979). That is usually achieved by reducing overall host density (Wobeser Citation1994), but it can also be achieved by reducing the number of susceptible hosts through immunisation (vaccination). Density can be reduced quickly through killing hosts (lethal control) or trapping and removing them; or more slowly through biological controls aimed at either increasing “natural” mortality, or suppressing reproduction. To date, lethal control of the host has been the only tactic used at large operational scales to reduce TB levels in possums. In common with Australia, the only country so far to have eradicated TB from a wildlife host (buffalo Bubalis bubalus), use of intensive lethal control of possums in New Zealand to control TB is possible because the primary host is an introduced species rather than a native one (Nugent and Cousins Citation2014).
Lethal control strategies
Lethal control is achieved using a variety of methods, depending on land tenure, habitat and topography but, in broad terms, there are two main approaches. The first is major periodic reductions in possum density (nowadays typically >90%) at about 5-yearly intervals. This is usually achieved though aerial poisoning, and is usually applied in large tracts of forest and/or remote but vegetated mountain land. Epidemiologically, the aim is to reduce the population to such low levels that it remains below the disease persistence threshold for at least the next 5 years, despite reproductive increase and reinvasion during that period. The second approach is used in more accessible forest and farmland, and involves more frequent but lesser reductions, usually at 1–2 yearly intervals, using ground-based trapping or poisoning. In principle the aim here is to reduce the population and then hold it more or less constant at some level below the disease persistence threshold although in practice this second “maintenance control” approach is often simply a low amplitude version of periodic or pulsed control.
Buffering strategy
A key strategy used to limit reinvasion of farmed areas by M. bovis-infected possums dispersing from uncontrolled populations in forested hinterlands has been to extend the on-farm control some distance into the forest, creating a buffer. The earliest buffers applied were typically 0.5–1.0 km wide, but these tended to be quickly repopulated (Coleman et al. Citation2000) and 3–5 km buffers are now the norm, even though a recent radio tracking study showed few of the possums at the back of a recently controlled buffer subsequently moved more than 600 m across the buffer over the course of a year (Pech et al. Citation2010).
Buffers were also used to contain disease within VRA. As early models suggested a 3 km wide buffer would reduce the flow of M. bovis-infected individuals across it by only 70% (Barlow Citation1991a,b), far wider containment buffers (10–15 km) were usually imposed (Tweddle and Livingstone Citation1994). Nowadays, similarly wide, or wider, areas are used to “buffer” eradication zones from areas in which TB remains unmanaged (Hutchings et al. Citation2011).
Control targets
By the mid-1990s, an early model (Barlow Citation1991a) was being used to set possum control targets. That model predicted that, firstly, the threshold density of susceptible possums for local TB elimination was about half the uncontrolled density, i.e. 50% of the carrying capacity, and, secondly, the density of susceptible possums could be reduced to that level by killing or sterilising about a quarter to a third of the population annually, i.e. at about the recruitment rate, or by immunising a slightly greater proportion annually. The model also predicted that periodic, intensive, lethal control, such as large-scale aerial poisoning every 6 years, would usually eliminate the disease within 2–3 such poisoning periods.
The Barlow model did not specify control targets in terms of absolute density, but rather in relation to carrying capacity. That was not operationally practical, as carrying capacity was not usually known. The operational need for a more tangible control target drove the adoption of targets based on an index of possum abundance, specifically the post-control residual trap catch index (RTCI), which is a percentage measure of nightly possum capture rate in systematically set leg-hold traps (Anonymous Citation2011). The RTCI is still widely used to measure the performance of contractors who work to reduce possum densities and the paradigm of performance-based contracting was central to the rapid improvements in control efficiency and effectiveness from the mid-1990s. An early version of the spatial possum model provided a critical step forward in the TB-possum management strategy, by converting the intangible targets related to carrying capacity to more operationally practical targets based on the RTCI by predicting that holding possums at 2% RTCI for 5–7 years was likely to lead to the eradication of TB from the possum population (Ramsey and Efford Citation2005). This lead to RTCI targets typically being set at 5% for an initial operation aiming to “knock down” the population from high density, and 2% or 1% for subsequent operations aiming to keep reduced populations at very low levels. These targets are much lower than the trap catch index (TCI) values for uncontrolled populations, which are typically above 50% in possums’ most preferred mixed forest habitat (such as that in the North Island) and often above 20% even in their less-preferred beech forest habitat (Nugent et al. Citation2010). The RTCI targets, therefore, represent possum population densities well below the disease persistence threshold predicted by Barlow, so achievement of these targets should usually result in rapid disease elimination.
Effect of lethal control on TB levels in possums
Early outcomes
The earliest attempts to manage TB in possums provided indirect but compelling evidence that possums were important hosts for M. bovis, and that they were transmitting infection to cattle. Implementation of possum control near Cape Foulwind in 1972, for example, saw the reactor rate in cattle fall from 12.3% in 1970 to zero by late 1976 (Hennessey Citation1986). Likewise, during the period 1984–1991, the annual incidence of TB in cattle fell to low levels (0.1%) in a West Coast area subject to possum control, but remained on average about 10 times higher in a nearby similar area with no possum control (Tweddle and Livingstone Citation1994). The effectiveness of those early operations is remarkable given that the percentage reductions were often low, by today's standards. For example, Pannet (Citation1991) recorded reductions of only 63–68% on Owhango Station, Wairarapa, after intensive possum control, which implies that it was relatively easy, in some cases, to reduce possum populations to below the population density threshold for disease persistence. However, funding for TB-related possum control operations virtually ceased during the 1978–1989 period, and TB rapidly became more widespread in possums and other wildlife (Livingstone et al. Citation2015).
1990s
After higher levels of control of possums with TB were re-imposed in about 1989, the subsequent evolutionary development of a national TB-management strategy was underpinned by a reliance on the predictions of the early Barlow epidemiological models to design possum control programmes and set control targets.
In the 1990s, an empirical test of a specific lethal-control regime was conducted at Hohotaka, in the central North Island, that was based on the Barlow models' predictions that eradication could be achieved by an initial knockdown of 75%, followed by annual maintenance control aimed at keeping the population at about 40% of uncontrolled levels. The outcomes of this trial were considered to be consistent with the Barlow models' predictions (Caley et al. Citation1999), even though TB largely disappeared from possums in most of the trial area within 2 years of initial control, which was more rapid than had been predicted. Although there was an apparent resurgence of TB in possums after 5 years, that was largely related to TB persistence in part of the landscape where possum control had neither been applied nor monitored; i.e. the apparent persistence was caused by control and surveillance failure rather than model failure. As TB disappeared quickly from possums even though possums were reduced to only about 7% RTCI (a mediocre level of control by today's standards), the results suggested that the population density threshold at Hohotaka was probably above 10% RTCI, which simulation modelling of the RTCI methodology suggests equates to ∼2 possums/ha (Ramsey et al. Citation2005).
The insight from the Hohotaka study and other studies (e.g. Coleman et al. Citation2005), that TB persistence could possibly be greatly extended by a failure of localised control, resulted in a body of research and modelling into the consequences of “patchiness” in possum density as a result of uneven control (Coleman and Fraser Citation2005; Fraser and Coleman Citation2005). Recent modelling suggests that patches as small as 10 ha with just one possum per hectare could result in TB persisting there for up to 5 years if one or two of them became infected (Barron and Warburton Citation2010).
Hauhungaroa case study
There are few other formally published data on the reduction in TB levels in possums following population reduction, but unpublished data for many areas are consistent with the Hohotaka outcomes. An example is the Hauhungaroa Ranges, central North Island, where the management goal is to prove, by 2026, that TB can be eradicated from wildlife in large forest tracts in which TB is long established (Anonymous Citation2009). In an early (1982–83) survey there, 2.1% of 6083 possums had TB lesions and 1.25% were confirmed histologically as TB-positive (Pfeiffer et al. Citation1995). In that survey, prevalence of infection was almost twice as high in adults than immature animals, and was 40% higher in males than females. Intensive possum control was imposed over much of the area in 1994–95 using aerial distribution of baits containing the toxin sodium fluoroacetate (1080), but a ∼19,000 ha central sector was left un-poisoned (Coleman et al. Citation2000). In a south-eastern sector of the area (the Waihaha catchment), the pre-control possum density was moderately high (TCI∼20%) with a TB prevalence of 1.2%. After control, TCI in that sector fell to zero. The TCI then re-increased to about 8% by 2000 (at which point control was repeated), but no TB was detected among 408 possums necropsied over the 5-year period 1995–1999 (Coleman et al. Citation2000). In contrast, in a western sector poisoned at the same time, control efficacy appears to have been lower, with TCI re-increasing to near pre-control levels by 2000. M. bovis-infected possums were detected in this western sector at both 2 and 5 years after control (Coleman et al. Citation2000), the latter close to the uncontrolled central sector. M. bovis-infected possums were also found at two other locations, one of which was also near the uncontrolled central sector, and the other was in a much smaller 300–400 m wide strip of stream-side area that was deliberately excluded from the 1994 poisoning operation (Nugent Citation2005). Thus the only evidence of TB persisting >2 years after the first imposition of 1080 control was in or near uncontrolled areas. In contrast to the low or zero prevalence in poisoned areas, however, a TB prevalence of close to 6% was recorded within the uncontrolled central sector over the 1997–2000 period (Nugent Citation2005), almost three times higher than the pre-control prevalence in 1982–83. Once again, prevalence in males in the uncontrolled area was twice that in females, and there was no evidence of pseudovertical transmission since no males weighing <1.7 kg and no females <2.8 kg were found to be infected.
The whole of the Hauhungaroa Range was poisoned again in 2000/01, but with indications that control in part of the central, previously unpoisoned, sector was ineffective (Nugent and Whitford Citation2008), and TB was detected in possums from that area in autumn 2005 (de Lisle et al. Citation2009). In winter 2005, a particularly intensive high-specification aerial 1080 poisoning operation successfully reduced possum relative abundance to extremely low levels (TCI 0.05% compared to 20–30% before control) over the whole range (Coleman et al. Citation2007). No possums with TB have been detected in subsequent necropsy surveys within the central sector (n>200; Nugent et al. Citation2014). In line with the decline of TB in possums, infection prevalence in wild deer in managed parts of the eastern range declined from 37% in 1993–94 to near zero levels by 2003 (Nugent Citation2005). In contrast, in pigs the decline was slow until effective control was achieved in the central sector in 2005 (Nugent et al. Citation2011). However, TB was recorded in hunter-killed pigs and deer (one of each) in 2013 (Nugent et al. Citation2014). In summary, although the current programme was initiated in 1994, the Hauhungaroa area as a whole was not under universal-coverage intensive control until 2005. TB was confirmed to still be present in possums in 2005, but has not been identified since then. However, given the persistence of disease in possums until 2005, it is still likely to be present in a few deer (Barron et al. Citation2013), and so may occasionally continue to be found in deer, or in pigs that feed on deer carcasses.
Current situation
The only remaining large area of farmland (albeit extremely rough farmland) where possums are not yet under control is in the northern South Island high country, where possum carrying capacity is low, and based on a 2005–06 survey, the TB prevalence in possums is also low (∼1%) (Byrom et al. Citation2008). In unfarmed areas, the main unmanaged but probably M. bovis-infected possum populations are in deep forest areas more than 3–5 km from farmland, such as on the West Coast and in Kahurangi National Park, the Rimutaka Range in the southern North Island, and parts of the Kaweka, Kaimanawa, and the southern Urewera Ranges in the eastern central North Island.
The remaining ∼8 million ha of the 40% of New Zealand designated as possibly containing M. bovis-infected possums is under some form of possum control. In the 5 years to mid-2012, 606 surveys of TB in possums were conducted within these areas. Of the 79,000 possums necropsied in areas subjected to sustained intensive possum control, none were confirmed infected (). Excluding survey zones that were very large (>25000 ha) or very small (<1000 ha), the mean zone size was ∼5000 ha and the mean sampling intensity per survey was 0.035 possums/ha. Such surveys are likely to sample 25–50% of the population (B. WarburtonFootnote3, pers. comm.), suggesting mean possum densities of <0.15/ha in most of the areas surveyed. This low density figure partly reflects reduced possum densities following control, and partly the inclusion in the figures of zone areas representing large tracts of non-possum habitat (e.g. open pasture). Even allowing for a low TB-detection sensitivity of just 75% at necropsy, we can be 99% confident that the true TB prevalence in possums across all such areas is below 0.02%.
Can TB be eradicated from possums?
By 2013, only ∼5% of the land within the main VRA had been declared TB free, based on empirical surveillance indicating a high (>95%) probability of TB freedom (Livingstone et al. Citation2015); however the data in suggest the predictions of the original Barlow model and its successors that the host population density threshold for TB persistence in possums must be moderate rather than low are accurate. Thus, TB is not sustained at low possum population levels and the disease persistence threshold could actually be ∼25% of carrying capacity (typically equating to RTCI of 5–10% in forested areas). If this is true, then TB could be quickly eliminated from possums by the level of high-intensity control that is currently being utilised, which is aiming to reduce the population to below 1–2% RTCI.
Therefore, the key requirements for large-scale TB eradication appear to be that, firstly, possum control is applied in ways that maintain very low, even possum densities at whole-landscape scales for at least 7 years, and the current operational targets of mean RTCI of 1 or 2% coupled with individual line maxima appear to be appropriate for this purpose. As it is likely to take populations at least 5 years to recover to above the disease persistence threshold, this equates to density being held below the disease persistence threshold for at least 12 years. Secondly, the risk of reintroduction of TB by immigrant possums is minimised by also controlling possums to low levels in 10–15 km wide buffers between eradication zones and adjacent to unmanaged M. bovis-infected populations. Thirdly, possum numbers are kept low for at least 15 years in areas where there is a risk of disease spillback from wild deer, pigs or ferrets. The main mechanism by which deer-to-possum spillback is thought to occur may have been largely eliminated by changes in commercial hunting practices since 1993 (Nugent Citation2011), so this risk is possibly close to being negligible, but the precautionary principle is likely to require it be taken into consideration in some areas. Likewise, the possibility of spillback after extended intra-specific TB persistence in ferrets will be a constraint in a few areas. We consider that the main uncertainties lie in the second and third requirements above. Specifically, what width of buffer and what level of control within that buffer are required to eliminate the risk of TB re-establishment by an M. bovis-infected immigrant, and what duration of control is required to eliminate spillback risk?
There are other uncertainties. TB in cattle and possums persists in some locations despite intensive management, with the Karamea area (northern West Coast region) being the most obvious example. There, possums on farmland have been controlled to targets of <2% RTCI since the early 2000s and aerial control has been applied in the adjacent forest on four occasions, most recently (2008) to a buffer-zone distance of at least 5 km from farmland (Warburton et al. Citation2012). Despite that, TB prevalence in the ∼60 cattle herds there remains high with 20–30% herds infected in the period 2008–12 and, in 2012, M. bovis-infected possums were found on farmland. Whatever the explanation, the continued persistence of disease in this small area suggests that the TB persistence threshold there may be lower, or immigration rates higher, than assumed for elsewhere in the country. While regional anomalies such as this make TB eradication more difficult, the ability to achieve near zero densities of possums with current tools (Coleman et al. Citation2007) makes it likely that it is still technically feasible, albeit probably at higher cost.
Despite these uncertainties, local eradication of disease from possums appears to be straightforward in the majority of cases, particularly given that managers now have the ability to reduce possum numbers to near zero levels and to maintain them at those levels for extended periods when required. We conclude that, although far from complete, current understanding of TB-possum epidemiology, and current management strategies and tactics, are sufficient to achieve local, regional, and even national, eradication of the disease from possums.
Making that not only technically feasible but also affordable will require improvements in the cost-effectiveness of possum control and in surveillance of TB in wildlife, including improved diagnostics. Better understanding is also required of a number of eco-epidemiological issues, all of them complex and difficult. These include the duration of spillback risk, particularly from ferrets; the frequency and distance over which infected possums spread TB, particularly on the West Coast; and further refinement and empirical validation of possum-TB models.
| LPA | = | Lymphocyte proliferation assay |
| RTCI | = | Residual Trap Catch Index |
| TB | = | Bovine tuberculosis |
| TCI | = | Trap Catch Index |
| VRA | = | Vector Risk Area |
Acknowledgements
This review was contracted by TBfree NZ (Project R10735-01), with co-funding from the Ministry of Business, Innovation and employment (Contact C09X0803). We thank the personal correspondents in this manuscript (Dr Paul Livingstone of TBfree NZ and Bruce Warburton of Landcare Research) for their information, and Dr Michelle Cooke for access to unpublished data. We also thank Frank Cross, Paul Livingstone and others for their input and comments on early drafts.
Notes
1M.M. Cooke, Palmerston North, NZ
2P. Livingstone, TBfree New Zealand, Wellington, NZ.
3B. Warburton, Landcare Research, Lincoln, NZ.
*Non-peer-reviewed
References
- Aldwell FE, Keen DL, Parlane NA, Skinner MA, de Lisle GW, Buddle BM. Oral vaccination with Mycobacterium bovis BCG in a lipid formulation induces resistance to pulmonary tuberculosis in brushtail possums. Vaccine 22, 70–6, 2003 doi: 10.1016/S0264-410X(03)00539-5
- Anderson RM, May RM. Population biology of infectious diseases: Part I. Nature 280, 361–67, 1979 doi: 10.1038/280361a0
- Anderson DP, Ramsey DSL, Nugent G, Bosson M, Livingstone P, Martin PAJ, Sergeant E, Gormley AM, Warburton B. A novel approach to assess the probability of disease eradication from a wild-animal-reservoir host. Epidemiology and Infection 141, 1509–521, 2013 doi: 10.1017/S095026881200310X
- Anderson DP, Ramsey DSL, de Lisle GW, Bosson M, Cross ML. Development of integrated surveillance systems for the management of tuberculosis in New Zealand wildlife. New Zealand Veterinary Journal 63 (Suppl. 1), 89–97, 2015
- *Anonymous. National Bovine Tuberculosis Pest Management Strategy - Amendment Proposal Sept 2009. http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Anonymous.%20National%20Bovine%20Tuberculosis%20Pest%20Management%20Strategy%20-%20Amendment%20Proposal%20Sept%202009.%20Animal%20Health%20Board,%20Wellington,%20New%20Zealand,%202009.pdf (accessed 02 September 2014). TBfree New Zealand, Wellington, NZ, 2009
- *Anonymous. A1 Possum population monitoring using the Trap-Catch Method. http://www.npca.org.nz/images/stories/NPCA/PDF/a1_monittrapc_201110_web.pdf (accessed 06 June 2014). National Pest Control Agencies, Wellington, NZ, 2011
- *Anonymous. Animal Health Board Annual Report 2011/2012. http://www.tbfree.org.nz/Portals/0/Annual%20Report%20web.pdf (accessed 26 June 2014). TBfree New Zealand, Wellington, NZ, 2012.
- Arthur A, Ramsey D, Efford M. Impact of bovine tuberculosis on a population of brushtail possums (Trichosurus vulpecula Kerr) in the Orongorongo Valley, New Zealand. Wildlife Research 31, 389–95, 2004 doi: 10.1071/WR03097
- Barlow N. Control of endemic bovine Tb in New Zealand possum populations - results from a simple model. The Journal of Applied Ecology 28, 794–809, 1991a doi: 10.2307/2404208
- Barlow N. A spatially aggregated disease/host model for bovine Tb in New Zealand possum populations. Journal of Applied Ecology, 28, 777–93, 1991b doi: 10.2307/2404207
- Barlow N. Non-linear transmission and simple models for bovine tuberculosis. Journal of Animal Ecology 69, 703–13, 2000a doi: 10.1046/j.1365-2656.2000.00428.x
- *Barlow N. Models for possum management. In: Montague T. (ed). The Brushtail Possum: Biology, Impact and Management of an Introduced Marsupial. Pp. 208–19. Manaaki Whenua Press, Lincol, NZ, 2000b
- *Barron M. Extending and validating the Landcare Research Possum-TB Model. Animal Health Board Project No. R-10736. http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Barron%20M.%20Extending%20and%20validating%20the%20Landcare%20Research%20Possum-TB%20Model.pdf (accessed 02 September 2014). TBfree New Zealand, Wellington, NZ, 2012
- *Barron MC, Warburton B. Identifying habitat patches with low risk for TB persistence. http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Barron%20MC,%20Warburton%20B.%20Identifying%20habitat%20patches%20with%20low%20risk%20for%20TB%20persistence.%20Animal%20Health%20Board%20Project%20No.%20R-10721%20(Module%203.%20Landcare%20Research%20Contract%20Report%20LC762.%20Lincoln,%20NZ,%202010.pdf) (accessed 04 September 2014). TBfree New Zealand, Wellington, NZ, 2010
- Barron MC, Pech RP, Whitford J, Yockney IJ, de Lisle GW, Nugent G. Longevity of Mycobacterium bovis in brushtail possum (Trichosurus vulpecula) carcasses, and contact rates between possums and carcasses. New Zealand Veterinary Journal 59, 209–17, 2011 doi: 10.1080/00480169.2011.595905
- Barron MC, Nugent G, Cross ML. Importance and mitigation of the risk of spillback transmission of Mycobacterium bovis infection for eradication of bovine tuberculosis from wildlife in New Zealand. Epidemiology and Infection 141, 1394–406, 2013 doi: 10.1017/S0950268812002683
- Bolliger A, Bolliger W. Experimental transmission of tuberculosis to Trichosurus vulpecula. American Journal of Botany 35, 182–183, 1948
- Buddle BM, Aldwell FE, Pfeffer A, Delisle GW. Experimental Mycobacterium bovis Infection in the Brushtail Possum (Trichosurus vulpecula) - Pathology, hematology and lymphocyte stimulation responses. Veterinary Microbiology 38, 241–54, 1994 doi: 10.1016/0378-1135(94)90005-1
- Buddle BM, Nolan A, McCarthy A, Heslop J, Aldwell F, Jackson R, Pfeiffer D. Evaluation of three serological assays for the diagnosis of Mycobacterium bovis infection in brushtail possums. New Zealand Veterinary Journal 43, 91–5, 1995 doi: 10.1080/00480169.1995.35860
- Buddle B, de Lisle G, Griffin J, Hutchings S. Epidemiology, diagnostics, and management of tuberculosis in domestic cattle and deer in New Zealand in the face of a wildlife reservoir. New Zealand Veterinary Journal 63 (Suppl. 1), 19–27, 2015
- *Byrom A, Nugent G, Yockney I, Poutu N, Whitford J, McKenzie J, Shepherd J, Porphyre T. Animal Health Board Project No. R-80629 Cost-effective control of Tb in the Northern South Island High Country (NSIHC): Identifying the habitats and vector species requiring control. http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Byrom%20A,%20Nugent%20G,%20Yockney%20I,%20Poutu%20N,%20Whitford%20J,%20McKenzie%20J,%20Shepherd%20J,%20Porphye%20T.%20Cost-effective%20control%20of%20Tb%20in%20the%20Northern%20South%20Island%20High%20Country%20(NSIHC).pdf) (accessed 04 September 2014). TBfree New Zealand, Wellington, NZ, 2008
- Caley P. Is the spatial distribution of tuberculous possums influenced by den “quality”?. New Zealand Veterinary Journal 44, 175–8, 1996 doi: 10.1080/00480169.1996.35967
- Caley P. Bovine tuberculosis in brushtail possums: models, dogma and data. New Zealand Journal of Ecology 30, 25–34, 2006
- Caley P, Spencer NJ, Cole RA, Efford MG. The effect of manipulating population density on the probability of den-sharing among common brushtail possums, and the implications for transmission of bovine tuberculosis. Wildlife Research 25, 383–92, 1998 doi: 10.1071/WR97029
- Caley P, Hickling G, Cowan P, Pfeiffer D. Effects of sustained control of brushtail possums on levels of Mycobacterium bovis infection in cattle and brushtail possum populations from Hohotaka, New Zealand. New Zealand Veterinary Journal 47, 133–42, 1999 doi: 10.1080/00480169.1999.36130
- Caley P, Coleman JD, Hickling GJ. Habitat-related prevalence of macroscopic Mycobacterium bovis infection in brushtail possums (Trichosurus vulpecula), Hohonu Range, Westland, New Zealand. New Zealand Veterinary Journal 49, 82–7, 2001 doi: 10.1080/00480169.2001.36208
- Clout M, Gaze P. Brushtail possums (Trichosurus vulpecula Kerr) in a New Zealand beech (Nothofagus) forest. New Zealand Journal of Ecology 7, 147–55, 1984
- Coleman J. Distribution, prevalence, and epidemiology of bovine tuberculosis in brushtail possums, Trichosurus vulpecula, in the Hohonu Range, New Zealand. Wildlife Research 15, 651–63, 1988 doi: 10.1071/WR9880651
- *Coleman J, Caley P. Possums as a reservoir of bovine Tb. In: Montague T (ed). The Brushtail Possum: Biology, Impact and Management of an Introduced Marsupial. pp. 92–104. Manaaki Whenua Press, Lincoln, New Zealand, 2000
- Coleman JD, Cooke MM. Mycobacterium bovis infection in wildlife in New Zealand. Tuberculosis 81, 191–202, 2001 doi: 10.1054/tube.2001.0291
- *Coleman J, Fraser W. Bovine Tb persistence in low-density possum populations - the patchiness problem. The 13th Australasian Vertebrate Pest Conference. Pp 81–6, 2005
- Coleman JD, Jackson R, Cooke MM, Grueber L. Prevalence and spatial distribution of bovine tuberculosis in brushtail possums on a forest-scrub margin. New Zealand Veterinary Journal 42, 128–32, 1994 doi: 10.1080/00480169.1994.35802
- Coleman J, Cooke M, Jackson R, Webster R. Temporal patterns in bovine tuberculosis in a brushtail possum population contiguous with infected cattle in the Ahaura Valley, Westland. New Zealand Veterinary Journal 47, 119–24, 1999a doi: 10.1080/00480169.1999.36127
- Coleman JD, Cooke MM, Jackson R, Webster R. Temporal patterns in bovine tuberculosis in a brushtail possum population contiguous with infected cattle in the Ahaura Valley, Westland. New Zealand Veterinary Journal 47, 119–24, 1999b doi: 10.1080/00480169.1999.36127
- *Coleman J, Fraser KW, Nugent G. Optimal buffer widths for control of possums in the Hauhungaroa Range: 1994/95–19998/99. Population recovery of possums and wild deer and Tb prevalence in possums, wild deer, and cattle. http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Coleman%20J,%20Fraser%20KW,%20Nugent%20G.%20Optimal%20buffer%20widths%20for%20control%20of%20possums%20in%20the%20Hauhungaroa%20Range%20.%20Population%20recovery%20of%20possums%20and%20wild%20deer%20and%20Tb%20prevalence%20in%20possums,%20wild%20deer,%20a.pdf (accessed 04 September 2014). TBfree New Zealand, Wellington, NZ, 2000
- *Coleman JD, Coleman MC, Nugent G, Yockney I. Animal Health Board Project No. R-50634 Identifying the causes of, and solutions to, vector-related Tb persistence near Featherston. http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Coleman%20JD,%20Coleman%20MC,%20Nugent%20G,%20Yockney%20I.%20Identifying%20the%20causes%20of,%20and%20solutions%20to,%20vector-related%20Tb%20persistence%20near%20Featherston.%20Landcare%20Research%20Contract%20Report%20LC0506005.%202005.pdf (accessed 04 September 2014). TBfree New Zealand, Wellington, NZ, 2005
- Coleman J, Fraser K, Nugent G. Costs and benefits of pre-feeding for possum control. New Zealand Journal of Zoology 34, 185–93, 2007 doi: 10.1080/03014220709510077
- Collins D, De Lisle G, Gabric D. Geographic distribution of restriction types of Mycobacterium bovis isolates from brush-tailed possums (Trichosurus vulpecula) in New Zealand. The Journal of Hygiene (London) 96, 431–8, 1986 doi: 10.1017/S0022172400066201
- *Cooke MM. Pathogenesis of tuberculosis in the brushtail possum, Trichosurus vulpecula. PhD thesis, Massey University, Palmerston North, NZ, 2000
- Cooke MM, Jackson R, Coleman JD, Alley MR. Naturally occurring tuberculosis caused by Mycobacterium bovis in brushtail possums (Trichosurus vulpecula): II. Pathology. New Zealand Veterinary Journal 43, 315–21, 1995 doi: 10.1080/00480169./1995.35912
- Cooke M, Buddle B, Aldwell F, McMurray D, Alley M. The pathogenesis of experimental endo-bronchial Mycobacterium bovis infection in brushtail possums (Trichosurus vulpecula). New Zealand Veterinary Journal 47, 187–92, 1999 doi: 10.1080/00480169.1999.36141
- Cooke MM, Gear AJ, Naidoo A, Collins DM. Accidental Mycobacterium bovis infection in a veterinarian. New Zealand Veterinary Journal 50, 36–8, 2002 doi: 10.1080/00480169.2002.36248
- Corner LAL. The role of wild animal populations in the epidemiology of tuberculosis in domestic animals: how to assess the risk. Veterinary Microbiology 112, 303–12, 2006 doi: 10.1016/j.vetmic.2005.11.015
- Corner LAL, Norton S. Resolution of Mycobacterium bovis infection in wild brushtail possums (Trichosurus vulpecula). New Zealand Veterinary Journal 51, 40–2, 2003 doi: 10.1080/00480169.2003.36329
- Corner LAL, Presidente PJA. Mycobacterium bovis infection in the brush-tailed possum (Trichosurus vulpecula): I. Preliminary observations on experimental infection. Veterinary Microbiology 5, 309–21, 1980 doi: 10.1016/0378-1135(80)90030-9
- Corner LAL, Presidente PJA. Mycobacterium bovis infection in the brush-tailed possum (Trichosurus vulpecula): II. Comparison of experimental infections with an Australian cattle strain and a New Zealand possum strain. Veterinary Microbiology 6, 351–66, 1981 doi: 10.1016/0378-1135(81)90028-6
- Corner LAL, Norton S, Buddle BM, Morris RS. The efficacy of bacille Calmette-Guérin vaccine in wild brushtail possums (Trichosurus vulpecula). Research in Veterinary Science 73, 145–52, 2002a doi: 10.1016/S0034-5288(02)00038-3
- Corner LAL, Pfeiffer DU, de Lisle GW, Morris RS, Buddle BM. Natural transmission of Mycobacterium bovis infection in captive brushtail possums (Trichosurus vulpecula). New Zealand Veterinary Journal 50, 154–62, 2002b doi: 10.1080/00480169.2002.36302
- Corner LAL, Buddle BM, Morris RS. Experimental infection of brushtail possums (Trichosurus vulpecula) with Mycobacterium bovis by conjunctival instillation. The Veterinary Journal 166, 177–84, 2003a doi: 10.1016/S1090-0233(02)00311-8
- Corner LAL, Pfeiffer DU, Morris RS. Social-network analysis of Mycobacterium bovis transmission among captive brushtail possums (Trichosurus vulpecula). Preventive Veterinary Medicine 59, 147–67, 2003b doi: 10.1016/S0167-5877(03)00075-8
- Corner LAL, Stevenson MA, Collins DM, Morris RS. The re-emergence of Mycobacterium bovis infection in brushtail possums (Trichosurus vulpecula) after localised possum eradication. New Zealand Veterinary Journal 51, 73–80, 2003c doi: 10.1080/00480169.2003.36343
- *Cowan PE. Denning habits of common brushtail possums, Trichosurus vulpecula, in New Zealand lowland forest. Wildlife Research 16, 63–78, 1989 doi: 10.1071/WR9890063
- *Cowan PE. Brushtail possum. In: CM King (ed). The Handbook of New Zealand Mammals. 2nd Edtn. Pp 56–80. Oxford University Press, Auckland, New Zealand, 2005
- *Cowan PE, Clout M. Possums on the move: activity patterns, home ranges, and dispersal. In: Montague T (ed). The Brushtail Possum: Biology, Impact and Management of an Introduced Marsupial. Pp 24–34. Manaaki Whenua Press, Lincoln, New Zealand, 2000
- de Lisle G, Yates G, Collins D, MacKenzie R, Crews K, Walker R. A study of bovine tuberculosis in domestic animals and wildlife in the MacKenzie Basin and surrounding areas using DNA fingerprinting. New Zealand Veterinary Journal 43, 266–71, 1995 doi: 10.1080/00480169./1995.35905
- de Lisle GW, Yates GF, Coleman JD. Isolation of Mycobacterium bovis from brushtail possums with non-visible lesions. New Zealand Veterinary Journal 57, 221–4, 2009 doi: 10.1080/00480169.2009.36905
- *Efford M. Possum density, population structure, and dynamics. In: Montague TL (ed). The Brushtail Possum: Biology, Impact and Management of an Introduced Marsupial. Pp 47–61. Manaaki Whenua Press, Lincoln, New Zealand, 2000
- Ekdahl M, Smith B, Money D. Tuberculosis in some wild and feral animals in New Zealand. New Zealand Veterinary Journal 18, 44–45, 1970
- *Fletcher T, Selwood L. Possum reproduction and development. In: Montague TL (ed). The Brushtail Possum. Biology, Impact and Management of an Introduced Marsupial. Pp 62–81. Manaaki Whenua Press, Lincoln, New Zealand, 2000
- *Fraser KW, Coleman JD. Animal Health Board Project No. R-10536 Patchiness of possums after control as a determinant of short-term persistence of Tb. http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Fraser%20KW,%20Coleman%20JD.%20Patchiness%20of%20possums%20after%20control%20as%20a%20determinant%20of%20short-term%20persistence%20of%20Tb.%20Landcare%20Research%20Contract%20Report%20LC0405037,%20Lincoln,%20NZ,%202005.pdf (accessed 04 September 2014). TBfree New Zealand, Wellington, NZ, 2005
- Green W, Coleman J. Den sites of possums, Trichosurus vulpecula, and frequency of use in mixed hardwood forest in Westland, New-Zealand. Wildlife Research 14, 285–92, 1987 doi: 10.1071/WR9870285
- Hardstaff JL, Bulling MT, Marion G, Hutchings MR, White PCL. Impact of external sources of infection on the dynamics of bovine tuberculosis in modelled badger populations. BMC Veterinary Research 8 (92), 1–10, 2012
- *Hennessey S. Epidemiological studies of tuberculosis in cattle and possums – studies involving the central North Island endemic area. Surveillance 13 (3) 20–2, 1986
- *Hickling G. Clustering of tuberculosis infection in brushtail possum populations: implications for epidemiological simulation models. In: Griifin F, de Lisle G (eds). Tuberculosis in Wildlife and Domestic Animals. Pp 174–7. University of Otago Press, Dunedin, New Zealand, 1995
- Hickling GJ, Pekelharing CJ. Intrinsic rate of increase for a brushtail possum population in rata/kamahi forest, Westland. New Zealand Journal of Ecology 12, 117–20, 1989
- *Hutchings S, Hancox N, Livingstone P. Approaches to eradication of tuberculosis from wildlife in New Zealand: a revised pest management strategy. Vetscript 24 (8), 8–12, 2011
- *Jackson R. Transmission of tuberculosis (Mycobacterium bovis) by possums. PhD thesis, Massey University, Palmerston North, NZ, 1995
- Jackson R, Cooke MM, Coleman JD, Morris RS. Naturally occurring tuberculosis caused by Mycobacterium bovis in brushtail possums (Trichosurus vulpecula): I. An epidemiological analysis of lesion distribution. New Zealand Veterinary Journal 43, 306–14, 1995a doi: 10.1080/00480169./1995.35911
- Jackson R, Cooke MM, Coleman JD, Morris RS, de Lisle GW, Yates GE. Naturally occurring tuberculosis caused by Mycobacterium bovis in brushtail possums (Trichosurus vulpecula): III. Routes of infection and excretion. New Zealand Veterinary Journal 43, 322–7, 1995b doi: 10.1080/00480169./1995.35913
- Jackson R, deLisle GW, Morris RS. A study of the environmental survival of Mycobacterium bovis on a farm in New Zealand. New Zealand Veterinary Journal 43, 346–52, 1995c doi: 10.1080/00480169./1995.35918
- Ji WH, Sarre SD, Craig JL, Clout MN. Denning behavior of common brushtail possums in populations recovering from density reduction. Journal of Mammalogy 84, 1059–67, 2003 doi: 10.1644/BOS-030
- Ji WH, White PCL, Clout MN. Contact rates between possums revealed by proximity data loggers. Journal of Applied Ecology 42, 595–604, 2005 doi: 10.1111/j.1365-2664.2005.01026.x
- Julian AF. Tuberculosis in the possum Trichosurus vulpecula. Proceedings of the First Symposium on Marsupials in New Zealand 74, 163–74, 1981
- *King C. The Handbook of New Zealand Mammals. 2nd Edtn. Oxford University Press, Melbourne, Australia, 2005
- *Lehane R. Beating the Odds in a Big Country: The Eradication of Bovine Brucellosis and Tuberculosis in Australia. CSIRO Publishing, Melbourne, Australia, 1996
- Livingstone P, Nugent G, de Lisle G, Hancox N. Toward eradication: the effect of Mycobacterium bovis infection in wildlife on the evolution and future direction of bovine tuberculosis management in New Zealand. E-published ahead of print, New Zealand Veterinary Journal 63 (Suppl. 1), 4–18, 2015
- *Lugton IW. The contribution of wild mammals to the epidemiology of tuberculosis (Mycobacterium bovis) in New Zealand. PhD thesis, Massey University, Palmerston North, NZ, 1997
- Lugton IW, Wilson PR, Morris RS, Nugent G. Epidemiology and pathogenesis of Mycobacterium bovis infection of red deer (Cervus elaphus) in New Zealand. New Zealand Veterinary Journal 46, 147–56, 1998 doi: 10.1080/00480169.1998.36079
- Lyashchenko KP, Greenwald R, Esfandiari J, Chambers MA, Vicente J, Gortazar C, Santos N, Correia-Neves M, Buddle BM, Jackson R, O'Brien DJ, Schmitt S, Palmer MV, Delahay RJ, Waters WR. Animal-side serologic assay for rapid detection of Mycobacterium bovis infection in multiple species of free-ranging wildlife. Veterinary Microbiology 132, 283–92, 2008 doi: 10.1016/j.vetmic.2008.05.029
- *Mackereth G. The Waipawa endemic area: the epidemiological picture. Proceedings of New Zealand Veterinary Association Deer Branch Course 10, 222–8, 1993
- *Montague TL. (ed). The Brushtail Possum: Biology, Impacts and Management of an Introduced Marsupial. Manaaki Whenua Press, Lincoln, New Zealand, 2000
- Moore J. Tuberculosis in an Australian opossum. Veterinary Journal 8, 283, 1903
- Morgan D, Nugent G, Warburton B. Benefits and feasibility of local elimination of possum populations. Wildlife Research 33, 605–14, 2006 doi: 10.1071/WR06055
- Morris RS, Pfeiffer DU. Directions and issues in bovine tuberculosis epidemiology and control in New Zealand. New Zealand Veterinary Journal 43, 256–65, 1995 doi: 10.1080/00480169./1995.35904
- Morris RS, Pfeiffer DU, Jackson R. The epidemiology of Mycobacterium bovis infections. Veterinary Microbiology 40, 153–77, 1994 doi: 10.1016/0378-1135(94)90053-1
- Norton S, Corner LAL, Morris RS. Ranging behaviour and duration of survival of wild brushtail possums (Trichosurus vulpecula) infected with Mycobacterium bovis. New Zealand Veterinary Journal 53, 293–300, 2005 doi: 10.1080/00480169.2005.36563
- *Nugent G. The role of wild deer in the epidemiology and management of bovine tuberculosis in New Zealand. PhD Thesis, Lincoln University, Lincoln, NZ, 2005
- Nugent G. Maintenance, spillover and spillback transmission of bovine tuberculosis in multi-host wildlife complexes: A New Zealand case study. Veterinary Microbiology 151, 34–42, 2011 doi: 10.1016/j.vetmic.2011.02.023
- *Nugent G, Cousins DV. Zoonotic tuberculosis in Australia and New Zealand. In: Thoen CO, Steele JH, Kaneene JB (eds). Zoonotic Tuberculosis: Mycobacterium bovis and Other Pathogenic Mycobacteria. 3rd Edtn. Pp 221–234. John Wiley and Sons, 2014
- *Nugent G, Whitford J. Animal Health Board Project No. R-10678 Is residual Tb infection in deer and pig populations important? http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Nugent%20G,%20Whitford%20J.%20Is%20residual%20Tb%20infection%20in%20deer%20and%20pig%20populations%20important.pdf (accessed 04 September 2014). TBfree New Zealand, Wellington, NZ, 2007
- *Nugent G, Whitford EJ. Animal Health Board Project No. R-10688 Confirmation of the spatial scale and duration of spillback risk from Tb-infected pigs. http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Nugent%20G,%20Whitford%20EJ.%20Confirmation%20of%20the%20Spatial%20Scale%20and%20Duration%20of%20Spillback%20Risk%20from%20Tb-infected%20Pigs.pdf (accessed 04 September 2104). TBfree New Zealand, Wellington, NZ, 2008
- *Nugent G, Sweetapple P, Coleman J, Suisted P. Possum feeding patterns: dietary tactics of a reluctant folivore. In: Montague T (ed). The Brushtail Possum: Biology, Impact and Management of an Introduced Marsupial. Pp 10–23. Manaaki Whenua Press, Lincoln, NZ, 2000
- *Nugent G, Ramsey D, Caley P. Animal Health Board Project No. R-10627. Enhanced early detection of Tb through use and integration of wildlife data into the national surveillance model. http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Nugent%20G,%20Ramsey%20D,%20Caley%20P.%20Enhanced%20early%20detection%20of%20Tb%20through%20use%20and%20integration%20of%20wildlife%20data%20into%20the%20national%20surveillance%20model.pdf (accessed June 3 2013). TBfree New Zealand, Wellington, NZ, 2006
- Nugent G, Whitford J, Sweetapple P, Duncan R, Holland P. Effect of one-hit control on the density of possums (Trichosurus vulpecula) and their impacts on native forest. Science for Conservation 304, 1–64, 2010
- Nugent G, Whitford J, Yockney IJ, Cross ML. Reduced spillover transmission of Mycobacterium bovis to feral pigs (Sus scrofa) following population control of brushtail possums (Trichosurus vulpecula). Epidemiology and Infection 140, 1036–47, 2011 doi: 10.1017/S0950268811001579
- Nugent G, Whitford EJ, Yockney I, Perry M, Tompkins DM, Holtslag N, Cross ML. Percutaneous interdigital injection of Mycobacterium bovis as a model for tuberculous lesion development in wild brushtail possums (Trichosurus vulpecula). Journal of Comparative Pathology 148, 33–42, 2013 doi: 10.1016/j.jcpa.2012.05.006
- *Nugent G, Sweetapple P, Yockney I, Barron M, Latham C. TBfree New Zealand R-140731 Progress toward eradication of TB from wildlife in the Hauhungaroa Ranges. http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Nugent%20G,%20Sweetapple%20P,%20Yockney%20I,%20Barron%20M,%20Latham%20C.%20Progress%20toward%20eradication%20of%20TB%20from%20wildlife%20in%20the%20Hauhungaroa%20Ranges.pdf (accessed 04 September 2014). TBfree New Zealand, Wellington, NZ, 2014
- *O'Hara PJ, Julian AF, Ekdahl MO. Tuberculosis in the Opossum (Trichosurus vulpecula): An Experimental Study. Tuberculosis seminar, Hamilton, New Zealand, 1976
- Palmer MV, Waters WR, Whipple DL. Shared feed as a means of deer-to-deer transmission of Mycobacterium bovis. Journal of Wildlife Diseases 40, 87–91, 2004 doi: 10.7589/0090-3558-40.1.87
- *Pannet G. Field experiences with TB control in New Zealand. Symposium on Tuberculosis. Publication No 132. Veterinary Continuing Education, Palmerston North, New Zealand, 1991
- Parkes JP, Nugent G, Warburton B. Commercial exploitation as a pest control tool for introduced mammals in New Zealand. Wildlife Biology 2, 171–7, 1996
- Paterson BM, Morris RS. Interactions between beef cattle and simulated tuberculous possums on pasture. New Zealand Veterinary Journal 43, 289–93, 1995 doi: 10.1080/00480169./1995.35908
- Pech R, Byrom A, Anderson D, Thomson C, Coleman M. The effect of poisoned and notional vaccinated buffers on possum (Trichosurus vulpecula) movements: minimising the risk of bovine tuberculosis spread from forest to farmland. Wildlife Research 37, 283–92, 2010 doi: 10.1071/WR09161
- *Pfeiffer D. The role of a wildlife reservoir in the epidemiology of bovine tuberculosis. PhD thesis, Massey University, Palmerston North, NZ, 1994
- Pfeiffer D, Stern M, Morris R. PossPOP—a geographical simulation model of bovine tuberculosis infection in a wildlife population. Veterinarian 18, 313–5, 1994a
- Pfeffer A, Buddle BM, Aldwell FE. Tuberculosis in the brushtail possum (Trichosurus vulpecula) after intratracheal inoculation with a low-dose of Mycobacterium bovis. Journal of Comparative Pathology 111, 353–63, 1994b doi: 10.1016/S0021-9975(05)80094-5
- Pfeiffer DU, Hickling GJ, Morris RS, Patterson KP, Ryan TJ, Crews KB. The epidemiology of Mycobacterium bovis infection in brushtail possums (Trichosurus vulpecula Kerr) in the Hauhungaroa ranges, New Zealand. New Zealand Veterinary Journal 43, 272–80, 1995 doi: 10.1080/00480169./1995.35906
- Porphyre T, Stevenson M, Jackson R, McKenzie J. Influence of contact heterogeneity on TB reproduction ratio R in a free-living brushtail possum Trichosurus vulpecula population. Veterinary Research 39, 31, 2008 doi: 10.1051/vetres:2008007
- Ragg JR, Mackintosh CG, Moller H. The scavenging behaviour of ferrets (Mustela furo), feral cats (Felis domesticus), possums (Trichosurus vulpecula), hedgehogs (Erinaceus europaeus) and harrier hawks (Circus approximans) on pastoral farmland in New Zealand: Implications for bovine tuberculosis transmission. New Zealand Veterinary Journal 48, 166–75, 2000 doi: 10.1080/00480169.2000.36188
- Ramsey D. Effects of fertility control on behavior and disease transmission in brushtail possums. Journal of Wildlife Management 71, 109–16, 2007 doi: 10.2193/2005-699
- Ramsey D, Cowan P. Mortality rate and movements of brushtail possums with clinical tuberculosis (Mycobacterium bovis infection). New Zealand Veterinary Journal 51, 179–85, 2003 doi: 10.1080/00480169.2003.36361
- *Ramsey DSL, Efford M. Animal Health Board Project No. R-10619 Eliminating Tb - Results from a spatially explicit, stochastic model. http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Ramsey%20DSL,%20Efford%20M.%20Eliminating%20Tb%20-%20results%20from%20a%20spatially%20explicit,%20stochastic%20model.pdf (accessed 04 September 2014). TBfree New Zealand, Wellington, NZ, 2005
- Ramsey D, Efford MG. Management of bovine tuberculosis in brushtail possums in New Zealand: predictions from a spatially explicit, individual-based model. Journal of Applied Ecology 47, 911–9, 2010 doi: 10.1111/j.1365-2664.2010.01839.x
- Ramsey D, Spencer N, Caley P, Efford M, Hansen K, Lam M, Cooper D. The effects of reducing population density on contact rates between brushtail possums: implications for transmission of bovine tuberculosis. Journal of Applied Ecology 39, 806–18, 2002 doi: 10.1046/j.1365-2664.2002.00760.x
- Ramsey D, Efford M, Ball S, Nugent G. The evaluation of indices of animal abundance using spatial simulation of animal trapping. Wildlife Research 32, 229–37, 2005 doi: 10.1071/WR03119
- Ramsey D, Coleman JD, Coleman MC, Horton P. The effect of fertility control on the transmission of bovine tuberculosis in wild brushtail possums. New Zealand Veterinary Journal 54, 218–23, 2006 doi: 10.1080/00480169.2006.36700
- *Ramsey DSL, Nugent G, Bosson M. Animal Health Board Project No. R-10677 Identifying the optimal frequency of control required to eradicate Tb under various RTCI targets. http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Ramsey%20DSL,%20Nugent%20G,%20Bosson%20M.%20Identifying%20the%20optimal%20frequency%20of%20control%20required%20to%20eradicate%20Tb%20under%20various%20RTCI%20targets.pdf (accessed 04 September 2014). TBfree New Zealand, Wellington, NZ, 2008
- Ramsey D, Aldwell FE, Cross ML, de Lisle GW, Buddle BM. Protection of free-living and captive possums against pulmonary challenge with Mycobacterium bovis following oral BCG vaccination. Tuberculosis 89, 163–8, 2009 doi: 10.1016/j.tube.2008.11.002
- Roberts MG. The dynamics and control of bovine tuberculosis in possums. Mathematical Medicine and Biology 9, 19–28, 1992 doi: 10.1093/imammb/9.1.19
- Rouco C, Norbury GL, Smith J, Byrom AE, Pech RP. Population density estimates of brushtail possums (Trichosurus vulpecula) in dry grassland in New Zealand. New Zealand Journal of Ecology 37, 12–7, 2012
- Sauter CM, Morris RS. Behavioural studies on the potential for direct transmission of tuberculosis from feral ferrets (Mustela furo) and possums (Trichosurus vulpecula) to farmed livestock. New Zealand Veterinary Journal 43, 294–300, 1995 doi: 10.1080/00480169./1995.35909
- Schmitt SM, O'Brien DJ, Bruning-Fann CS, Fitzgerald SD. Bovine tuberculosis in Michigan wildlife and livestock. Annals of the New York Academy of Sciences 969, 262–8, 2002 doi: 10.1111/j.1749-6632.2002.tb04390.x
- *Tompkins D, Buddle BM, Ramsey DSL, Aldwell FE. Animal Health Board Project No. R-10630 Efficacy of an oral Tb vaccine on wild possum populations. http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Tompkins%20D,%20Buddle%20BM,%20Ramsey%20DSL,%20Aldwell%20FE.%20Efficacy%20of%20an%20oral%20Tb%20vaccine%20on%20wild%20possum%20populations.pdf (accessed 04 September 2014). TBfree New Zealand, Wellington, NZ, 2007
- Tweddle NE, Livingstone P. Bovine tuberculosis-control and eradication programs in Australia and New-Zealand. Veterinary Microbiology 40, 23–39, 1994 doi: 10.1016/0378-1135(94)90044-2
- *Warburton B, Cowan P, Shepherd J. How many possums are now in New Zealand following control and how many would there be without it? http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Warburton%20B,%20Cowan%20P,%20Shepherd%20J.%20How%20many%20possums%20are%20now%20in%20New%20Zealand%20following%20control%20and%20how%20many%20would%20there%20be%20without%20it.pdf (accessed 04 September 2014). TBfree New Zealand, Wellington, NZ, 2009
- *Warburton B, Anderson D, Nugent G, Whitford J, Neill M. Animal Health Board Project No. R-10726 Identification of risk factors associated with new and persistent infection in cattle herds at Karamea http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Warburton%20B,%20Anderson%20D,%20Nugent%20G,%20Whitford%20J,%20Neill%20M.%20Identification%20of%20risk%20factors%20associated%20with%20new%20and%20persistent%20infection%20in%20cattle%20herds%20at%20Karamea.pdf (accessed 04 September 2014). TBfree New Zealand, Wellington, NZ, 2012
- Wedlock DN, Aldwell FE, Keen D, Skinner MA, Buddle BM. Oral vaccination of brushtail possums (Trichosurus vulpecula) with BCG: immune responses, persistence of BCG in lymphoid organs and excretion in faeces. New Zealand Veterinary Journal 53, 301–6, 2005 doi: 10.1080/00480169.2005.36564
- *Wobeser GA. Investigation and Management of Disease in Wild Animals. Plenum Publishing Corporation, New York, USA, 1994
- Yockney IJ, Nugent G, Latham MC, Perry M, Cross ML, Byrom AE. Comparison of ranging behaviour in a multi-species complex of free-ranging hosts of bovine tuberculosis in relation to their use as disease sentinels. Epidemiology and Infection 141, 1407–16, 2013 doi: 10.1017/S0950268813000289
