Abstract
In New Zealand, wild deer and feral pigs are assumed to be spillover hosts for Mycobacterium bovis, and so are not targeted in efforts aimed at locally eradicating bovine tuberculosis (TB) from possums (Trichosurus vulpecula), the main wildlife host. Here we review the epidemiology of TB in deer and pigs, and assess whether New Zealand's TB management programme could be undermined if these species sometimes achieve maintenance host status.
In New Zealand, TB prevalences of up to 47% have been recorded in wild deer sympatric with tuberculous possums. Patterns of lesion distribution, age-specific prevalences and behavioural observations suggest that deer become infected mainly through exposure to dead or moribund possums. TB can progress rapidly in some deer (<10%), but generalised disease is uncommon in wild deer; conversely some infected animals can survive for many years. Deer-to-deer transmission of M. bovis is rare, but transmission from tuberculous deer carcasses to scavengers, including possums, is likely. That creates a small spillback risk that could persist for a decade after transmission of new infection to wild deer has been halted.
Tuberculosis prevalence in New Zealand feral pigs can reach 100%. Infections in lymph nodes of the head and alimentary tract predominate, indicating that TB is mostly acquired through scavenging tuberculous carrion, particularly possums. Infection is usually well contained, and transmission between pigs is rare.
Large reductions in local possum density result in gradual declines (over 10 years) in TB prevalence among sympatric wild deer, and faster declines in feral pigs. Elimination of TB from possums (and livestock) therefore results in eventual disappearance of TB from feral pigs and wild deer. However, the risk of spillback infection from deer to possums substantially extends the time needed to locally eradicate TB from all wildlife (compared to that which would be required to eradicate disease from possums alone), while dispersal or translocation of pigs (e.g. by hunters) creates a risk of long-distance spread of disease. The high rate at which pigs acquire M. bovis infection from dead possums makes them useful as sentinels for detecting TB in wildlife.
It is unlikely that wild deer and feral pigs act as maintenance hosts anywhere in New Zealand, because unrestricted year-round hunting keeps densities low, with far less aggregation than on New Zealand farms. We conclude that active management of wild deer or feral pigs is not required for local TB eradication in New Zealand.
Introduction
Eradication of tuberculosis (TB) from a multi-species wildlife host complex requires that the disease be managed in all maintenance host species. By definition, maintenance hosts are, if unmanaged, able to sustain the disease independently of other species, whereas, in the long term, spillover hosts do not. Host status is often ascribed to species as if it was a fixed characteristic, but it is not. Rather, host status is a local or regional characteristic that varies with host density and/or with variation in transmission mechanisms and other factors that affect the basic reproductive rate of the disease (Nugent Citation2011).
In New Zealand, introduced brushtail possums (Trichosurus vulpecula) are the most important wild animal maintenance host for TB (Morris and Pfeiffer Citation1995; Coleman and Cooke Citation2001; Nugent et al. Citation2015). In contrast, wild deer (predominantly red deer, Cervus elaphus) and feral pigs (Sus scrofa) are considered to be spillover hosts. Therefore, these species have not been targeted for TB management or control despite the sometimes high prevalences of TB among feral pigs, in particular, often being far higher than the prevalence among sympatric possums (Nugent Citation2011). However, there is strong evidence that wild boar (the conspecific progenitor of feral pigs) are maintenance hosts for TB in parts of Spain (Naranjo et al. Citation2008; Boadella et al. Citation2012), as are white-tailed deer (Odocoileus virginianus) in Michigan, United States of America (USA; O'Brien et al. Citation2011), red deer in parts of Europe (Gortazar et al. Citation2012), and fallow deer (Dama dama) on deer farms in New Zealand and elsewhere (Robinson et al. Citation1989; Griffin et al. Citation2004). Given these differences in host status, if the spillover assumption is not universally valid, the decision not to include active control of TB in wild deer and feral pigs could, potentially, undermine New Zealand's overall TB control and eradication programme. The primary aim of this review is to assess that risk and evaluate its potential importance, based on a summary of the ecology and management of these species in New Zealand and the pathology and epidemiology of TB in them.
Ecology and epidemiology of TB in wild deer and feral pigs
Status
Neither wild deer nor feral pigs are native to New Zealand; both were introduced by early European settlers in the eighteenth or nineteenth centuries and are now widespread. As in America and Europe, these animals are valued as a hunting resource (Figgins and Holland Citation2012) and, since the 1970s, deer have also been domesticated. There were ∼1 million farmed deer New Zealand in 2012 (Anonymous Citation2012a). In June 2012, just five deer farms were confirmed as infected with Mycobacterium bovis (Anonymous Citation2012b), down from over 361 farms in 1987. As introduced species, wild deer and pigs are viewed by conservationists as pests due to their unwanted impacts on indigenous biodiversity (Anonymous Citation2001). Deliberate or incidental killing of deer and pigs as part of TB control and surveillance programmes is not, therefore, as controversial in New Zealand as it is in some other countries.
Wild deer as hosts of TB
Demography and ecology
Seven taxa of wild deer occur in New Zealand (Nugent et al. Citation2001), with a total population of ∼250,000 (Nugent and Fraser Citation1993). Red deer are predominant (∼80% of the 1988 harvest), followed by fallow deer and sika deer (C. nippon) (Nugent Citation1992a). These are the only three deer species known to have been infected with M. bovis in New Zealand. All three are temperate species with similar biology – an autumn mating season leading to an early summer birthing season when a single fawn is born. Females usually reproduce annually from 2 years of age, but males do not reach full maturity until 6–8 years. When not limited by food or resources, deer populations can increase by about 35% annually or higher with immigration (Forsyth et al. Citation2010). Deer can live for more than two decades, but most New Zealand populations are heavily and continuously hunted with no restrictions on age or sex of the animals harvested. As a result, the mean age of deer shot by New Zealand hunters is usually <3 years, with few males surviving to full maturity (and very few beyond 10 years) and few females beyond 15 years (Nugent and Fraser Citation2005).
Wild deer occupy >120,000 km2 of New Zealand, which corresponds to 44% of the country (Fraser et al. Citation2000). Deer are present in most of the 10.5 million hectares of land where TB occurs in wildlife, which are designated as Vector Risk Areas (VRA). However, the deer are mostly confined by hunting to the more heavily forested parts of the VRA, where carrying capacities are probably 15–30 deer/km2 (Nugent and Fraser Citation2005). However, helicopter-based commercial harvesting, introduced in the 1960s, has reduced deer densities in most places, and to near-zero densities in most open areas lacking substantial cover (Challies Citation1991). Therefore, the overall average deer density (all species) in forests is probably <5 deer/km2, with densities in the 2–5 deer/km2 range in the South Island and 5–15 deer/km2 in the North Island (Nugent Citation1992b). Densities of sika and fallow deer tend to be higher than these all-species averages, with extreme local densities of up to 40 deer/km2 recorded occasionally (Nugent and Yockney Citation2004).
Large-scale population control of deer is feasible with the techniques available, but this is currently not part of the national TB management plan. In addition to lethal control measures, vaccination could be used to manage TB in wild deer. Inoculation with live BCG (Bacillus Calmette-Guérin, a non-virulent form of M. bovis) vaccine is effective in protecting red deer and white-tailed deer, and the availability of an effective oral formulation potentially makes it feasible to deliver the vaccine to free-ranging wild deer (Griffin et al. Citation2001; Nol et al. Citation2008). However, the lack of major impediments to lethal control makes it unlikely that vaccination of wild deer would be considered as a viable alternative in New Zealand.
Overall, the densities of wild deer in New Zealand are much lower than in places where spread of TB between deer has been confirmed such as on deer farms and hunting estates in New Zealand and overseas, where densities are typically some hundreds of deer/km2 and the incidence of TB can be very high (Robinson et al. Citation1989; Griffin et al. Citation2004); or in Michigan, USA, where the disease emerged in white-tailed deer during the 1990s after deer densities increased to ∼20 wild deer/km2 as a result of supplemental feeding (O'Brien et al. Citation2002); or in Spain where densities of red deer averaged 19 deer/km2 (range <1–66 deer/km2) across a number of areas, mostly hunting estates (Acevedo et al. Citation2008).
Year-round hunting in New Zealand has resulted in low-density deer populations that are widely dispersed with deer roaming as individuals or in small family groups. In contrast, hunting seasons overseas are typically short in duration, and outside the hunting seasons deer are frequently able to aggregate into larger herds. This natural aggregation can be intensified by supplemental feeding and at water supplies (both natural and artificial), resulting in increased interactions between deer and an increased likelihood of TB transmission (Vicente et al. Citation2007a). In Michigan, supplemental feeding has been considered the crucial factor for TB emergence in wild white-tailed deer there, but not elsewhere in the USA (Miller et al. Citation2003).
Home range size and dispersal distances help determine the scale over which deer could spread TB. In New Zealand, farm escapes or illegal liberations (Fraser et al. Citation2000) are a potential mechanism for long-distance spread of TB. Overseas and New Zealand data indicate that, where cover and food are adequate, female red deer occupy ranges of a few km2, while the ranges of males tend to be substantially larger (Nugent et al. Citation2003a). In mixed forest in the West coast region of the South Island the home range sizes of six young female red deer varied between 1.7 km2 and 3.8 km2, but were much larger for two males at 21 and 26 km2 (Nugent et al. Citation2003a). In unforested high country in New Zealand, adult male ranges can be up to 190 km2 (Knowles Citation1997; Yockney et al. Citation2013). Fallow deer ranges are roughly half as large as that of red deer, and sika deer are intermediate (Nugent et al. Citation2001). Few female red deer disperse more than 4 km, and although most males do disperse, only 5% move more than 10 km (Nugent et al. Citation2003a).
TB origins and source
Tuberculosis was first recorded in wild deer in New Zealand in 1954 on the West Coast (Livingstone et al. Citationforthcoming) and was widespread by the 1980s in both wild and farmed deer (de Lisle and Havill Citation1985; Lugton et al. Citation1998), with infections reported from red, sika and fallow deer (Cooke et al. Citation1999). The susceptibility of deer to TB varies widely among individuals and is influenced by climate, nutrition, age and genetic factors (Griffin and Buchan Citation1994; Griffin and Mackintosh Citation2000). Unlike cattle, farmed deer in New Zealand rarely exhibit clinical signs of the disease before the last 1–2 weeks of life (Beatson Citation1985; Griffin and Buchan Citation1994).
Before the 1960s, TB was not widespread in New Zealand wildlife, but deer probably became infected through occasional direct contact with M. bovis-infected cattle, or indirectly via shared feeding areas. For example, there are reports from the mid-1900s that New Zealand farmers would sometimes capture wild fawns, raise them on milk from M. bovis-infected dairy herds, then release them back into the wild, potentially (but unknowingly) creating a reservoir of wildlife infection (P. LivingstoneFootnote1 pers. comm.). In Michigan, USA, occasional outbreaks in cattle herds continue to occur as a consequence of TB transmission from wild white-tailed deer populations (O'Brien et al. Citation2011), and indirect deer-to-cattle transmission via shared feedstuffs has been demonstrated (Palmer et al. Citation2000). Wild deer originally infected by farmed cattle may have been the indirect conduit by which TB established in New Zealand's possum population in the 1960s (Morris and Pfeiffer Citation1995). After the 1960s, however, commercial aerial hunting reduced deer densities to much lower levels (Nugent et al. Citation2001), especially in open grassland areas; therefore, the habitat overlap between cattle and wild deer is now minimal and the likelihood of TB transmission between these species is low.
TB Prevalence
Before the 1990s, overseas reports of TB in wild deer populations indicated prevalences of below 6% (Clifton-Hadley and Wilesmith Citation1991). Since then, much higher prevalences have been reported among red deer in Spain with an average of 14%, range 0–66%, reported from 21 hunting estates (Vicente et al. Citation2006). High local prevalences have also been reported in France (up to 24% among red deer in the 2005–2006 hunting season; Zanella et al. Citation2008), and among fallow deer in England (18.5% prevalence among 65 deer examined between 1971 and 1996; Delahay et al. Citation2002). Observations during the 1980s of high prevalences of TB in farmed and wild deer in New Zealand led to speculation that wild deer could be maintenance hosts of TB (Morris and Pfeiffer Citation1995). However, there were early indications that TB transmission between wild deer was rare, specifically that wild fawns were rarely infected even when a high proportion of their mothers were (Nugent and Lugton Citation1995; Lugton et al. Citation1998). The absence of infection in fawns, however, does not reflect resistance to infection, which was demonstrated when Palmer et al. (Citation2002) induced severe infection in young white-tailed deer fawns by feeding them with TB-contaminated milk. Furthermore, in a large New Zealand outbreak in farmed red deer, over 95% of 5-month-old fawns were reported to be infected (Griffin et al. Citation2004).
There have been no longitudinal studies of M. bovis infection in New Zealand deer, but between 1993 and 2003 periodic cross-sectional surveys of TB prevalence were conducted in the Hauhungaroa and western Kaimanawa Ranges in the central North Island, and in the Hochstetter and Omoto Ranges in the West Coast region of the South Island (Nugent Citation2005). In those studies, the overall TB prevalence was 8–37% in areas where deer were sympatric with uncontrolled tuberculous possum populations; prevalence increased steadily with age for about 3 years after independence, and the increase was faster in males. This suggests that either the incidence of new infection is more or less constant with little loss of infection (through TB-induced mortality or resolution of infection) or, less likely, that the risk and incidence somehow increases with age. After about 4 years of age however, age-specific prevalence stabilised and then appeared to decline in old age, with the decline thought to reflect a combination of TB-induced mortality and some loss of detectable infection through the resolution of overt disease (Nugent Citation2005); consistent with that, a lower proportion of infected adult deer had typical TB lesions compared with sub-adult deer. An initial increase in the prevalence of TB-like lesions with age has also been reported among red deer in Spain (Vicente et al. Citation2006), and in an outbreak among red deer in a small (91 km2) isolated forest area in France (Zanella et al. Citation2008). In the French study, the TB prevalence in juveniles remained constant (11% and 14%) between 2002 and 2005, while that in adults increased from 13% to 32%.
In New Zealand, the prevalence of infection is similar in male and female deer, with a higher prevalence in young males (≤2 years of age) offset by a lower prevalence in older males (>5 years; Nugent Citation2005). In contrast, in Michigan, USA, male white-tailed deer ≥2 years of age were more likely to be infected than females of similar age (O'Brien et al. Citation2002). Similarly, in Spain, the prevalences of infection in sub-adult and adult female red deer were found be lower than the prevalences for males of the same age class (Vicente et al. Citation2006, Citation2007b).
As part of the present review, we obtained and collated unpublished historical statistics from databases held by the Ministry for Primary Industries (or their predecessors), and TBfree New Zealand, on the numbers of commercially harvested wild deer and feral pigs diagnosed as tuberculous during post-mortem meat inspection, or from formal surveys of local TB prevalence in wildlife. The deer records are presented here and the pig data in the pig section below. These records show that from mid 1994 to mid 1997, 209 tuberculous wild deer were identified among a total of 80,457 inspected (0.26% prevalence overall). In contrast, for the period mid-2008 to mid-2012, there were just 26 tuberculous wild deer detected among 74,701 inspected (0.03% prevalence), although this rate still exceeds that in livestock (which was 0.004% of 4.6 m farmed cattle and deer tested between July 2011 and June 2012; TBfree New Zealand Ltd, unpublished data). All 26 TB-positive wild deer identified between 2008 and 2012 were from north Canterbury or the West Coast regions in the South Island, where there are substantial areas in which possum control has not yet been imposed. By contrast, in areas subject to intensive possum control, none of 872 wild deer from 10 formal wildlife TB surveys conducted between mid 2008 and mid 2012 (mostly in the southern and central North Island) were confirmed infected, although eight animals had gross but culture-negative lesions suggestive of TB (Anonymous Citation2012b).
The impact of possum control on TB prevalence in wild deer is most evident in a western central part of Hauhungaroa Range in the North Island. It was here that the highest prevalence ever recorded in wild New Zealand red deer (20/44, 45%) was measured in 1999 (Nugent Citation2005), just before possum control was imposed for the first time. By 2011, after a decade of possum control, prevalence had declined to 0% (0/37) (Nugent et al. Citation2012).
Lethality
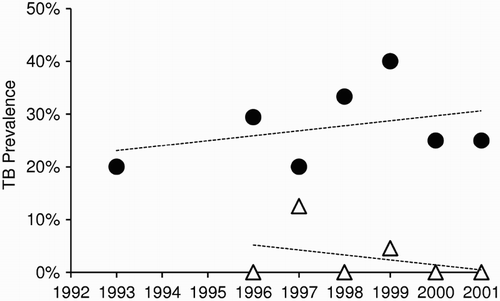
Occasional observations by researchers and New Zealand hunters of young wild deer with that are too weak to flee and have been assumed to be tuberculous, suggest that TB progresses rapidly to death in some wild deer. Infected fawns on deer farms can also die before 12 months of age (Beatson et al. Citation1984). However, there is wide genetic variation in susceptibility to disease in farmed red deer (Mackintosh et al. Citation2000), with only a few (∼5–10%) appearing to be highly susceptible (Griffin and Mackintosh Citation2000). The same appears to be true of wild deer, with indications that some deer can survive for many years with M. bovis infection. This was inferred from areas where possum control had reduced the incidence of new infections in young deer to low levels (and eventually to zero), but the prevalence of infection in adult females remained unchanged, suggesting there is little loss of infection from either additional TB-induced mortality or from resolution (). As noted by Buchan and Griffin (Citation1990), this parallels the long latent phase post-infection followed by potential reactivation late in life that is typical of M. bovis infection in humans (Barry et al. Citation2009). The crucial consequence of deer carrying a latent M. bovis infection is the risk that they can act as a temporal vector, potentially carrying disease through time for a decade or more.
Figure 1. Trends in the prevalence of Mycobacterium bovis infection in female deer between 1993 and 2001 in central North Island areas of New Zealand. The upper series (circles) comprises all annual cohorts of adult and sub-adult females that were born before 1993 and exposed to uncontrolled M. bovis-infected possum populations. The lower series (triangles) comprises adult and sub-adult females born after the implementation of possum control measures in 1993. Adapted from Nugent (Citation2005)
Pathology and lesion distribution
Although gross tuberculous lesions in deer vary in shape and form, the most frequent presentation is well-encapsulated abscesses that are detected by lymph node (LN) enlargement; the LN often contain several granulomas. The material within lesions can be liquefactive, caseous or, particularly in older deer, highly calcified (to the point of being bone-like).
In contrast to a study in Spain, in which 57% of red deer with TB lesions presented with generalised disease and the percentage of generalised cases increased with age (Vicente et al. Citation2007b), generalised TB is comparatively uncommon in wild deer in New Zealand. In the central North Island, about a quarter of M. bovis-infected deer had no visible lesions, compared to two thirds of deer from two areas of the West Coast region in South Island (Nugent Citation2005). In those surveys, the deer with one or more visible lesions usually presented with a single lesion <1 cm in diameter, and many of these lesions were in the head of the animals, in either the tonsils or the retropharyngeal, parotid or submandibular LN. Of 42 deer with only a single gross lesion, Nugent (Citation2005) reported that 42% had infection in the retropharyngeal LN of the head, compared to 15% in the mesenteric LN of the alimentary tract, 10 and 6% in the lung-associated mediastinal and bronchial LN respectively, and 6% in the popliteal LN of the hind leg. If, as is often assumed, the presence of a single lesion usually reflects a relatively early stage of infection, this lesion distribution suggests wild New Zealand deer acquire TB by multiple routes (ingestion, inhalation, and perhaps also occasionally via skin breakage). Lesion distribution resulting from intra-specific transmission among New Zealand farmed deer and in wild white-tailed deer in Michigan, USA (Hathaway et al. Citation1994; O'Brien et al. Citation2001) is broadly similar to that recorded in wild deer in New Zealand, but with lesser involvement of the mesenteric LN. In contrast, in a recent TB outbreak in red deer in France (Zanella et al. Citation2008), there was an unusual predominance of mesenteric lesions thought to reflect ingestion of M. bovis during investigation by deer of offal from hunter-killed tuberculous deer and/or wild boar. However, in Spain a high prevalence of abdominal TB lesions has also been reported among wild red deer, e.g. 54% and 41% of M. bovis-infected red and fallow deer, respectively (Martín-Hernando et al. Citation2010), and in such cases alimentary tract infection is thought to more likely reflect transmission by food and/or water.
Transmission to deer
In New Zealand, the absence of M. bovis infection among wild red deer fawns suggests that deer-to-deer transmission is rare. Furthermore, as fawns share the same foods as their mothers from soon after birth, indirect transmission from other host species via feedstuffs, water, or other environmental contamination must also occur rarely. Deer aside, the other common potential sources of infection for wild deer in New Zealand are from cattle, ferrets (Mustela furo), pigs and possums. However, it is known that wild deer become infected in “deep” forest where they have little opportunity to interact with either ferrets or livestock (Nugent Citation2005), which makes it unlikely that those species are major sources of infection in all cases; and, as explained in the next section of this review, pigs are regarded as end-hosts of TB in New Zealand unless their carcasses are scavenged, reducing their likelihood of transmission of infection to wild deer. Therefore, the inference is that most of the TB observed in wild deer in New Zealand is acquired from possums. This is considered to result from deer aggressively investigating terminally ill possums (Sauter and Morris Citation1995).
Infection via the palatine tonsils in the buccal cavity, which drain into the retropharyngeal LN, is considered the primary route of transmission to both wild and farmed deer in New Zealand (Lugton et al. Citation1998). Supporting this, experimental inoculation of the tonsils of captive red deer with virulent M. bovis produces a pattern of TB that is clinically similar to that seen in naturally infected farmed deer (Mackintosh and Griffin Citation1994). Infection through inhalation of infectious aerosols appears to be of minor importance, because lung lesions are uncommon in wild deer in New Zealand. Furthermore, historically, coughing was rarely seen in infected farmed deer, whereas in cattle, where thoracic lesions are more common, it is a common feature of the disease (Beatson Citation1985).
Tonsillar infection may occur when the animal is eating, drinking, licking, muzzling or grooming (Mackintosh and Griffin Citation1994). We consider that the predominance of infection in the LN of the head, but lesser prevalences in the LN of the thorax and of the alimentary tract, implicates licking and muzzling of some source of infection, such as dead or dying tuberculous possums, as the predominant mechanism of transmission of TB to wild deer in New Zealand, rather than ingestion with food or inhalation of airborne aerosols. Similar patterns of lesion distribution are observed among deer from countries without possums, which suggests that some form of infection involving the oral cavity can occur intra-specifically, either by licking of shared food supplies (Palmer et al. Citation2004) or by visiting communal water sources (Vicente et al. Citation2007b), or perhaps during interactions such as nose-to-nose muzzling that occur frequently at such shared sites.
Transmission from deer
Deer can excrete M. bovis, but in 58 naturally infected wild deer in New Zealand, excretion was minimal with viable M. bovis bacilli isolated from only 8% of oropharyngeal swabs, 2% of tracheal and nasal swabs, 2% of faecal samples and 0% of urine samples (Lugton Citation1997). Most of the positive samples in the study came from four deer that had generalised disease. The higher percentage of positive oropharyngeal swabs was interpreted as evidence that excretion via the tonsils, and therefore in saliva, may be the most common route of excretion (Lugton Citation1997).
On farms, a few infected deer appear to become highly infectious to other deer through development of severe disease, often involving open sinuses from peripheral lymph nodes that drain bacilli directly onto the skin (Lugton Citation1997; Griffin and Mackintosh Citation2000). Rapid spread of TB among farmed deer appears to always involve such severely infected individuals and probably occurs through direct contact with bacilli-laden discharges (Lugton et al. Citation1998). Pseudo-vertical transmission is another potential pathway for intraspecific transmission, as fawns have been experimentally infected by feeding them with contaminated milk (Palmer et al. Citation2002). However, no cases of gross lesions in mammary tissue have been reported from wild deer in New Zealand.
As there is little evidence of either direct or indirect transmission between wild New Zealand deer, indirect transmission from wild deer to farmed deer, cattle or possums is presumably also uncommon. However, cattle have occasionally acquired infection from farmed deer (Hennessey Citation1986). Transmission apparently occurred via pasture contamination in three cases and across a fence in another case. In all four cases, there was a high prevalence of infection in the sympatric or adjacent deer herds.
The main route of interspecific transmission from infected wild deer is likely to be other animals scavenging their carcasses; predominantly pigs, ferrets, and possums. Pigs have been filmed feeding on deer carcasses (Nugent Citation2005) and ferrets on pig carcasses (Yockney et al. Citation2008), so the latter would presumably also scavenge deer carcasses. Importantly, possums occasionally scavenge meat and carcasses (Ragg et al. Citation2000) and have been filmed feeding on pig and deer remains on several occasions, albeit usually only briefly (Nugent Citation2005; Nugent et al. Citation2006). Such feeding has been suggested as the route by which possums first became infected in the 1960s (Nugent Citation2011). The presence of a single large mesenteric lesion in a possum trapped at a site where tuberculous deer carcasses had been left several months previously (Nugent et al. Citation2003a) provides some circumstantial support for this possibility. Furthermore, the detection of new M. bovis infection in possums within 2 years of M. bovis-infected farmed deer of South Island origin being moved to a previously TB-free North Island area (Mackereth Citation1993) provides compelling evidence of TB transmission from deer to possums.
Overall, wild New Zealand deer are most likely to transmit M. bovis to scavenging wildlife, and only rarely to other herbivores (including livestock). Of the potential scavengers, Nugent (Citation2005) has suggested that individual pigs have the greatest likelihood of becoming infected from feeding on tuberculous deer carcasses simply because they consume much more of the carcass per encounter than small scavengers such as ferrets, although the latter in turn consume far more than occasional scavengers such as possums (Nugent Citation2005). However, the rate of M. bovis transmission from deer to scavengers will depend on the relative density and susceptibility to infection of the particular scavengers. Hence, although the likelihood of an individual possum actively scavenging from a tuberculous deer carcass is probably lower than the commensurate risk of pigs or ferrets doing so, the much higher densities of possums (than pigs or ferrets) in typical forested deer habitat, and their seemingly high susceptibility to low doses of M. bovis (Nugent et al., Citation2013), greatly offset this lower risk.
Host status
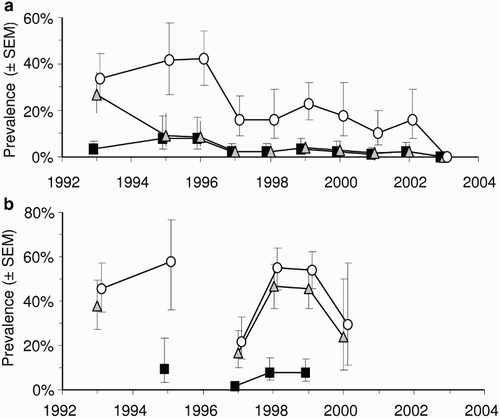
Intraspecific transmission of M. bovis has been demonstrated between experimentally infected and uninfected, captive white-tailed deer, both when they were in direct contact, and when they were not in direct contact but were sharing the same housing facilities (Palmer et al. Citation2001). Transmission has also been recorded between experimentally infected red deer and uninfected red deer in a paddock (Mackintosh and Griffin Citation1994). As already noted, however, there is circumstantial evidence from fawns that deer-to-deer transmission is rare in the wild in New Zealand. In an experiment aimed at determining the host status of wild deer in New Zealand, trends in the prevalence of TB in deer were compared between two areas in which possums (but not deer or pigs) were controlled (Nugent Citation2005). TB prevalence did not decline significantly in areas where possums were not controlled, but declined to near zero levels, over 8 years, in areas where possums were controlled (). This outcome strongly suggests that deer are spillover rather than maintenance hosts in New Zealand, and that most, if not all, infection observed in deer was being acquired from possums in the Nugent (Citation2005) study. The same study also showed that, at a local scale (200–300 ha), the prevalence of TB in deer was strongly and positively related to the local presence of TB in possums, with the simple presence or absence of infection in sympatric possums being a stronger predictor than actual prevalence.
Figure 2. Mean prevalence (±SEM) of Mycobacterium bovis infection in deer killed and necropsied in the Hauhungaroa Range of the central North island of New Zealand from 1993–2003. (a) Data from the eastern side of the range where intensive possum control was applied for the first time in 1994 and (b) Data from the contiguous western side of the range where possums were not controlled during this time. Squares=0–1 year-olds, triangles=1–2 year-olds, circles=>2 year olds. Adapted from Nugent (Citation2005)
In contrast to the New Zealand situation, the persistence of TB in white-tailed deer in Michigan, USA, for 20 years leaves no doubt that deer there are true maintenance hosts. That appears to reflect a combination of generally higher deer densities than in New Zealand, particularly in the most heavily affected areas, and far greater aggregation of deer resulting from the presence of artificial feeding sites. The threshold density for TB persistence there (in conjunction with reduced but continued supplemental feeding) appears to be ∼12 deer/km2 (O'Brien et al. Citation2011). In Spain and France, the situation is complicated by the frequent co-occurrence of red deer and wild boar, especially on hunting estates, where the TB prevalences in both species are closely correlated (Vicente et al. Citation2006). A major reduction in deer density at Brotonne, France, resulted in an eventual reduction in TB prevalence in wild red deer (Hars et al. Citation2010), implying maintenance host status in that case, but wild boar densities were also reduced so the cause and the effect in this situation are not entirely clear. In Spain, reduction in wild boar numbers in a 30 km2 hunting estate resulted in modest decreases in TB prevalence among a high density (30–80 deer/km2) sympatric fallow deer herd (García-Jiménez et al. Citation2013), suggesting that the deer were acting primarily as spillover hosts, even at those high densities. Similarly, in another Spanish study, reductions in wild boar density were inferred to have resulted in reduced infection in sympatric red deer (Boadella et al. Citation2012). As transmission from infected deer to feral pigs/wild boar through consumption of carcasses (or the carcass remnants of hunter-killed deer) seems inevitable, and these two Spanish studies indicate some form of reverse transmission from wild boar to deer is possible, it is hypothesised that the two species may co-amplify the infection in each other.
Feral pigs as hosts of TB
Demography and ecology
Feral pigs are widely, but patchily, distributed in New Zealand. They occupied ∼34% (93,000 km2) of New Zealand in 1996 after having substantially expanded their distributions in Otago, Southland and on the West Coast of the South Island over the preceding decades (Fraser et al. Citation2000). These expansions stemmed partly from natural spread, but mostly from illegal liberations to facilitate recreational hunting.
Pigs occupy a diverse range of habitats, including native forest, plantations, rough farmland, and tussock and shrub land. They require protein-rich foods for successful reproduction and are omnivorous, eating fruits, roots, invertebrates, small vertebrates, and carrion. Possum carrion comprised 15% of pig diets in native forest in the central North Island (Thomson and Challies Citation1988). Pigs often feed in family groups; Coleman et al. (Citation2005) filmed an adult sow eating virtually all of a possum carcass, and later returning with a litter of five 1–2-month-old piglets that all investigated the litter for remains. As M. bovis-infected possum carcasses carry a large burden of infective material, a single-infected possum carcass clearly has the potential to infect whole groups of scavenging pigs, which would amplify the numbers of infected animals in an area.
Extremely high pig densities were recorded in the mid 1900s in New Zealand with 123 pigs/km2 (Wodzicki Citation1950), but such high densities have not been reported since (McIlory Citation2005). Densities of 3–8 pigs/km2 and 12–43 pigs/km2 were recorded in heavily hunted and un-hunted areas, respectively, near Murchison, South Island in the 1980s (McIlroy Citation1989). Densities are maintained at these reduced levels by year-round, ground-based, recreational shooting while hunting other big game or, more commonly, by using dog teams trained to trail and corner pigs. In open areas, helicopter-based shooting was historically used to harvest pigs commercially, to control numbers, and/or collect samples for TB surveillance. Because feral pigs are gregarious, deliberately released “Judas” pigs have also been used to help locate resident pigs for TB surveillance in management or research studies (Knowles Citation1994; Yockney and Nugent Citation2006). This involves capturing or rearing feral pigs, fitting them with a radio-transmitting collars and releasing them into the wild. Radio telemetry is then used to relocate them on a number of subsequent occasions, and any resident uncollared pigs that they have become associated with are shot. This Judas method facilitates location and shooting of feral pigs at approximately twice the rate of untargeted hunting (Nugent et al., Citation2014). With the available range of hunting techniques, it is feasible to maintain feral pig populations at very low densities in most areas, but deliberate control of feral pig populations has not been applied for TB management purposes in New Zealand. Furthermore, preventive vaccination of feral pigs has not been tested as a control measure in New Zealand, although this is being investigated overseas using either BCG or preparations of killed M. bovis bacilli as potential oral-delivery vaccines (Garrido et al. Citation2011; Beltrán-Beck et al. Citation2012).
There are usually no restrictions on the numbers, age or sex of pigs harvested, at least on public conservation land, and the continual hunting pressure generally prevents pigs aggregating into large groups. On some private lands and plantation forests, pigs are managed for hunting more actively though the use of closed seasons, and pig numbers are supplemented with regular releases of animals raised in captivity. The latter are usually TB-tested before release and necropsied on recovery, which helps to confirm that TB is absent from wildlife in those particular areas.
Nationwide, extrapolation from the estimated harvest of 100,000 pigs in 1988 (Nugent Citation1992a) using an exponential rate of increase of 60% (see below) suggests a population size of ∼110,000 at that time, which corresponds to ∼1 pig/km2 across the entire area occupied by feral pigs. These New Zealand densities appear to be 1–2 orders of magnitude lower than the densities of up to 90 pigs/km2 recorded for M. bovis-infected boar populations in supplementally fed and watered hunting estates in Spain (Acevedo et al. Citation2007).
Feral pigs have a high potential reproductive rate, being able to produce up to three litters over 14–16 months, with a mean litter size of 6.2 (range 1–11, Dzieciołowski et al. Citation1992). The large litter size results in potentially larger family groups than are seen for deer. Survival of piglets is extremely variable, exceeding 90% when conditions are good, but <10% when they are poor (McIlory Citation2005). Pigs have a shorter life span than wild deer, particularly in heavily hunted populations, where the mean age at harvest can be less than 1 year (Dzieciolowski and Clarke Citation1989). With high but extremely variable birth and death rates, the exponential rate of population increase can vary widely. For example, fluctuations between 29–118% per annum have been reported from Australia (Choquenot et al. Citation1996), which would result in booms and busts in pig numbers over time.
Adult feral pigs in New Zealand appear relatively sedentary when food and cover are adequate (McIlroy Citation1989), but may be more nomadic in open areas (Knowles Citation1994). In five New Zealand studies in largely forested areas, average home range size varied between 70 and 1170 ha (Nugent et al. Citation2003b). Knowles (Citation1994) recorded much larger ranges (up to 23,410 ha) among 14 pigs in open mountain land.
There are few data on pig dispersal in New Zealand. In a piglet movement study at Mt White, Canterbury, no long-distance dispersal occurred, home range size ranged from 0.1–14.9 km2, and the maximum distance between successive daily locations of any juvenile was 5.8 km (Barber Citation2004). In contrast, Knowles (Citation1994) recorded much larger movements (up to 30 km) by individual pigs in largely unforested landscapes in central Otago. Nugent et al. (Citation2002) studied 16 pigs deliberately released (as uninfected sentinels) into a forested area on the West Coast; within 6 months all were relocated and killed, by which time all but one were recovered within 13 km of their release points (the one exception was a female that had shifted 35 km). After 6 months of natural TB exposure, all 16 of these pigs had become infected with M. bovis, and the pattern of lesion development indicated that they had probably acquired infection within their first 2 months of release, suggesting, consequently, that the female pig that had shifted 35 km had acquired infection before she dispersed. Overall, pig home range sizes and dispersal distances are usually, but not always, larger than those of deer, particularly in habitats with limited cover.
TB origins and source
In New Zealand and globally, TB was historically common in domestic pigs, often as a result of feeding milk or offal from infected cattle (Nuttall Citation1986; Cousins Citation2001). However, TB was not identified in feral pigs in New Zealand until 1964 (Ekdahl et al. Citation1970). Since then, it has been reported in feral pigs from most areas where TB is endemic in wildlife (de Lisle Citation1994). Furthermore, there are no areas where the prevalence of TB is known to be high in feral pigs, but low or zero in other sympatric or nearby hosts.
Prevalence of TB in feral pigs
The first formal surveys in New Zealand quickly identified high prevalences of TB in some feral pig populations; for example, 31–32% recorded in central Otago between 1989 and 1993 (Wakelin and Churchman Citation1991; Knowles Citation1994). The infected animals were clustered in space, with up to 100% prevalence recorded within some family groups. Subsequent cross-sectional surveys of pigs in areas where tuberculous possums were present reported total-sample prevalences of 42–78%, with 100% of some age-classes infected in some areas (Nugent et al. Citation2011a).
The Ministry for Primary Industries and TBfree New Zealand databases on commercial pig and deer harvest and survey data, as referred to for deer above, indicate that, at a national scale, overall TB prevalence is low among feral pigs in New Zealand; of 12,698 feral pigs harvested commercially in the 3 years to mid 1997, only 133 (1.0%) were tuberculous. Since 2000, commercial harvesting of feral pigs has largely ceased so there are no comparable recent figures. However, in local-area surveys conducted in the 5 years to mid 2012 involving some 13,000 feral pigs, the TB prevalence was 0.2% (n=7,245) in areas long subject to intensive possum control, compared to 0.4% (n=5,645) in less intensively managed areas (TBfree New Zealand Ltd, unpublished data).
In several other countries, TB has been found in feral pigs in areas where the disease occurs in other hosts. In Australia, Corner et al. (Citation1981) documented high prevalences of TB (48–85%) in feral pigs that shared habitat with tuberculous cattle (Bos taurus) and water buffalo (Bubalus bubalis). On Molokai, Hawaii, a 20% prevalence of TB was recorded in feral pigs in 1980, and TB had previously been found in both cattle and wild axis deer (Axis axis) (Essey et al. Citation1981). In semi-domestic, free-ranging black pigs in Sicily, M. bovis was isolated from 3% of pigs within an area of 1260 km2, and from 27% of pigs in two infected herds of ∼3000 animals each (Di Marco et al. Citation2012).
Tuberculosis has become increasingly common in wild boar throughout much of Europe, most notably in Spain, Portugal, France and Italy, but also in England and at low levels throughout much of Eastern Europe (Gortazar et al. Citation2012). In Spain, Portugal, and France, this increasing prevalence is attributed to characteristics of Mediterranean habitats, such as dry summers causing animals to aggregate at the few remaining watering sites. However, changing patterns of land use are also implicated, particularly the development of hunting estates where wild boar are maintained at high densities and managed in ways that promote aggregation at feeders and waterholes (Santos et al. Citation2012). On such estates, and a few natural areas where wild boar are protected, the prevalence of TB-like lesions is frequently higher than 50% and can reach 100% (Vicente et al. Citation2006).
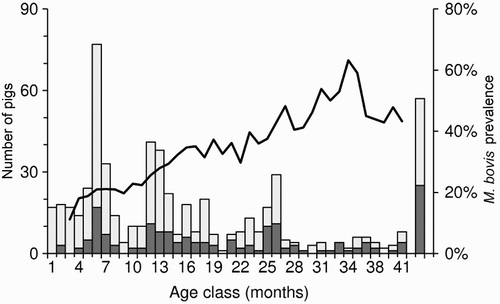
In New Zealand, no M. bovis infection has yet been found in feral piglets that are less than 2-months of age, which suggests little or no vertical or pseudovertical transmission; after 2 months, however, TB prevalence increases with age (). Like deer, this increase suggests an accumulation of infection over time, i.e. pigs acquire infection far more rapidly than they die of TB or resolve infection. In the northern high country of the South Island, the incidence of infection in young pigs exceeded three new cases per year of pig exposure (Nugent and Whitford Citation2008a). In some parts of that area, M. bovis prevalence approached 100% in 1–2-year-old pigs, but was lower in older pigs (Nugent et al. Citation2011a). That decline cannot be attributed to better survival of uninfected sub-adult pigs as there appears to have been none; the implication is, rather, that some pigs are able to resolve detectable infection.
Figure 3. Five-month running average prevalence of Mycobacterium bovis infection (line) and sample size (columns) for 592 pigs aged 1–41 months that were necropsied between 1994 and 2008 in three regions of New Zealand. The far right bar represents all adult pigs >3.5 years old (∼10% of the sample). The dark shading represents the number of tuberculosis-positive animals. Adapted from data presented in Nugent and Whitford (Citation2008b), with permission
In studies of European wild boar, some authors reported increased prevalence rates with age (Vicente et al. Citation2006), but others reported higher prevalence rates and more generalised and severe infection in juveniles (Gortázar et al. Citation2008; Santos et al. Citation2009). The latter could reflect greater TB-induced mortality in young pigs where a combination of factors such as greater genetic susceptibility, high density and inadequate food and/or water places the population under greater stress than experienced by most feral pig populations in New Zealand. Prevalence is usually similar between the sexes, but in one Portuguese study prevalence was much higher in females (Santos et al. Citation2009).
Pathology and lesion distribution
Of all the domestic livestock species, the pig is considered to be the most susceptible to infection with M. bovis (Francis Citation1958); however, there may be some variation in susceptibility. For instance in wild boar, the progenitor of pigs, a high level of genetic heterozygosity is associated with reduced susceptibility to infection and slower progression of disease in infected individuals (Acevedo-Whitehouse et al. Citation2005). Young domestic and feral pigs rapidly develop severe lesions in the LN of the head, lungs and abdominal organs, but with time progressive fibrosis and calcification occur (Lugton Citation1997). Active lesions may even regress and resolve into fibrous tissue, with few or no detectable bacilli present (Ray et al. Citation1972; Bollo et al. Citation2000). In general, numbers of viable bacilli in lesions in feral pigs in New Zealand appear to be low, especially compared to those in possums and ferrets (Cooke et al. Citation1999). In a North Canterbury study, samples from 15/43 (35%) of >3-year-old feral pigs with suspect TB lesions were negative on first culture (Nugent et al. Citation2011a). The culture negative samples were typically from lesions that were either heavily calcified or were fibrotic with few or no necrotic foci. However, for a sub-sample these (n = 10), five proved positive after a second re-culture, indicating that low numbers of viable bacilli were present that were on the borderline of detectability using mycobacterial culture methods.
In New Zealand, almost all pigs with visible lesions have infection in the sub-maxillary LN, and those without head lesions usually have gross lesions in the alimentary tract (Wakelin and Churchman Citation1991; Lugton Citation1997; Nugent et al. Citation2011a). This distribution is likely to be a consequence of pigs ingesting tuberculous carrion. The above-mentioned studies show that generalised disease affecting many body regions is usually rare in New Zealand. By contrast, in a Spanish study of wild boar, LN in the head were the most frequently affected (107/116 animals, 92%) but, unlike New Zealand observations, 38% of boar also had TB lesions in the lungs (Martín-Hernando et al. Citation2007).
Lethality
Of the large mammalian hosts, pigs (particularly adults) appear to be the least detrimentally affected by M. bovis infection. Consistent with that, New Zealand pigs rarely show clinical signs of TB. In a study in Hochstetter Forest, West Coast region, South Island, all of 16 released pigs that became M. bovis-infected within 2 months of their first exposure to natural infection, were still in good condition when the animals were killed up to 21 months later, and none had external signs of disease (Nugent et al. Citation2002). The ability to tolerate infection, coupled with the accumulation of infection with age, implies that TB-induced mortality is low in feral New Zealand pigs, at least within the first year infection.
Transmission to pigs
The predominance of lesions in the head and mesenteric LN in feral pigs in New Zealand is likely to result from scavenging tuberculous carrion, such as possums (Nuttall Citation1986; de Lisle Citation1994; Lugton Citation1997). Ramsey and Cowan (Citation2003) suggested that about 80% of M. bovis-infected possums die in places accessible to pigs. In one 400 ha South Island high country area containing over 20 pigs, pigs found and scavenged 32 of 40 possum carcasses that had been randomly placed in the area (Barber 2004). Based on broad best guesses as to pig and possum densities on Molesworth Station, Marlborough region, Nugent and Whitford (Citation2008a) estimated that an individual uninfected pig would have a high (0.38–0.74) probability of finding and becoming M. bovis-infected if a tuberculous possum carcass was located within its home range.
Pseudo-vertical transmission (post-partum transmission between sows and their dependent offspring) has been suggested as an explanation for the clustering of infection in family groups of piglets (Knowles Citation1994; Lugton Citation1997). However, horizontal transmission can sometimes occur from an early age, as demonstrated by a litter of eight ∼2-month old piglets killed in Hochstetter Forest that were all infected with the same M. bovis strain, while their mother was infected with a different strain (Nugent et al. Citation2003b). Pigs may also become infected via environmental contamination (Albiston et al. Citation1954), although such contamination is thought to be rare in New Zealand (Lugton Citation1997).
Deer carcasses are also readily scavenged by pigs (Nugent Citation2005). However, pigs appear less likely to cannibalise the carcasses of other pigs, or to feed on ferret carcasses. Cannibalism does occur (Nugent et al. Citation2003b), but in video-monitoring of 23 sets of pig remains (carcass, or viscera, or carcass with viscera exposed) in the South Island high country, Yockney and Nugent (Citation2003) demonstrated that none were scavenged by other pigs. In a related trial, no ferret carcasses placed in pig habitat in either summer or winter were scavenged by pigs (Byrom Citation2004).
Transmission from pigs
Low numbers of viable bacilli in lesions, coupled with the relatively high level of organised and encapsulated infection seen in affected LN of many older pigs (Francis 1958), suggest excretion of M. bovis by pigs is uncommon. Correspondingly, Lugton (Citation1997) found no bacilli in the urine, faeces or nasal cavities of four tuberculous feral pigs with widespread and large lesions. Nevertheless, there are occasional reports in New Zealand of grossly diseased feral pigs with draining abscesses (Lugton Citation1997). Therefore, environmental contamination may occasionally occur, and transmission to herbivores (cattle, deer, possums) via pasture is theoretically possible in New Zealand (Lugton Citation1997).
In Mediterranean Europe, TB often occurs in cattle herds in the vicinity of infected wild boar or semi-feral pig populations (Richomme et al. Citation2010; Vieira-Pinto et al. Citation2011; Di Marco et al. Citation2012; Rodríguez-Prieto et al. Citation2012) suggesting that transmission from pigs to cattle can occur. In contrast, in New Zealand, the main route of transmission from infected pigs to other hosts is more likely to be via carcasses of pigs that have died naturally or have been killed by hunters. Ferrets are likely to be the most at-risk species; in one trial, 10 of 13 sets of pig remains left in ferret-prone habitat were scavenged by ferrets feeding in family groups of up to five animals (Yockney and Nugent Citation2003). In that trial, a possum was also filmed feeding on mesenteric tissue from a pig, indicating a possible spillback mechanism from pigs to possums. Supporting that, a field study of three possum populations investigated scavenger contact using pig or deer meat containing dye-impregnated LN showed that 0–7% of the possums in each population had ingested the dye (Nugent and Whitford Citation2007).
Host status
Feral pigs have long been regarded as spillover or end hosts of TB in New Zealand, based on their ability to become infected without developing overt clinical disease, in contrast to most other wildlife in New Zealand (Coleman and Cooke, Citation2001). End-host status is supported by evidence from the Northern Territories of Australia, where TB prevalence in feral pigs declined following the removal of infected cattle and buffalo (true maintenance hosts) without any additional control of pigs (McInerney et al. Citation1995). TB transmission between live pigs can occur, at least in captivity (Ray et al. Citation1972), but only limited transmission between captive feral pigs has been observed in New Zealand, even when the animals were kept at densities far higher than those seen in the wild (Nugent et al. Citation2011b). In a formal test of the host status of feral pigs in New Zealand, the TB prevalence in such pigs fell substantially in three areas after intensive possum, but not pig, control was applied at geographic scales far greater than most pig home range sizes (Nugent et al. Citation2011a). In three other areas where neither possums nor pigs were controlled, there were only small or no declines in TB prevalence levels among the feral pigs. Furthermore, the declines in prevalence occurred rapidly after possum control was instigated, with near-zero prevalences recorded within 2–3 years in some areas. The rapidity of the decline in TB prevalence is surprising given the likelihood that some pigs can survive for longer than 2–3 years in an infected state. This suggests very high rates of population turnover due to continual hunting pressure, perhaps coupled with rapid resolution of detectable infection when pigs are not continually exposed to re-infection. The rapid decline in TB prevalence among pigs contrasts sharply with the much slower declines observed in wild deer after possum control measures are instigated. This indicates that infected pigs are far less likely to act as temporal vectors of TB in New Zealand than infected wild deer.
The reverse experiment, in which the density of wild boar was reduced without reducing numbers of the other common TB hosts, has been conducted in Spain. The results of this work resulted in a decline in TB prevalence among the boar, suggesting boar are maintenance hosts for TB in that country (Boadella et al. Citation2012). Further evidence of maintenance host status in overseas studies is presented by the persistence of a high prevalence of TB in wild boar on hunting estates where they have been isolated from livestock and other wild ungulates (Naranjo et al. Citation2008; Gortazar et al. Citation2012). Furthermore, more frequent generalisation of infection to multiple anatomical regions was also recorded among the boar in these studies, including lesion development in the lungs (particularly in young animals). In Sicily, free-ranging domestic pigs are also considered to be maintenance hosts as there is evidence of strains of M. bovis being present in pigs that are not present in sympatric cattle (Di Marco et al. Citation2012). In contrast, in France, wild boar in the Brotonne Forest are considered to be primarily spillover hosts, although disease management measures are undertaken there to prevent boar from scavenging the offal of other pigs (Zanella et al. Citation2012).
In conclusion, the spillover host status of feral pigs in New Zealand is likely to be the result of comparatively low intra-specific contact rates, which in turn result from the unrestricted year-round New Zealand hunting system reducing average densities to low levels and keeping the pigs widely dispersed. In contrast, wild boar and feral pigs in Europe occur are at much higher densities, and can be aggregated further by the use of artificial feeders or at watering points during hot dry Mediterranean summers, sufficient to facilitate intra-specific M. bovis transmission.
Roles of wild deer and feral pigs in the New Zealand TB control programme
Wild deer
Deer are now considered to be density-dependent maintenance hosts of TB, and the density threshold for disease persistence via deer-to-deer transmission alone is greater than the densities at which deer routinely occur in the wild in New Zealand. Therefore, direct management of the disease in deer (by density reduction or vaccination) is not seen as an essential prerequisite for local eradication of the disease from wildlife.
Nonetheless, the ability of deer to survive in an infected state for a decade or more imposes an important temporal constraint on the length of time believed to be required to locally eradicate TB from wildlife. This is because there is a presumed (but as yet not fully proven) risk of spillback infection from deer to possums. The strategic approach to local eradication of TB is predicated on reducing possum densities to very low levels, then ceasing control as soon as possible (to avoid on-going costs). This can be often achieved in as little as 5–10 years (Barron et al. Citation2013). But if possum control was stopped as soon as the population was deemed free of TB, possum densities could increase quickly to above the threshold density at which TB can persist indefinitely in that population, at which point spillback infection to possums (from a deer infected more than 10–15 years previously) could therefore result in the M. bovis re-establishing in the possum population
Modelling this potential mechanism of long-duration spillback risk, Barron et al. (Citation2013) predicted that the risk of M. bovis spillback from deer to possums could persist for up to 14 years after the initial reduction in possum population. The modelling also predicted that reducing deer densities by 80% would have only a small impact in reducing the duration of the risk of spillback from deer to possums. Therefore, the current possum control regime for the forested areas in which most M. bovis-infected deer typically reside involves applying control measures for at least 10 years, after which the possum population would take 5–10 years to return to pre-control levels. That combined period exceeds the predicted duration of any perceived spillback risk from deer, mitigating the threat of TB re-establishing in possums, but at the cost of extended possum control. The temporal constraint imposed by M. bovis infection in wild deer is that it largely precludes the strategic possibility of speeding up eradication from possums by applying more intensive possum control, at least in areas where wild deer and possums co-exist.
There is also a risk that deer might spread TB beyond the boundaries of the areas in which disease is already established in possums and, through spillback, establish a new focus of infection in uninfected possum populations. Infected deer have the potential to carry TB with them when they disperse, and young males can disperse for over 30 km (Nugent Citation2005). Spatial data on TB prevalence in deer in the Hauhungaroa Range in the central North Island indicate the scale of TB spread by deer (Nugent Citation2005). Possum control in the eastern half of the area in 1994 greatly reduced transmission from possums to deer in the eastern half, but not in the contiguous area immediately to the west where no possum control measures were taken. Over time an east-west prevalence gradient developed within the eastern half, with a high TB prevalence on its western boundary and a declining prevalence with increasing distance eastward from that boundary, i.e. with increasing distance from an uncontrolled possum population. In female deer, prevalence fell to near zero levels within 3 km eastward of the western boundary, compared to about 5 km in males.
The parts of New Zealand known or believed to contain tuberculous wildlife are formally designated as VRA. The boundaries of these are typically set at distances of 15–20 km beyond the known or suspected location of infected possums, creating possum management buffer zones aimed at preventing TB spread beyond the VRA. As the buffers zones are substantially wider than the gradient described above, and because the prevalence of TB in possums (and therefore deer) near the edges of these VRA is usually low, so the risk of spread of TB by deer across the buffer zones seems likely to be very low.
Beyond the risks posed by being infected with M. bovis, deer have some functional utility in the New Zealand TB management programme. As spillover hosts, they can be used as sentinels to help assess the distribution of infection in wildlife or, where efforts are being made to eradicate TB from possums, to help assess the likelihood that TB is absent from possums in that area. For the latter, a spatially explicit Bayesian framework has been developed for calculating the probability that possums in an area are free of TB (Anderson et al. Citation2013, Citationforthcoming). This framework makes use of negative TB diagnoses not only from possums themselves, but also from ferrets, pigs, and deer. Comparison of the densities and TB prevalence and incidence rates for possums, deer, pigs, and ferrets, indicates that deer are up to 98% less sensitive TB-sentinels than pigs (Nugent Citation2005; Nugent and Whitford Citation2008a). This is presumably because pigs are likely to actively seek out possum carcasses, and then consume all of the infectious material they contain. It is also often more expensive to obtain deer as surveillance samples, but deer may still act as useful sentinels in areas where there are few pigs or ferrets (Nugent and Whitford Citation2008a).
Feral pigs
This review re-affirms the long-standing belief that feral pigs in New Zealand are spillover hosts for TB. In contrast, wild boar in Mediterranean Europe are maintenance hosts, and this is likely to be a consequence of differences in climate and ecology, and wildlife management systems between the two regions resulting in differing average densities and degrees of aggregation. Therefore, pig control or vaccination is not considered to be essential for TB eradication in New Zealand. However, feral pigs are not always end-hosts, they do occasionally pass on infection under the following circumstances: (i) to other pigs (but at too low a rate to sustain on-going infection); (ii) to cattle and deer (through environmental contamination, albeit rarely); (iii) to ferrets (through scavenging, which may occur frequently where they share habitat); (iv) to possums (also through scavenging). This suite of possible transmission routes from pigs to other species is inconsequential in areas where TB is prevalent in possums, and any residual risk of M. bovis transmission from pigs will almost completely disappear within a few years of TB being eradicated from possums.
Nonetheless, pigs do pose some risks to the success of TB control measures. For instance, pigs may disperse TB widely, both naturally and with human assistance. The natural dispersal risks are much the same as noted above for deer, but the less stable home ranges of pigs and their greater propensity to disperse over long distances suggest that the “risk radius” for pigs is probably greater than that for deer. Some evidence for that comes from the Hauhungaroa Range study (Nugent Citation2005), where intensive possum control was initiated in the 7 km-wide eastern section in 1994, but not fully in the contiguous western section until 2005. Despite this control, the M. bovis prevalence in older pigs remained high throughout the 7 km-wide eastern side until 2005 (Nugent et al. Citation2011a) whereas in deer, by contrast, there were no new infections in the easternmost half of the east side. Together, these observations imply that a source of infection (tuberculous possums) persisted in the western section until at least 2005, and that the wider-ranging pigs had acquired infection there and had relocated back eastwards bearing disease (i.e. they had moved up to at least 7 km) whereas the deer had ranged <4 km and consequently remained disease-free.
Adding to the risk of geographic spread of TB posed by pigs naturally dispersing, both live pigs and pig carcasses are often moved long distances by hunters. Illegal releases of M. bovis-infected pigs in TB-free areas offer a plausible explanation for some TB outbreaks that have occurred outside known infected areas. This hunter-related dispersal risk appears to be greater for pigs than for deer, because pigs are usually transported with the head on the body. The head of the pig is discarded later, sometimes in areas that are freely accessible to possums and ferrets (Yockney et al. Citation2008).
Pigs, in conjunction with ferrets and deer, may also increase the cost of eradicating TB from wildlife by obscuring the fact that TB has been eradicated from possums in an area. One plausible scenario involves an infected deer dying a number of years after the disease has disappeared from possums and this initially causing new infection in young pigs and/or ferrets, followed by the infected pigs themselves subsequently dying and additionally passing the disease to ferrets, both of these actions potentially amplifying the spillback risk posed by deer. If possum numbers are kept low, that risk is likely to be negligible, but if infection is detected in pigs or ferrets, the precautionary principle may force managers to continue expensive possum control for longer than actually required.
Those risks and problems aside, pigs are likely to play an increasingly important role in TB management in New Zealand, as the strategic aim is currently to eradicate TB from wildlife over at least 2.5 million ha (25% of the VRA) by 2026 (Hutchings et al. Citation2011). Pigs are recognised as being the most cost-effective sentinels for helping to confirm TB freedom in a specific area, particularly in forested areas where direct surveillance of low-density possum populations is difficult and expensive (Nugent and Whitford Citation2008a). Their utility as sentinels reflects their large home range size, their propensity for scavenging possum carrion, their high susceptibility to acquiring infection and presenting detectable disease, and the ability to obtain samples at low cost in many areas by collecting pigs heads from recreational hunters. A sampling intensity of just one feral pig per km2 (i.e. 3–4 pigs per pig home range) is sufficient to provide 95% confidence that TB is not present in possums over the same area (Nugent and Whitford Citation2008a). The high sensitivity of pigs as sentinels has supported the deliberate release of radio-tagged TB-free pigs for disease surveillance purposes (Nugent et al. Citation2002), and sometimes these pigs' movements have been tracked by radio-collaring them with GPS-transmitting devices to better define the area “surveyed” (Nugent et al. Citation2011c), and sometimes to help locate non-collared sympatric resident pigs for sampling (Yockney and Nugent Citation2006; Nugent et al., Citation2014).
Concluding summary
Although deer can independently maintain TB when their densities are very high (as on farms), and while increased transmission can be facilitated by local management practices and conditions (as in Spain, France, and Michigan), there is no evidence that such high densities facilitating independent M. bovis maintenance occur in wild deer in New Zealand. The elimination of TB from possums (and livestock) is therefore expected to result in the eventual disappearance of the disease from wild deer, and additional deer-control measures will not be necessary for TB eradication.
However, infected deer can survive for more than a decade, which imposes a temporal constraint on the duration of time for which local possum control needs to be maintained. Deer can also disperse for long distances, which can result in the need for wide containment buffers around areas containing infected possums and sympatric deer. Fortunately, possum control is usually maintained for at least 10 years, so this constraint is more apparent than real. Modelling studies also suggest that further deer control measures would be unlikely to be a cost effective means of reducing the risk of spillback from deer to possums (Barron et al. Citation2013).
The same is largely true for feral pigs, except that their shorter lifespan results in a shorter duration of the risk for local spillback to possums. The other main epidemiological risk connected to feral pigs lies in the potential for long-distance spread of M. bovis via the transportation of infected pigs or pig carcasses by hunters. This risk has diminished substantially as possum control has expanded to cover more than 80% of the M. bovis-infected areas of New Zealand.
The most important gaps in current knowledge involve a lack of understanding about both the duration of time for which the spillback risk persists in deer, and how frequently scavengers such as pigs and ferrets acquire infection from aged deer. That knowledge could be important for guiding decisions about when to stop possum control. It would be particularly useful in those situations where several years of surveillance in a given area provide a high level of confidence that M. bovis infection is absent from possums, but the disease is then detected in an aged deer or in a young pig or ferret.
We conclude that wild deer and feral pigs are important but play a secondary role in the complex epidemiology of TB in New Zealand. The subsidiary roles they play are largely dependent on their interaction with other hosts, particularly possums. The major future involvement of pigs and deer in the active management of TB in New Zealand will lie predominantly in their use as sentinels. However, occasional unexplained incidents of TB in deer, pigs or ferrets in areas close to being, or having recently been, declared TB-free could feasibly continue, either as a result of illegal translocation and release M. bovis-infected pigs or via long-term persistence of viable M. bovis in deer. In reality, such occurrences are rarely likely to be of epidemiological significance, but the uncertainty about local TB freedom that they cause is likely to be too great to be entirely ignored, resulting in substantial precautionary expenditure on TB control measures.
| LN | = | Lymph node |
| TB | = | Tuberculosis |
| VRA | = | Vector Risk Area |
Acknowledgements
Funding to support the drafting of this review was provided by TBfree New Zealand (Project R10735-01), with co-funding from the Ministry of Business, Innovation and Employment (Contact C09X0803); Christian Gortazar additionally acknowledges support from Plan Nacional I+D+i AGL2011-30041 (MINECO, Spain) for his contribution to this review. We thank Frank Cross, Phil Cowan, and Paul Livingstone and others for their input and comments on early drafts.
Notes
1P. Livingstone, TBfree New Zealand, Wellington, NZ.
*Non-peer-reviewed
References
- Acevedo P, Vicente J, Hofle U, Cassinello J, Ruiz-Fons F, Gortázar C. Estimation of European wild boar relative abundance and aggregation: A novel method in epidemiological risk assessment. Epidemiology and Infection 135, 519–27, 2007 doi: 10.1017/S0950268806007059
- Acevedo P, Ruiz-Fons F, Vicente J, Reyes-Garcia AR, Alzaga V, Gortázar C. Estimating red deer abundance in a wide range of management situations in Mediterranean habitats. Journal of Zoology 276, 37–47, 2008 doi: 10.1111/j.1469-7998.2008.00464.x
- Acevedo-Whitehouse K, Vicente J, Gortazar C, Hofle U, Fernandez-De-Mera IG, Amos W. Genetic resistance to bovine tuberculosis in the Iberian wild boar. Molecular Ecology 14, 3209–17, 2005 doi: 10.1111/j.1365-294X.2005.02656.x
- Albiston HE, Pullar EM, Grayson AR. The epidemiology of tuberculosis in Victorian pigs. Australian Veterinary Journal 30, 364–76, 1954 doi: 10.1111/j.1751-0813.1954.tb05399.x
- Anderson DP, Ramsey DSL, Nugent G, Bosson M, Livingstone P, Martin PAJ, Sergeant E, Gormley AM, Warburton B. A novel approach to assess the probability of disease eradication from a wild-animal-reservoir host. Epidemiology and Infection 141, 1509–21, 2013 doi: 10.1017/S095026881200310X
- Anderson DP, Ramsey DSL, de Lisle GW, Bosson M, Cross ML, Nugent G. Development of integrated surveillance systems for the management of Mycobacterium bovis in New Zealand wildlife. New Zealand Veterinary Journal, (forthcoming)
- *Anonymous. Department of Conservation’s policy statement on deer control. http://doc.govt.nz/Documents/about-doc/policies-and-plans/deer-control-policy.pdf (accessed 05 September 2014). Department of Conservation, Wellington, NZ, 2001
- *Anonymous. Situation and Outlook for Primary Industries (SOPI) 2012. http://www.mpi.govt.nz/news-resources/publications? title=Situation%20and%20Outlook%20for%20Primary%20Industries%20(SOPI)%202012 (accessed 08 July 2013). Ministry of Primary Industries, Wellington, NZ, 2012a
- *Anonymous. Animal Health Board Annual Report 2011/2012. http://www.tbfree.org.nz/Portals/0/Annual%20Report%20web.pdf (accessed 26 June 2014). TBfree New Zealand, Wellington, NZ, 2012b
- *Barber KS. Ecological aspects affecting the use of feral pigs (Sus scrofa) as sentinels of Tb (Mycobacterium bovis) in high country farm habitat. MSc thesis thesis, Lincoln University, Canterbury, NZ, 2004
- Barron MC, Nugent G, Cross ML. Importance and mitigation of the risk of spillback transmission of Mycobacterium bovis infection for eradication of bovine tuberculosis from wildlife in New Zealand. Epidemiology and Infection 141, 1394–1406, 2013 doi: 10.1017/S0950268812002683
- Barry CE, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, Schnappinger D, Wilkinson RJ, Young D. The spectrum of latent tuberculosis: Rethinking the biology and intervention strategies. Nature Reviews Microbiology 7, 845–55, 2009
- *Beatson N. Tuberculosis in red deer in New Zealand. Royal Society of New Zealand Bulletin 22, 147–50, 1985
- *Beatson NS, Hutton JB, de Lisle GW. Tuberculosis – test and slaughter. In: Proceedings of a Deer Course for Veterinarians. New Zealand Veterinary Association Deer Branch Course No. 1. Pp 18–27, 1984
- Beltrán-Beck B, Ballesteros C, Vicente J, de la Fuente J, Gortázar C. Progress in oral vaccination against tuberculosis in its main wildlife reservoir in Iberia, the Eurasian wild boar. Veterinary Medicine International, doi:10.1155/2012/978501, 2012
- Boadella M, Vicente J, Ruiz-Fons F, de la Fuente J, Gortázar C. Effects of culling Eurasian wild boar on the prevalence of Mycobacterium bovis and Aujeszky's disease virus. Preventive Veterinary Medicine 107, 214–21, 2012 doi: 10.1016/j.prevetmed.2012.06.001
- Bollo E, Ferroglio E, Dini V, Mignone W, Biollatti B, Rossi L. Detection of Mycobacterium tuberculosis complex in lymph nodes of wild boar (Sus scrofa) by a target-amplified test system. Journal of Veterinary Medicine, Series B 47, 337–342, 2000 doi: 10.1046/j.1439-0450.2000.00354.x
- Buchan G, Griffin J. Tuberculosis in domesticated deer (Cervus elaphus): A large animal model for human tuberculosis. Journal of Comparative Pathology 103, 11–22, 1990 doi: 10.1016/S0021-9975(08)80131-4
- *Byrom AE. Animal Health Board Project No. R-10618 Spread of Tb by ferrets in the northern South Island high country. http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Byrom%20AE.%20Spread%20of%20Tb%20by%20ferrets%20in%20the%20northern%20South%20Island%20high%20country.%20Landcare%20Research%20contract%20report%20LC0304146.%20Landcare%20Research,%20Lincoln,%20New%20Zealand,%202004.pdf (accessed 05 September 2014). TBfree New Zealand, Wellington, NZ, 2004
- Challies CN. Status and future management of the wild animal recovery industry. New Zealand Forestry 36, 10–7, 1991
- *Choquenot D, McIlroy JC, Korn T. Managing vertebrate pests: feral pigs. Australian Government Publishing Service, Canberra, Australia, 1996
- Clifton-Hadley R, Wilesmith J. Tuberculosis in deer: a review. Veterinary Record 129, 5–12, 1991 doi: 10.1136/vr.129.1.5
- Coleman JD, Cooke MM. Mycobacterium bovis infection in wildlife in New Zealand. Tuberculosis 81, 191–202, 2001 doi: 10.1054/tube.2001.0291
- *Coleman JD, Coleman MC, Nugent G, Yockney I. Animal Health Board Project No. R-50634 Identifying the causes of, and solutions to, vector-related Tb persistence near Featherston. http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Coleman%20JD,%20Coleman%20MC,%20Nugent%20G,%20Yockney%20I.%20Identifying%20the%20causes%20of,%20and%20solutions%20to,%20vector-related%20Tb%20persistence%20near%20Featherston.%20Landcare%20Research%20Contract%20Report%20LC0506005.%202005.pdf (accessed 05 September 2014). TBfree New Zealand, Wellington, NZ, 2005
- Cooke MM, Alley MR, Duignan PJ, Murray A. Tuberculosis in wild and feral animals in New Zealand. Infectious Disease Review 1, 241–7, 1999
- Corner L, Barrett R, Lepper A, Lewis V, Pearson C. A survey of mycobacteriosis of feral pigs in the Northern Territory. Australian Veterinary Journal 57, 537–42, 1981 doi: 10.1111/j.1751-0813.1981.tb00428.x
- Cousins D. Mycobacterium bovis infection and control in domestic livestock. Revue scientifique et technique (International Office of Epizootics) 20, 71, 2001
- Delahay RJ, De Leeuw ANS, Barlow AM, Clifton-Hadley RS, Cheeseman CL. The status of Mycobacterium bovis infection in UK wild mammals: A review. Veterinary Journal 164, 90–105, 2002 doi: 10.1053/tvjl.2001.0667
- *de Lisle G. Mycobacterial infections in pigs. Surveillance 21 (4), 23–5, 1994
- de Lisle G, Havill P. Mycobacteria isolated from deer in New Zealand from 1970–1983. New Zealand Veterinary Journal 33, 138–140, 1985 doi: 10.1080/00480169.1985.35198
- Di Marco V, Mazzone P, Capucchio MT, Boniotti MB, Aronica V, Russo M, Fiasconaro M, Cifani N, Corneli S, Biasibetti E, Biagetti M, Pacciarini ML, Cagiola M, Pasquali P, Marianelli C. Epidemiological significance of the domestic black pig (Sus scrofa) in maintenance of bovine tuberculosis in Sicily. Journal of Clinical Microbiology 50, 1209–18, 2012 doi: 10.1128/JCM.06544-11
- Dzieciolowski RM, Clarke CMH. Age structure and sex ratio in a population of harvested feral pigs in New Zealand. Acta Theriologica 37, 259–270, 1989 doi: 10.4098/AT.arch.92-24
- Dzieciołowski RM, Clarke CMH, Frampton CM. Reproductive characteristics of feral pigs in New Zealand. Acta Theriologica 37, 259–270, 1992 doi: 10.4098/AT.arch.92-24
- Ekdahl MO, Smith BL, Money DFL. Letters to the editor. New Zealand Veterinary Journal 18, 44–5, 1970 doi: 10.1080/00480169.1970.33860
- *Essey M, Payne R, Himes E, Luchsinger D. Bovine tuberculosis surveys of axis deer and feral swine on the Hawaiian island of Molokai. Proceedings of the United States Animal Health Association. Pp 538–49, 1981
- Figgins G, Holland P. Red deer in New Zealand: Game animal, economic resource or environmental pest?. New Zealand Geographer 68, 36–48, 2012 doi: 10.1111/j.1745-7939.2012.01219.x
- Forsyth DM, Allen RB, Marburg AE, MacKenzie DI, Douglas MJ. Population dynamics and resource use of red deer after release from harvesting in New Zealand. New Zealand Journal of Ecology 34, 277–87, 2010
- *Francis J. Tuberculosis in animals and man: A study in comparative pathology. Cassell, Bristol, England, 1958
- Fraser KW, Cone JM, Whitford EJ. A revision of the established ranges and new populations of 11 introduced ungulate species in New Zealand. Journal of the Royal Society of New Zealand 30, 419–437, 2000 doi: 10.1080/03014223.2000.9517633
- García-Jiménez WL, Fernández-Llario P, Benítez-Medina JM, Cerrato R, Cuesta J, García-Sánchez A, Gonçalves P, Martínez R, Risco D, Salguero F. Reducing Eurasian wild boar (Sus scrofa) population density as a measure for bovine tuberculosis control: Effects in wild boar and a sympatric fallow deer (Dama dama) population in Central Spain. Preventive Veterinary Medicine 110, 435–46, 2013 doi: 10.1016/j.prevetmed.2013.02.017
- Garrido JM, Sevilla IA, Beltrán-Beck B, Minguijón E, Ballesteros C, Galindo RC, Boadella M, Lyashchenko KP, Romero B, Geijo MV. Protection against tuberculosis in Eurasian wild boar vaccinated with heat-inactivated Mycobacterium bovis. PLoS one 6, e24905, 2011 doi: 10.1371/journal.pone.0024905
- Gortázar C, Torres MJ, Vic e nte J, Acevedo P, Reglero M, de La Fuente J, Negro JJ, Aznar-Martín J. Bovine tuberculosis in Doñana Biosphere Reserve: The role of wild ungulates as disease reservoirs in the last Iberian lynx strongholds. PLoS one 3, e2776, 2008 doi: 10.1371/journal.pone.0002776
- Gortazar C, Delahay RJ, McDonald RA, Boadella M, Wilson GJ, Gavier-Widen D, Acevedo P. The status of tuberculosis in European wild mammals. Mammal Review 42, 193–206, 2012 doi: 10.1111/j.1365-2907.2011.00191.x
- Griffin JFT, Buchan GS. Aetiology, pathogenesis and diagnosis of Mycobacterium bovis in deer. Veterinary Microbiology 40, 193–205, 1994 doi: 10.1016/0378-1135(94)90055-8
- Griffin JFT, Mackintosh CG. Tuberculosis in deer: Perceptions, problems and progress. Veterinary Journal 160, 202–219, 2000 doi: 10.1053/tvjl.2000.0514
- Griffin J, Chinn D, Rodgers C, Mackintosh C. Optimal models to evaluate the protective efficacy of tuberculosis vaccines. Tuberculosis 81, 133–139, 2001 doi: 10.1054/tube.2000.0271
- Griffin JFT, Chinn DN, Rodgers CR. Diagnostic strategies and outcomes on three New Zealand deer farms with severe outbreaks of bovine tuberculosis. Tuberculosis 84, 293–302, 2004 doi: 10.1016/j.tube.2003.11.001
- Hars J, Richomme C, Boschiroli ML. La tuberculose bovine dans la faune sauvage en France. Bulletin épidémiologique/Spécial zoonoses 38, 28–31, 2010
- *Hathaway SC, Ryan TJ, de Lisle GW, Johnstone AC. Post mortem meat inspection for tuberculosis in deer, with some implications for animal health surveillance. Proceedings of a Deer Course for Veterinarians. New Zealand Veterinary Association Deer Branch Course No. 10. Pp 92–105, 1994
- *Hennessey S. Epidemiological studies of tuberculosis in cattle and possums – Studies involving the central North Island endemic area. Surveillance 13 (3), 20–22, 1986
- *Hutchings S, Hancox N, Livingstone P. Approaches to eradication of tuberculosis from wildlife in New Zealand: A revised pest management strategy. Vetscript 24 (8), 8–12, 2011
- *Knowles GJE. MAF Quality Management Contract Report 73/90 Use of the Judas pig methodology for controlling tuberculosis in feral pigs (Sus scrofa). http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Knowles%20GJE.%20Use%20of%20the%20Judas%20pig%20methodology%20for%20controlling%20tuberculosis%20in%20feral%20pigs%20(Sus%20scrofa).pdf (accessed 05 September 2014). TBfree New Zealand, Wellington, NZ, 1994
- *Knowles GJE. Movement patterns of red deer (Cervus elaphus) in Otago. http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Knowles%20GJE.%20Movement%20patterns%20of%20red%20deer%20(Cervus%20elaphus)%20in%20Otago.pdf (05 September 2014). TBfree New Zealand, Wellington, NZ, 1997
- Livingstone PG, Nugent G, de Lisle GW, Hancox N. Toward eradication: the effect of Mycobacterium bovis infection in wildlife on the evolution and future direction of bovine tuberculosis management in New Zealand. New Zealand Veterinary Journal, (forthcoming)
- *Lugton IW. The contribution of wild mammals to the epidemiology of tuberculosis (Mycobacterium bovis) in New Zealand. PhD thesis thesis, Massey University, Palmerston North, NZ, 1997
- Lugton I, Wilson P, Morris R, Nugent G. Epidemiology and pathogenesis of Mycobacterium bovis infection of red deer (Cervus elaphus) in New Zealand. New Zealand Veterinary Journal 46, 147–56, 1998 doi: 10.1080/00480169.1998.36079
- *Mackereth G. The Waipawa endemic area: The epidemiological picture. Proceedings of New Zealand Veterinary Association Deer Branch Course 10. Pp 222–8, 1993
- *Mackintosh CG, Griffin JFT. Epidemiological aspects of deer tuberculosis research. In: Proceedings of a Deer Course for Veterinarians, No. 11, Deer Branch of the New Zealand Veterinary Association. Pp 106–13, 1994
- Mackintosh CG, Qureshi T, Waldrup K, Labes RE, Dodds KG, Griffin JFT. Genetic resistance to experimental infection with Mycobacterium bovis in red deer (Cervus elaphus). Infection and Immunity 68, 1620–5, 2000 doi: 10.1128/IAI.68.3.1620-1625.2000
- Martín-Hernando MP, Höfle U, Vicente J, Ruiz-Fons F, Vidal D, Barral M, Garrido JM, de la Fuente J, Gortazar C. Lesions associated with Mycobacterium tuberculosis complex infection in the European wild boar. Tuberculosis 87, 360–7, 2007 doi: 10.1016/j.tube.2007.02.003
- Martín-Hernando M, Torres M, Aznar J, Negro J, Gandía A, Gortázar C. Distribution of lesions in red and fallow deer naturally infected with Mycobacterium bovis. Journal of Comparative Pathology 142, 43–50, 2010 doi: 10.1016/j.jcpa.2009.07.003
- McIlroy JC. Aspects of the ecology of feral pigs (Sus scrofa) in the Murchison area, New Zealand. New Zealand Journal of Ecology 12, 11–22, 1989
- *McIlory JC. Feral pig. In: King CM (ed). The Handbook of New Zealand Mammals. 2nd Edtn. Pp 334–45. Oxford University Press, Melbourne, Australia, 2005
- McInerney J, Small K, Caley P. Prevalence of Mycobacterium bovis infection in feral pigs in the Northern Territory. Australian Veterinary Journal 72, 448–51, 1995 doi: 10.1111/j.1751-0813.1995.tb03486.x
- Miller R, Kaneene JB, Fitzgerald SD, Schmitt SM. Evaluation of the influence of supplemental feeding of white-tailed deer (Odocoileus virginianus) on the prevalence of bovine tuberculosis in the Michigan wild deer population. Journal of Wildlife Diseases 39, 84–95, 2003 doi: 10.7589/0090-3558-39.1.84
- Morris RS, Pfeiffer DU. Directions and issues in bovine tuberculosis epidemiology and control in New Zealand. New Zealand Veterinary Journal 43, 256–265, 1995 doi: 10.1080/00480169./1995.35904
- Naranjo V, Gortazar C, Vicente J, de la Fuente J. Evidence of the role of European wild boar as a reservoir of Mycobacterium tuberculosis complex. Veterinary Microbiology 127, 1–9, 2008 doi: 10.1016/j.vetmic.2007.10.002
- Nol P, Palmer MV, Waters WR, Aldwell FE, Buddle BM, Triantis JM, Linke LM, Phillips GE, Thacker TC, Rhyan JC. Efficacy of oral and parenteral routes of Mycobacterium bovis bacille Calmette-Guerin vaccination against experimental bovine tuberculosis in white-tailed deer (Odocoileus virginianus): A feasibility study. Journal of Wildlife Diseases 44, 247–259, 2008 doi: 10.7589/0090-3558-44.2.247
- Nugent G. Big-game, small-game, and gamebird hunting in New Zealand: hunting effort, harvest, and expenditure in 1988. New Zealand Journal of Zoology 19, 75–90, 1992a doi: 10.1080/03014223.1992.10422312
- *Nugent G. Forest Research Institute Report No. 92/3 The conservation role of commercial deer hunting. http://www.landcareresearch.co.nz/__data/assets/pdf_file/0018/77022/conservation-role-deer-hunting.pdf (accessed 05 September 2014). Landcare Research, Lincoln, NZ, 1992b
- *Nugent G. The role of wild deer in the epidemiology and management of bovine tuberculosis in New Zealand. PhD Thesis, Lincoln University, Lincoln, NZ, 2005
- Nugent G. Maintenance, spillover and spillback transmission of bovine tuberculosis in multi-host wildlife complexes: A New Zealand case study. Veterinary Microbiology 151, 34–42, 2011 doi: 10.1016/j.vetmic.2011.02.023
- Nugent G, Fraser K. Pests or valued resources? Conflicts in management of deer. New Zealand Journal of Zoology 20, 361–6, 1993 doi: 10.1080/03014223.1993.10420359
- *Nugent G, Fraser KW. Red deer. In: King CM (ed). The Handbook of New Zealand Mammals. 2nd Edtn. Pp 410–20. Oxford University Press, Melbourne, Australia, 2005
- *Nugent G, Lugton I. Prevalence of bovine tuberculosis in wild deer in the Hauhungaroa Range, North Island, New Zealand. In: Griffin F, de Lisle G (eds). Tuberculosis in Wildlife and Domestic Animals. Pp 155–7. University of Otago Press, Dunedin, New Zealand, 1995
- *Nugent G, Whitford J. Animal Health Board Project No. R-10678 Is residual Tb infection in deer and pig populations important? http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Nugent%20G,%20Whitford%20J.%20Is%20residual%20Tb%20infection%20in%20deer%20and%20pig%20populations%20important.pdf (accessed 05 September 2014). TBfree New Zealand, Wellington, NZ, 2007
- *Nugent G, Whitford J. Animal Health Board Project No. R-10652 Relative utility of Tb hosts as sentinels for detecting Tb. http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Nugent%20G,%20Whitford%20J.%20Relative%20utility%20of%20Tb%20hosts%20as%20sentinels%20for%20detecting%20Tb.pdf (accessed 05 September 2014). TBfree New Zealand, Wellington, NZ, 2008a
- *Nugent G, Whitford J. Animal Health Board Project no. R-10688 Confirmation of the spatial scale and duration of spillback risk from Tb-infected pigs. http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Nugent%20G,%20Whitford%20EJ.%20Confirmation%20of%20the%20Spatial%20Scale%20and%20Duration%20of%20Spillback%20Risk%20from%20Tb-infected%20Pigs.pdf (accessed 02 September 2014). TBfree, New Zealand, Wellington, NZ, 2008b
- Nugent G, Yockney I. Fallow deer deaths during aerial 1080 poisoning of possums in the Blue Mountains, Otago, New Zealand. New Zealand Journal of Zoology 31, 185–92, 2004 doi: 10.1080/03014223.2004.9518371
- Nugent G, Fraser KW, Asher GW, Tustin KG. Advances in New Zealand mammalogy 1990–2000: Deer. Journal of the Royal Society of New Zealand 31, 263–98, 2001 doi: 10.1080/03014223.2001.9517654
- Nugent G, Whitford J, Young N. Use of released pigs as sentinels for Mycobacterium bovis. Journal of Wildlife Diseases 38, 665–77, 2002 doi: 10.7589/0090-3558-38.4.665
- *Nugent G, Byrom A, Arthur T, Whitford J, Yockney I. Animal Health Board Project No. R-10576 Risk of Tb persistence and spread: The role of deer, pigs, and ferrets. http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Nugent%20G,%20Byrom%20A,%20Arthur%20T,%20Whitford%20J,%20Yockney%20I.%20Risk%20of%20Tb%20persistence%20and%20spread%20The%20role%20of%20deer,%20pigs,%20and%20ferrets.pdf (accessed 05 September 2014). TBfree New Zealand, Wellington, NZ, 2003a
- *Nugent G, Reddiex B, Whitford J, Yockney I. Animal Health Board Project No. R-10577 Pig as hosts of bovine tuberculosis in New Zealand – a review. http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Nugent%20G,%20Reddiex%20B,%20Whitford%20J,%20Yockney%20I.%20Pig%20as%20hosts%20of%20bovine%20tuberculosis%20in%20New%20Zealand%20_%20a%20review.pdf (accessed 05 September 2014). TBfree New Zealand, Wellington, NZ, 2003b
- *Nugent G, Ramsey D, Caley P. Animal Health Board Project No. R-10627. Enhanced early detection of Tb through use and integration of wildlife data into the national surveillance model. http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Nugent%20G,%20Ramsey%20D,%20Caley%20P.%20Enhanced%20early%20detection%20of%20Tb%20through%20use%20and%20integration%20of%20wildlife%20data%20into%20the%20national%20surveillance%20model.pdf (accessed 05 September 2014). TBfree New Zealand, Wellington, NZ, 2006
- Nugent G, Whitford J, Yockney I, Cross ML. Reduced spillover transmission of Mycobacterium bovis to feral pigs (Sus scrofa) following population control of brushtail possums (Trichosurus vulpecula). Epidemiology and Infection 140, 1036–47, 2011a doi: 10.1017/S0950268811001579
- Nugent G, Yockney I, Whitford E. Intraspecific transmission of Mycobacterium bovis among penned feral pigs in New Zealand. Journal of Wildlife Diseases 47, 364–72, 2011b doi: 10.7589/0090-3558-47.2.364
- *Nugent G, Yockney I, Whitford J. Animal Health Board R-10629_02 Eliminating TB from Molesworth Station: II. Persistence of TB on Molesworth Station two years after aerial 1080 baiting. http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Nugent%20G,%20Yockney%20I,%20Whitford%20J.%20Eliminating%20TB%20from%20Molesworth%20Station%20II.%20Persistence%20of%20TB%20on%20Molesworth%20Station%20two%20years%20after%20aerial%201080%20baiting.pdf (accessed 05 September 2014). TBfree New Zealand, Wellington, NZ, 2011c
- *Nugent G, Morriss G, Fitzgerald N, Innes J. Animal Health Board joint Project Nos. 10710 and 10743 Bait aggregation and deer repellent effects on efficacy, and non-target impacts on deer and birds, during aerial 1080 baiting: Hauhungaroa 2011. http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Nugent%20G,%20Morriss%20G,%20Fitzgerald%20N,%20Innes%20J.%20Bait%20aggregation%20and%20deer%20repellent%20effects%20on%20efficacy,%20and%20non-target%20impacts%20on%20deer%20and%20birds,%20during%20aerial%201080%20baiting%20Hauhungaroa%202011.pdf (accessed 05 September 2014). TBfree New Zealand, Wellington, NZ, 2012
- Nugent G, Whitford J, Yockney I, Perry M, Tompkins DM, Holtslag N, Cross ML. Percutaneous interdigital injection of Mycobacterium bovis as a model for tuberculous lesion development in wild brushtail possums (Trichosurus vulpecula). Journal of Comparative Pathology 148, 33–42, 2013 doi: 10.1016/j.jcpa.2012.05.006
- Nugent G, Yockney I, Whitford J, Cross ML. Assessing the effectiveness of tuberculosis management in brushtail possums (Trichosurus vulpecula), through indirect surveillance of Mycobacterium bovis infection using released sentinel pigs. Veterinary Medicine International, doi:10.1155/2014/361634, 2014
- Nugent G, Buddle BM, Knowles GJE. Epidemiology and control of Mycobacterium bovis infection in brushtail possums (Trichosurus vulpecula), the primary wildlife host of bovine tuberculosis in New Zealand. New Zealand Veterinary Journal, 2015
- *Nuttall WO. Tuberculosis of pigs. Surveillance 13 (1), 2–4, 1986
- O'Brien DJ, Fitzgerald SD, Lyon TJ, Butler KL, Fierke JS, Clarke KR, Schmitt SM, Cooley TM, Berry DE. Tuberculous lesions in free-ranging white-tailed deer in Michigan. Journal of Wildlife Diseases 37, 608–13, 2001 doi: 10.7589/0090-3558-37.3.608
- O'Brien DJ, Schmitt SM, Fierke JS, Hogle SA, Winterstein SR, Cooley TM, Moritz WE, Diegel KL, Fitzgerald SD, Berry DE, et al. Epidemiology of Mycobacterium bovis in free-ranging white-tailed deer, Michigan, USA, 1995–2000. Preventive Veterinary Medicine 54, 47–63, 2002 doi: 10.1016/S0167-5877(02)00010-7
- O'Brien DJ, Schmitt SM, Fitzgerald SD, Berry DE. Management of bovine tuberculosis in Michigan wildlife: current status and near term prospects. Veterinary Microbiology 151, 179–87, 2011 doi: 10.1016/j.vetmic.2011.02.042
- Palmer MV, Whipple DL, Payeur JB, Alt DP, Esch KJ, Bruning-Fann CS, Kaneene JB. Naturally occurring tuberculosis in white-tailed deer. Journal of the American Veterinary Medical Association 216, 1921–4, 2000 doi: 10.2460/javma.2000.216.1921
- Palmer MV, Whipple DL, Waters WR. Experimental deer-to-deer transmission of Mycobacterium bovis. American Journal of Veterinary Research 62, 692–6, 2001 doi: 10.2460/ajvr.2001.62.692
- Palmer MV, Waters WR, Whipple DL. Milk containing Mycobacterium bovis as a source of infection for white-tailed deer fawns (Odocoileus virginianus). Tuberculosis 82, 161–5, 2002 doi: 10.1054/tube.2002.0334
- Palmer MV, Waters WR, Whipple DL. Shared feed as a means of deer-to-deer transmission of Mycobacterium bovis. Journal of Wildlife Diseases 40, 87–91, 2004 doi: 10.7589/0090-3558-40.1.87
- Ragg JR, Mackintosh CG, Moller H. The scavenging behaviour of ferrets (Mustela furo), feral cats (Felis domesticus), possums (Trichosurus vulpecula), hedgehogs (Erinaceus europaeus) and harrier hawks (Circus approximans) on pastoral farmland in New Zealand: Implications for bovine tuberculosis transmission. New Zealand Veterinary Journal 48, 166–175, 2000 doi: 10.1080/00480169.2000.36188
- Ramsey D, Cowan P. Mortality rate and movements of brushtail possums with clinical tuberculosis (Mycobacterium bovis infection). New Zealand Veterinary Journal 51, 179–85, 2003 doi: 10.1080/00480169.2003.36361
- Ray J, Mallmann V, Mallmann W, Morrill C. Pathologic and bacteriologic features and hypersensitivity of pigs given Mycobacterium bovis, Mycobacterium avium, or group 3 Mycobacteria. American Journal of Veterinary Research 33, 1333–45, 1972
- Richomme C, Boschiroli ML, Hars J, Casabianca F, Ducrot C. Bovine tuberculosis in livestock and wild boar on the Mediterranean Island, Corsica. Journal of Wildlife Diseases 46, 627–31, 2010 doi: 10.7589/0090-3558-46.2.627
- Robinson RC, Phillips PH, Stevens G, Storm PA. An outbreak of Mycobacterium bovis infection in fallow deer (Dama dama). Australian Veterinary Journal 66, 195–7, 1989 doi: 10.1111/j.1751-0813.1989.tb09806.x
- Rodríguez-Prieto V, Martínez-López B, Barasona J, Acevedo P, Romero B, Rodriguez-Campos S, Gortázar C, Sánchez-Vizcaíno J, Vicente J. A Bayesian approach to study the risk variables for tuberculosis occurrence in domestic and wild ungulates in South Central Spain. BMC Veterinary Research 8, 148, 2012 doi: 10.1186/1746-6148-8-148
- Santos N, Correia-Neves M, Ghebremichael S, Källenius G, Svenson SB, Almeida V. Epidemiology of Mycobacterium bovis infection in wild boar (Sus scrofa) from Portugal. Journal of Wildlife Diseases 45, 1048–61, 2009 doi: 10.7589/0090-3558-45.4.1048
- *Santos N, Correia-Neves M, Almeida V, Gortázar C. Wildlife tuberculosis: A systematic review of the epidemiology in Iberian Peninsula. In: Cunha M (ed). Epidemiology Insights. http://www.intechopen.com/books/epidemiology-insights/wildlife-tuberculosis-a-systematic-review-of-the-epidemiology-in-the-iberian-peninsula (accessed 30 June 2014). InTech, Rijeka, Croatia, 2012
- Sauter CM, Morris RS. Behavioural studies on the potential for direct transmission of tuberculosis from feral ferrets (Mustela furo) and possums (Trichosurus vulpecula) to farmed livestock. New Zealand Veterinary Journal 43, 294–300, 1995 doi: 10.1080/00480169./1995.35909
- Thomson C, Challies CN. Diet of feral pigs in the podocarp-tawa forests of the Urewera Ranges. New Zealand Journal of Ecology 11, 73–8, 1988
- Vicente J, Höfle U, Garrido JM, Fernández-De-Mera IG, Juste R, Barral M, Gortazar C. Wild boar and red deer display high prevalences of tuberculosis-like lesions in Spain. Veterinary Research 37, 107–19, 2006 doi: 10.1051/vetres:2005044
- Vicente J, Höfle U, Fernández-De-Mera IG, Gortazar C. The importance of parasite life history and host density in predicting the impact of infections in red deer. Oecologia 152, 655–64, 2007a doi: 10.1007/s00442-007-0690-6
- Vicente J, Höfle U, Garrido JM, Fernandez-de-Mera IG, Acevedo P, Juste R, Barral M, Gortazar C. Risk factors associated with the prevalence of tuberculosis-like lesions in fenced wild boar and red deer in south central Spain. Veterinary Research 38, 451–464, 2007b doi: 10.1051/vetres:2007002
- Vieira-Pinto M, Alberto J, Aranha J, Serejo J, Canto A, Cunha MV, Botelho A. Combined evaluation of bovine tuberculosis in wild boar (Sus scrofa) and red deer (Cervus elaphus) from Central-East Portugal. European Journal of Wildlife Research 57, 1189–201, 2011 doi: 10.1007/s10344-011-0532-z
- Wakelin CA, Churchman OT. Prevalence of bovine tuberculosis in feral pigs in Central Otago. Surveillance 18, 19–20, 1991
- *Wodzicki KA. Introduced mammals of New Zealand. An ecological and economic survey. Department of Scientific & Industrial Research Bulletin 98. 1950
- *Yockney I, Nugent G. Animal Health Board Project No. R-10577 Scavenging of potentially tuberculous feral pig carcasses in the northern South Island high country. http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Yockney%20I,%20Nugent%20G.%20Scavenging%20of%20potentially%20tuberculous%20feral%20pig%20carcasses%20in%20the%20northern%20South%20Island%20high%20country.pdf (accessed 05 September 2014). TBfree New Zealand, Wellington, NZ, 2003
- *Yockney I, Nugent G. Animal Health Board Project No. R-80629(sub-project). Relative effectiveness of the Judas technique in rapidly reducing pig numbers in part of Molesworth Station: An operational trial. http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Yockney%20I,%20Nugent%20G.%20Relative%20effectiveness%20of%20the%20Judas%20technique%20in%20rapidly%20reducing%20pig%20numbers%20in%20part%20of%20Molesworth%20Station%20An%20operational%20trial.pdf (accessed 05 September 2014). TBfree New Zealand, Wellington, NZ, 2006
- *Yockney I, Morriss G, Nugent G. Animal Health Board Project No. R-10686 Reducing the risk of spillback infection from hunter kills. http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/Yockney%20I,%20Morriss%20G,%20Nugent%20G.%20Reducing%20the%20risk%20of%20spillback%20infection%20from%20hunter%20kills.pdf (accessed 05 September 2014). TBfree New Zealand, Wellington, NZ, 2008
- Yockney IJ, Nugent G, Latham MC, Perry M, Cross ML, Byrom AE. Comparison of ranging behaviour in a multi-species complex of free-ranging hosts of bovine tuberculosis in relation to their use as disease sentinels. Epidemiology and Infection 141, 1407–16, 2013 doi: 10.1017/S0950268813000289
- Zanella G, Durand B, Hars J, Moutou F, Garin-Bastuji B, Duvauchelle A, Fermé M, Karoui C, Boschiroli ML. Mycobacterium bovis in wildlife in France. Journal of Wildlife Diseases 44, 99–108, 2008 doi: 10.7589/0090-3558-44.1.99
- Zanella G, Bar-Hen A, Boschiroli M-L, Hars J, Moutou F, Garin-Bastuji B, Durand B. Modelling transmission of bovine tuberculosis in red deer and wild boar in Normandy, France. Zoonoses and Public Health 59, 170–8, 2012 doi: 10.1111/j.1863-2378.2011.01453.x
