Abstract
AIM: To explore how the inclusion of multi-host dynamics affects the predicted prevalence of bovine tuberculosis (TB) in possums and other host species following the current best practice for control of TB in large difficult and remote areas, to identify which host species are responsible for changes in predicted prevalence, and whether TB can persist in possum-free host communities.
METHODS: Multi-host TB models were constructed, comprising three host species with density-dependent population growth, density-dependent disease transmission and susceptible and infected classes. Models were parameterised for two case studies of current concern in New Zealand, namely chronic TB persistence in a possum-deer-pig complex in extensive forest, and in a possum-pig-ferret complex in unforested semi-arid shrub and grasslands. Persistence of TB in the face of best practice possum control was evaluated from model simulations, and the contribution of different hosts to persistence of TB was assessed by removing each host species in turn from the simulations. A sensitivity test explored how different parameter values affected modelled persistence of TB.
RESULTS: The forest multi-host model-predicted amplification of TB prevalence due to the presence of pigs. The presence of pigs and/or deer did not jeopardise the success of best practice possum control in eradicating TB from the system, as pigs and deer are effectively end-hosts for TB. Sensitivity analyses indicated these interpretations were robust to uncertainty in model parameter values. The grassland system model predicted that the multi-host species complex could potentially lead to failure of eradication of TB under possum-only control, due to TB persisting in ferret and pig populations in the absence of possum hosts through reciprocal scavenging, resulting in spillback transmission to possums once their populations had started to recover from control.
CONCLUSIONS: With respect to management of TB, for modelled forest habitats, 15 years of effective possum control was predicted to eradicate TB from the possum-deer-pig host community, indicating the current focus on possum-only control is appropriate for such areas. For grassland model systems, TB was predicted to persist in the ferret-pig host complex in the absence of possums, potentially jeopardising the effectiveness of possum-only control programmes. However this outcome depended on the occurrence and rate of pigs acquiring TB from ferrets, which is unknown. Thus some estimation of this transmission parameter is required to enable managers to assess if multi-host disease dynamics are important for their TB control programmes.
Introduction
When sympatric host species share the same infectious disease, multiple transmission pathways are possible (Woolhouse et al. Citation2001). Under such circumstances, the host community functions as a large heterogeneous host population, with implications for pathogen persistence and spread (Dobson Citation2004). The resulting complex dynamics may lead to both on-going pathogen presence in, and impact on, host populations in which they would not otherwise persist (Tompkins et al. Citation2003), and amplification of disease outbreaks when they occur (Craft et al. Citation2008). For management actions to control or eradicate infectious diseases in target host populations (be they culling, vaccination, or other steps to reduce transmission), such dynamics can increase the effort required in two key ways. First, in the face of intra- and inter-species disease transmission the magnitude of control effort applied to the primary host population to achieve the desired goal may need to be increased (e.g. Fitzpatrick et al. Citation2012). Second, in addition to primary hosts, control of secondary host populations may be necessary, for example, if the disease could persist in secondary host species and eradication was the goal (e.g. Olmstead and Rhode Citation2004). Alternatively, control of secondary hosts may provide a more cost-effective method to achieve disease eradication in an already managed species, by reducing inter-specific transmission as opposed to further reducing intra-specific transmission (e.g. Lembo et al. Citation2008).
Mycobacterium bovis, the causative agent of bovine tuberculosis (TB), has a very broad host range, which in New Zealand includes brushtail possums (Trichosurus vulpecula), red deer (Cervus elaphus), ferrets (Mustela furo), feral pigs (Sus scrofa), and hedgehogs (Erinaceus europaeus) (Nugent Citation2011). The brushtail possum has long been considered the primary wildlife maintenance host in which the disease can persist independently (Pfeiffer et al. Citation1995), although at very high densities ferrets can also potentially act as maintenance hosts (Caley and Hone Citation2005). Feral pigs and deer are considered to be spillover hosts as evidenced by the decline in prevalence of TB in pigs and deer that occurs when possums but not pigs or deer are intensively controlled (Nugent et al. Citation2015). In light of this, the wildlife management programme aimed at reducing TB transmission to domestic cattle and deer in New Zealand has focussed on lethal control of possums alone (Ryan et al. Citation2006). The national programme has now progressed to the stage where large areas are being declared “free” of TB in possums (Livingstone et al. Citation2015) but there are some areas where TB is still occasionally being found in deer, pigs or ferrets long after the initial imposition of intensive possum control. Examples include reports of M. bovis-infected pigs and deer in the eastern Hauhungaroa Ranges, in the Waikato region of New Zealand, in the 2008–2012 period, 14–18 years after initial possum control, and the continued detection of TB in ferrets in the northern Pisa Range, in the Otago region, despite a high likelihood TB has been locally eradicated from possums (Anonymous Citation2013). These events have prompted speculation about the combined role of wildlife species in maintaining M. bovis transmission and thus disease across landscapes (Nugent Citation2011). In the face of inter-specific transmission, a higher level of possum control may be required for disease eradication, and control may be needed for longer periods of time to safeguard against spillback transmission to possums from individuals of the other host species prior to it fading out in their populations. Worse, even if these spillover hosts cannot maintain TB in isolation at their natural abundances, there may be sufficient inter-species transmission for TB to persist within the collective host community.
These possibilities create a need to consider the disease dynamics of TB in wildlife in New Zealand in a multi-host context. However, models of the wildlife component have to-date focussed largely on the possum (Roberts Citation1996; Barlow Citation2000; Ramsey and Efford Citation2010), mostly aimed at exploring the question of how much possum population control is required (and for how long) to eliminate TB from this species. Therefore we developed multi-host models for two case studies of current concern for TB management; (i) chronic TB persistence in a possum-deer-pig complex in extensive forest (such as the Hauhungaroa Ranges) and (ii) in a possum-pig-ferret complex in unforested semi-arid shrub and grasslands, as on Molesworth Station, in the Marlborough region (Byrom et al. Citation2008; Nugent et al. Citation2011a). The aims of this modelling exercise were firstly, to explore how the inclusion of multi-host dynamics affects the predicted prevalence of TB in possums and other host species following the current best practice for control of TB in large, difficult-to-access and remote areas (i.e. three aerially delivered poison baiting operations spaced 5 years apart, each killing 95% of the possum population present), and secondly, to identify which host species are responsible for changes in predicted prevalence, and whether TB can persist in possum-free host communities.
Materials and methods
Our models were based on wildlife species interactions only. Farmed cattle and deer were not included as hosts in these models as they are not sympatric with the wildlife host complex in extensive forest. While beef cattle are sympatric with wildlife hosts in some grassland areas, we considered they did not contribute to TB dynamics as the number of infected animals is extremely low, these infected animals would have to escape detection by annual herd testing, die on the farm and their carcass left accessible to scavengers for any chance of infection to occur. Anecdotal evidence that cattle to possum infection is a very rare event, is the absence of TB in possums before the 1960s, despite well-established possum populations and heavily infected cattle co-occurring since the early 1900s (Nugent Citation2011).
Model construction
The same three-host TB model was constructed for each case study, but parameterised according to the wildlife host community present. Model structure was based on the possum-TB model of Barlow (Citation2000), comprising a susceptible and infectious host class with density-dependent population growth and density-dependent disease transmission:
where, for each host species j, Nj is the total host density, Sj is the density of susceptible hosts, Ij is the density of infectious hosts, αj is the additional host mortality rate due to infection and pj is the pseudo-vertical disease transmission rate. Density-dependence in birth and death rates was modelled using a theta-logistic function:
where bj and dj are the maximum birth and minimum death rates respectively, δj is the proportion of density-dependence in birth rates and θ is a shape parameter for the population growth rate. As per Barlow (Citation2000), intra-specific disease transmission among possums (common to both case studies) was modelled using non-linear contact rates C(Nj) and heterogeneous-mixing F(Sj,Ij):
where εj is a shape parameter describing the relationship with density, and kj is an over-dispersion parameter describing the mixing between susceptible and infectious individuals (where small values of k give more heterogeneity). For all other disease transmission events, both intra-specific within other species and inter-specific among species, contact rates were simply assumed to be linear with host density (ε = 0), and mixing homogeneous (k = 100), because we lacked data to parameterise these terms (resulting in the conventional density-dependent transmission term βSI).
Model parameterisation
In the forested Hauhungaroa Ranges (175.576198E, 38.649730S), red deer and feral pigs are the known TB hosts sympatric at moderate population densities with possums (Nugent et al. Citation1997). On the unforested semi-arid shrub and grasslands on Molesworth Station (173.063653E, 42.237301S), ferrets and feral pigs are the known hosts occurring sympatrically, at low-to-moderate densities in some parts of the area, with possums (Byrom et al. Citation2008; Nugent et al. Citation2011a). Demographic parameter values for the different host species at the two sites were obtained from the literature and are summarised in . Demographic stochasticity in the numbers of births was incorporated by drawing these quantities from a Poisson distribution. Demographic stochasticity in the numbers of hosts surviving (from natural hazards and/or disease) or the number of hosts escaping M. bovis infection was simulated by drawing those numbers from a binomial distribution at each time step.
Table 1. Estimated annual birth and mortality rates, rate of increase, carrying capacity and other parameters, derived from the literature for brushtail possums (Trichosurus vulpecula), feral ferrets (Mustela furo), feral pigs (Sus scrofa) and red deer (Cervus elaphus) in forest and grassland habitats, used in multi-host models to predict long-term persistence of tuberculosis.
Transmission of infectious M. bovis bacilli within the two host communities modelled most probably occurs through some form of close contact, either via some respiratory mechanism, orally via ingestion of infected material as a result of scavenging, or via the skin as a result of bite or scratch wounds (Nugent Citation2011). Corresponding disease transmission rates (β) were obtained from the literature, or estimated from published incidence rates or from published contact/scavenging rates, when available. In the absence of any such information, unknown rates were rescaled from known rates using the relative ratios of infected species home range sizes (assumed to approximate contact rates). For example, the deer to pig transmission rate was estimated by multiplying the possum to pig rate β = 2.4 by 0.025/3.5 where the numerator is the size of a possum home range and the denominator the size of a deer home range (both in km2). For possum to possum transmission rates we adopted the approach of Barlow (Citation1991, Citation1993, Citation2000) and assumed that the maximum contact rate per infectious possum βK was constant. While Barlow (Citation2000) assumed βK = 2.5, to generate equilibrium disease prevalence of approximately 5% prevalence, we used a lower value of βK = 1.56 to more realistically simulate the known landscape-scale infection levels in possums at our two case study sites (approximately 2% prevalence).
Transmission rates and their sources are detailed in . Where there was little or no evidence of pseudo-vertical disease transmission (e.g. as in ferrets, pigs and deer), then the rate (pj) was set to a nominal value of 0.01 (). If mortality due to disease was noted to be low, and infected individuals appeared to have the same mortality rates as non-infected individuals (e.g. pigs; Nugent et al. Citation2015) then the disease mortality rate (αj) was set to 0.05. The transmission rates estimated from scavenging rates (i.e. the percentage of carcasses consumed) are likely to be overestimates as (1) they do not try to estimate the probability of infection given scavenging (it is assumed to be 1.0), and (2) it is assumed that carcasses are contacted or scavenged within the period that M. bovis bacteria remain viable, as has been found for free-ranging possums encountering dead possums, and for free-ranging possums and ferrets visiting pig remains (Yockney and Nugent Citation2003; Barron et al. Citation2011).
Table 2. Main intra- and inter-specific transmission routes and estimated transmission rates (β in km2 per individual per annum) of Mycobacterium bovis, derived from the literature, for brushtail possums (Trichosurus vulpecula), ferrets (Mustela furo), pigs (Sus scrofa) and red deer (Cervus elaphus), used in multi-host models to predict long-term persistence of tuberculosis.
Table 3. Estimated disease mortality and pseudo-vertical transmission rates (per annum), derived from the literature, for brushtail possums (Trichosurus vulpecula), ferrets (Mustela furo), pigs (Sus scrofa) and red deer (Cervus elaphus), used in multi-host models to predict long-term persistence of tuberculosis.
For disease transmission simulated as occurring peri- or post-mortem (e.g. infection occurring via scavenging of carcasses), the number of infected individuals was multiplied by the combined (natural plus disease) mortality rate to approximate the supply of carcasses.
Model simulation
Simulations for the forest and grassland systems were run over areas of 916 km2 and 1800 km2 respectively, nominally representing the areas of the Hauhungaroa Ranges and Molesworth Station case studies. The model time-step was monthly. Each model simulation covered a period of 60 years, comprising a burn-in period of 20 years, at which time steady-state prevalence of TB (equilibrium) was assessed, followed by simulated possum control imposed in years 21, 26 and 31 to mimic the current best practice control regime. One thousand replicated simulations were run for each potential host community, i.e. all combinations of one, two or three host species, for each case study.
Results for each host community simulated were summarised as the median and 95th percentiles for the density of each host species present each year, the prevalence of TB in host species present each year, and the proportion of replicates where TB persisted within the host community. TB eradication within a host species was defined as the point in the simulation where the number of infected hosts/prevalence of TB in that species declined to zero. For each case study, these predictions were compared (1) to available data on post-control TB dynamics to assess the biological realism of predictions, (2) between the three-species community and the possum only community, to assess the impact of including multi-host dynamics on predicted control success, (3) between the three-species community and the two-species communities with different non-possum hosts dropped out, to assess which species was responsible for the differences observed, and (4) among all possum-free host community combinations to assess whether TB was predicted to persist in any of them.
Because of the high uncertainty in the disease-related parameter values (intra and inter-disease transmission, disease mortality and pseudo-vertical transmission rates) a global sensitivity analysis on this parameter set was conducted for each case study. We used the Fourier Amplitude Sensitivity Test (FAST) using the statistical software R (R Development Core Team, R foundation for Statistical Computing, Vienna, Austria). FAST is a variance decomposition method which samples the different parameters at different frequencies across the parameter space. Fourier analysis then estimates the strength of each parameter's frequency in the model output giving a partial variance for each parameter Si, defined as the proportion of the total output model variance explained by variation in input parameter i. For the disease transmission and mortality rates we defined 0.5 and 2 times the default rate as the minimum and maximum rates respectively. For the proportion of offspring pseudo-vertically infected we subtracted and added 0.25 to the default value, truncating the range to 0 or 1 where necessary. For the 15 disease-related parameters, FAST sampling selected 1,019 different combinations of parameter values. The effects of varying parameter values were assessed against prevalence of TB and persistence for each host species.
Results
Forest case study: Hauhungaroa Ranges
Comparison of model predictions with field data
Modelled prevalence of TB in possums (a) post-control was consistent with empirical evidence from the eastern Hauhungaroa Ranges, where use of sodium fluoroacetate (1080) in 1994 resulted in a decline in prevalence of TB in possums from 2/232 (0.86%) to undetectable levels (0/7 and 0/16) 1–2 years post-control (Nugent et al. Citation1997). Similarly in the central-western part of the range prevalence of TB fell from 6% in the 1997–2000 period to zero in more than 200 possums necropsied after 2010 (Nugent et al. Citation2015). Confirmed M bovis infection was last observed among possums in the central-western Hauhungaroa Ranges in 2005, 5 years after that part of the area was first controlled (de Lisle et al. Citation2009).
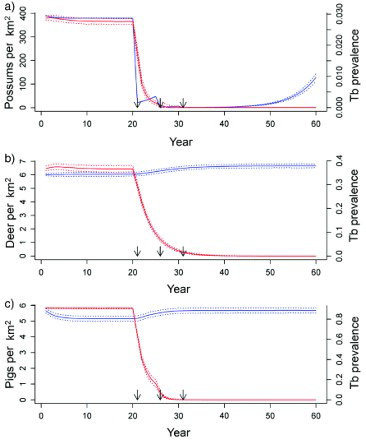
Figure 1. Predicted animal population density (blue lines) and proportional prevalence of tuberculosis (TB; red lines) from a three-host model in a forest habitat, with simulated possum control imposed at 21, 26 and 31 years for a) possum, b) deer, and c) pig populations. Solid lines are the median values and dotted lines are the 2.5 and 97.5 percentiles of model simulations. Arrows indicate the timing of possum-control operations.
Modelled prevalence of TB in deer (b) showed good agreement with data from the eastern parts of the Hauhungaroa Ranges, where prevalence declined from >30% in 1993 to near zero in 2003, 10 years after control of possums began in 1994 (Nugent Citation2005). Likewise, in the central western area, prevalence in deer declined from 45%, (n=44) in 1999 to 0% (n=37) in 2011 (Nugent et al. Citation2012a). However, a TB-positive deer was reported by a hunter in 2008 in the eastern part of the range, 14 years after the initial control there (Nugent et al. Citation2014).
Modelled prevalence of TB in pigs was predicted to fall rapidly after possum control (c). In reality, a decline in prevalence of TB did occur, but in the eastern parts of the Hauhungaroa Ranges (where initial possum control was instigated in 1994) the decline was not as rapid as predicted, remaining at around 54–78% during the 1995–2000 period. But after a second control operation in 2000 prevalence of TB in pigs fell to 33% during 2000–2005 (n=107), and following another operation in 2005 prevalence of TB in pigs declined to zero in 2007 (n=26; Nugent et al. Citation2012a). Since then, however, two TB-positive pigs have been shot between 2009 and 2013 (Nugent et al. Citation2014).
Impact of multi-host dynamics on control success
Predicted dynamics of TB in possums were similar in both the possum-only and three-host (possum/pig/deer) models (). Simulated possum control after 21, 26 and 31 years consistently eradicated TB from possum populations in both models, with possum-only simulations taking 7 years after the first possum control event for >95% of populations to be free of TB, compared with 10 years for the three-host model.
Specific influences of non-possum hosts on TB dynamics
Steady-state prevalence of TB in the possum and pig two-host model was much the same as in the three-host model (); 2.7% for possums and 91% for pigs, and decreased similarly in response to control, taking 10 years post-control for 95% of possum populations to be free of TB.
In contrast, in the possum and deer two-host model, steady-state prevalence of TB was lower in both species, namely 1.7% in possums (compared to 2.7% in the three-host model) and 27% in deer (compared to 37% in the three-host model). Hence, the influence of multi-host dynamics was driven mainly by the presence of pigs, as opposed to deer. There was no TB persistence in any model system that did not include possums.
Forest model sensitivity analysis
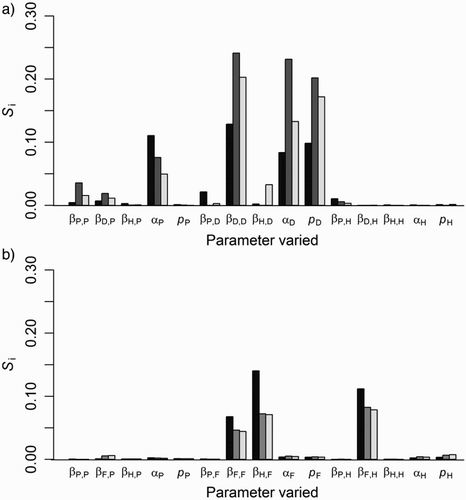
Steady-state prevalence of TB in possums was largely explained by the possum disease mortality rate (67% of the variance in model output), followed by the possum-to-possum transmission rate (18% of model variance), and was relatively insensitive to all the other parameters which collectively explained <1.5% of the variance in model output.
Variation in TB persistence in all three host species was mostly explained by the deer disease parameters, specifically the deer-to-deer transmission rate, disease mortality rate and the pseudo-vertical transmission rate which collectively explained 31, 67, and 51% of the variation in persistence in possum, deer and pig populations, respectively (a). The possum disease mortality rate also had some influence on persistence with lower mortality rates giving greater persistence. Overall model predictions were relatively robust to changes in parameter values with 95% of the sensitivity analysis parameter combinations predicting TB eradication (<5% persistence) from the possum populations following best practice possum control.
Figure 3. Proportional contribution to variance (sensitivity; Si) in the predicted persistence of tuberculosis (TB) for three-host simulation models, for a) forest and b) grassland habitats, contributed by variation in disease parameter values. TB persistence is the proportion of simulated populations of possums (P; black bars), deer (D), ferret (F) (both dark grey bars), or pigs (H; light grey bars) with infected individuals still present 20 years after three consecutive possum control operations. β is the disease transmission rate, α is the disease mortality rate and p is the pseudo-vertical transmission rate.
Tussock grassland case study: Molesworth Station
Comparison of model predictions with field data
The control programme for TB in possums on Molesworth Station is far less advanced than that for the Hauhungaroa Ranges, consequently there were no long-term empirical data to compare model predictions against. The simulation data for prevalence of TB in possums are presented in a.
Figure 4. Predicted animal population density (blue lines) and proportional prevalence of tuberculosis (TB; red lines) from a three-host model in a grasslands habitat, with simulated possum control imposed at 21, 26 and 31 years for a) possum, b) ferret, and c) pig populations. Solid lines are the median values and dotted lines are the 2.5 and 97.5 percentiles of model simulations. Arrows indicate the timing of possum control operations.
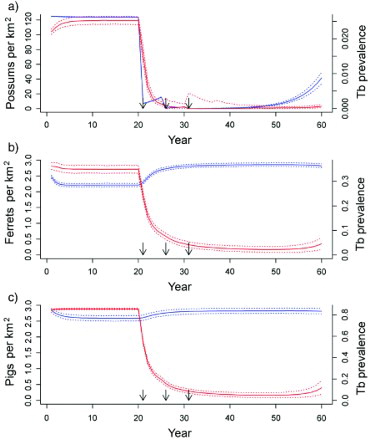
Modelled prevalence of TB among ferrets was higher (35% at equilibrium, b) compared with field data from Molesworth Station of 21% (n=407 ferrets, TB identified by cultured samples: Byrom et al. Citation2008). No data for prevalence of TB in ferrets after possum control were available for Molesworth Station, but in a similar semi-arid grassland habitat (Scargill Hills, Canterbury region) reducing possum abundance resulted in a reduction in the prevalence of macroscopic TB lesions in ferrets from 11% pre-possum-control (n=93) to 3% 1–2 years post-control (n=120) (Caley et al. Citation2001).
Modelled prevalence of TB in pigs (c) showed general agreement with the observed rapid decline of TB in pigs following possum control. The response of prevalence of TB in pigs to aerial 1080 poisoning of possums was measured for the Bullen's Hill (8700 ha) area of Molesworth Station in late October 2004, and for the south-east boundary of the station (17 800 ha) in late 2008. Prevalence in pigs from the Bullen's Hill area declined from 78% (n=9) prior to possum control to 50% (n=4) 1 year after control, and to 10% (n=31) 2 years after control (Nugent et al. Citation2012b). Similarly, prevalence in pigs surveyed from the south-east area decreased from 90% (n=30) prior to possum control in 2008 to 55% immediately post-control (n=22), 51% (n=53) in 2009, and 25% (n=63) in 2010/11 (Nugent et al. Citation2011b). Since 2011, however, prevalence of TB in pigs has plateaued at about 20%, possibly as a consequence of the apparent rapid recovery of the possum population there (Nugent et al. Citation2011b). The modelled prevalence of TB in pigs never fell below 5% on average, despite possum control (c).
Impact of multi-host dynamics on control success
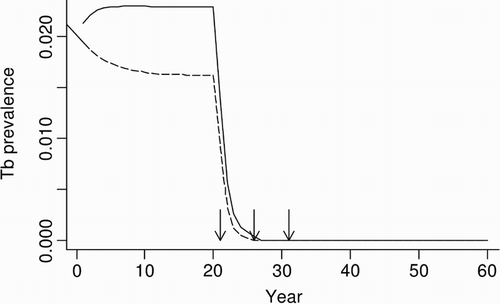
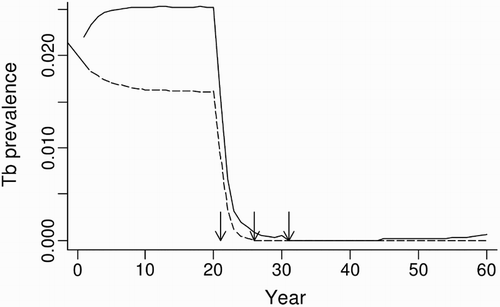
In the possum-only model for the grassland system, simulated possum control in years 21, 26 and 31 resulted in >95% of populations being free of TB 6 years after the first possum control event. In contrast, when multi-host dynamics were included, 7% of the simulated possum populations still carried TB in year 31, climbing to 100% of the populations with TB by year 60, albeit at a very low average prevalence of <0.1% (). This disease recovery in the model was due to the persistence of TB in both the ferret and pig populations followed by spillback transmission of M. bovis from these populations to the recovering possum population.
Figure 5. Predicted proportional prevalence of tuberculosis (TB) in possums from possum-only (dashed line) and three-host (solid line) model simulations in a grassland habitat, with simulated possum control imposed at 21, 26 and 31 years. Arrows indicate the timing of possum control operations.
Because of this persistence we also explored whether additional control of ferret and pig populations could lead to TB eradication. High annual culling rates (>60%) of either ferrets or pigs during the period of possum control, or lower annual culling rates (∼30%) sustained for a longer term (during and for 20 years following the possum control), were sufficient to achieve TB eradication from the three-host system. Annual culling of ferrets or pigs was needed to suppress their abundance because of their high potential rates of annual population increase (1.2 and 0.69, respectively).
Specific influences of non-possum hosts on TB dynamics
Tuberculosis was predicted to fade out of pig or ferret populations when they were modelled as single host systems. So when either species was combined with possums into a two-host model, TB was only maintained through spill-over transmission from possums. This meant that simulated best practice control of possums eliminated TB from both the possum and the secondary host. Removing pigs from the system had a larger impact on steady-state prevalence of TB than removing ferrets; pig removal was predicted to reduce prevalence of TB in possums from 2.5% to 1.9% and to reduce prevalence of TB in ferrets from 35% to 16%. However in the two-host pig and ferret model, TB was predicted to persist in the absence of possums via reciprocal scavenging at a steady-state prevalence of TB of 1.4% and 3% among pigs and ferrets, respectively. This provides a mechanism for the potential control failure identified in the three-host model; TB could persist in the pig/ferret complex in the absence of possum hosts and this would provide a source of spillback infection for possums once their numbers recovered again many years after control.
Tussock grassland model sensitivity analysis
Steady-state prevalence of TB in possums was highly sensitive to changes in the possum disease mortality rate, which explained 66% of the output variation, followed by the possum-to-possum transmission rate explaining 19% of the variation in possum prevalence, but was relatively insensitive to changes in the other parameters which collectively explained 1.1% of the total variation.
Persistence of TB in all three grassland host species was most sensitive to variation in the ferret-to-pig, pig-to-ferret and ferret-to-ferret disease transmission rates (b), with increasing transmission rates increasing the probability of TB persistence. The proportion of total variance in TB persistence explained by first order effects of all 15 parameters combined was lower for the grassland than the forest scenario (34 vs. 47% for possums).This was probably because the total number of individuals in the grassland simulations was much lower than in the forest simulations and small populations are more susceptible to chance losses (demographic stochasticity), and persistence is less predictable compared with larger populations which conform more to deterministic expectations.
Discussion
The multi-host TB models presented here, based on realistic population vital rates and plausible intra- and inter-specific transmission rates, predict that multi-host species complexes could potentially pose a risk to TB eradication strategies based on single-species (possum only) dynamics and control, and that level of risk depends on the combination of host species involved. Specifically, the simultaneous presence of moderate density ferret and pig populations in the grasslands case study predicted continued TB persistence even when the density of possums was zero or extremely low. This could result in spillback transmission to previously controlled possum populations after they had recovered from control, and, ultimately, TB eradication failure. In contrast, deer and pig populations in the forest case study acted as true spillover hosts and did not affect the predicted success of possum control in eradicating TB from the possum population.
The grassland case study demonstrates potential for both postulated multi-host effects, namely inter-specific transmission influencing TB dynamics in possums, and sufficient inter-species transmission for TB to potentially persist within the collective non-possum host community. The ability of ferrets and pigs to maintain TB within their collective populations was contingent upon sufficient pig-to-ferret and ferret-to-pig transmission, with long-term persistence of TB in the face of simulated possum control being most sensitive to these inter-specific transmission rates. Pigs and ferrets have some similar characteristics as TB hosts as they both have high population turnover, are scavengers of carcasses and are highly susceptible to oral-route infection with M. bovis. In addition, although ferrets are much more likely to succumb to the disease, there is some indication that “natural” mortality compensates for disease mortality (Caley et al. Citation2002), so the lifespan of infected and susceptible ferrets may be quite similar, as it is in pigs. The parameter value for pig-to-ferret transmission was derived from a scavenging estimate and may be too high as it assumed all scavenging of an infected pig carcass resulted in disease transmission. There were no estimates available for ferret-to-pig transmission rates and instead these were derived by rescaling encounter rates of possum carcasses by pigs using relative home range sizes. Again, this estimate does not take into account the chances of an individual becoming infected given they have ingested M. bovis-infected material, and it also assumes pigs are just as likely to scavenge a ferret as a possum carcass. There is anecdotal evidence that pigs may avoid feeding on ferret carcasses whereas they readily consume possum carcasses (Byrom Citation2004). If this is the case then the ferret-to-pig transmission rates were likely set too high and the sensitivity analysis indicated that a 50% reduction in this rate would result in TB not persisting in the ferret-pig complex alone.
Despite these uncertainties around parameter values, the modelling identified the grassland system host combination as the one most likely to require control of non-possums hosts in addition to possums to achieve TB eradication. Because ferrets and pigs were predicted to act a host complex (via reciprocal scavenging) and because transmission was assumed to be density-dependent, moderately intensive control of either species was able to reduce their combined abundance below the threshold for disease persistence (<5 per km2 for the simulated populations). In conjunction with best practice possum control, the additional control enabled eradication of TB from the area. The only constraint was that control had to be annual to overcome the high population rates of increase of both ferrets and pigs. If multi-host persistence is suspected, we suggest that it would be more effective to target ferrets in preference to pigs for additional host control because of their potential to act as maintenance hosts at high densities.
The forest case study showed evidence for the first postulated multi-host effect, namely that inter-specific transmission could influence TB dynamics in possums, but this was predicted to have little effect on the success of best practice TB control. The presence of deer had very little influence on TB dynamics in possums. This supports their long-inferred status as a largely inconsequential spillover hosts for the disease (Lugton et al. Citation1998), and that the long-term consequences of spillback transmission to possums is minimal, other than extending the duration of the possum control required somewhat (Barron et al. Citation2013). Pigs were predicted to have the most influence on prevalence of TB in possums, largely as a consequence of their disease amplification role; but because they are relatively short-lived, they did not have any great effect in extending the spillback risk to possums. Sensitivity analyses indicated these interpretations are robust to uncertainty in model parameter values. The use of pigs as disease sentinels is discussed elsewhere in this issue (Anderson et al. Citation2015; Nugent et al. Citation2015) but is well illustrated here by the amplification effects they had on TB prevalence in both case study habitats.
It is difficult to compare our model predictions of multi-species TB dynamics under possum control with those observed in the field because the possum control operations were not as consistent in reality as those modelled. For example, reduction of possum populations to very low density was not achieved in the western central part of the Hauhungaroa Ranges until 2005 when the third aerial control operation was done. The observations of M. bovis-infected deer and pigs shot in the Hauhungaroa Ranges 15–20 years after the initial possum control operation are consistent with inadequate initial control coverage and efficacy resulting in a longer tail of residual infection, particularly in a long-lived species such as deer. Taking into account the fact that 11 years was required before intensive possum control was achieved over the whole area, the model captured the responsiveness of TB in pigs to possum control, with TB in pigs declining to about 50% before the second control operation in both the modelled and measured population, and the modelled decline of prevalence of TB in deer closely mirrored that observed in the eastern and western parts of the area (Nugent et al. Citation2015).
The modelling approach taken here was a first attempt to characterise multi-host dynamics of TB for New Zealand wildlife using point estimates of disease-related rates derived from the literature, then assessing model predictions against field data where available. Sensitivity analysis identified ferret and pig intra- and inter-specific transmission rates for M. bovis as having the most influence on persistence of TB. Ideally future work should focus on empirical estimation of these transmission rates to determine if multi-host dynamics could be jeopardising TB eradication programmes. However field-based estimation of disease transmission rates is notoriously difficult and an alternative or complimentary approach could involve calibrating model predictions with observed disease prevalence patterns using approximate Bayesian computation (Beaumont Citation2010). These methods have been used to make inference on parameters for complex multi-parameter models such as ours (Toni et al. Citation2009; Rasmussen and Hamilton Citation2012).
With respect to management of TB, for modelled forest habitats, 15 years of effective possum control was predicted to eradicate TB from the possum-deer-pig host community, indicating the current focus on possum-only control is appropriate for such areas. For grassland model systems, TB was predicted to persist in the ferret-pig host complex in the absence of possums potentially jeopardising the effectiveness of possum-only control programmes. However this outcome depended on the occurrence and rate of pigs acquiring TB from ferrets, which is unknown. Thus some estimation of this transmission parameter is required to enable managers to assess if multi-host disease dynamics are important for their TB control programmes. In the interim, if TB is still being detected in ferrets and pigs following the implementation of effective possum control, managers should consider reducing the average annual density of these other hosts, particularly ferrets.
| FAST | = | Fourier Amplitude Sensitivity Test |
| TB | = | Tuberculosis |
Acknowledgements
This review was contracted by TBFreeNZ (Project R10735-01), with co-funding from the Ministry of Business, Innovation and employment (Contact C09X0803). We thank Graham Nugent for his substantial input on the control and disease histories for the case studies and Frank Cross for his editorial assistance.
Notes
*Non-peer-reviewed
References
- Anderson DP, Ramsey DSL, de Lisle GW, Bosson M, Cross ML. Development of an integrated surveillance system for the management of tuberculosis in New Zealand wildlife. New Zealand Veterinary Journal 63 (Suppl. 1), 89–97, 2015
- *Anonymous. Animal Health Board Annual report 2012/13. http://www.tbfree.org.nz/Portals/0/AHB%20Annual%20Report%20E-book%202012-13final.pdf (accessed 14 March 2014). TBfree New Zealand, Wellington, NZ, 2013
- Barlow ND. A spatially aggregated disease/host model for bovine Tb in New Zealand possum populations. The Journal of Applied Ecology 28, 777–93, 1991 doi: 10.2307/2404207
- Barlow ND. A model for the spread of bovine Tb in New Zealand possum populations. The Journal of Applied Ecology 30, 156–64, 1993 doi: 10.2307/2404279
- Barlow ND. Non-linear transmission and simple models for bovine tuberculosis. Journal of Animal Ecology 69, 703–13, 2000 doi: 10.1046/j.1365-2656.2000.00428.x
- Barlow ND, Norbury GL. A simple model for ferret population dynamics and control in semi-arid New Zealand habitats. Wildlife Research 28, 87–94, 2001 doi: 10.1071/WR99090
- Barron MC, Pech RP, Whitford J, Yockney IJ, de Lisle GW, Nugent G. Longevity of Mycobacterium bovis in brushtail possum (Trichosurus vulpecula) carcasses, and contact rates between possums and carcasses. New Zealand Veterinary Journal 59, 209–17, 2011 doi: 10.1080/00480169.2011.595905
- Barron MC, Nugent G, Cross ML. Importance and mitigation of the risk of spillback transmission of Mycobacterium bovis infection for eradication of bovine tuberculosis from wildlife in New Zealand. Epidemiology and Infection 141, 1394–406, 2013 doi: 10.1017/S0950268812002683
- Beaumont MA. Approximate Bayesian Computation in Evolution and Ecology. Annual Review of Ecology, Evolution, and Systematics 41, 379–406, 2010 doi: 10.1146/annurev-ecolsys-102209-144621
- *Byrom A. R-10618 Spread of Tb by ferrets in the northern South Island high country. http://www.tbfree.org.nz/Portalsxxx/0/2014AugResearchPapers/Byrom%20AE.%20Spread%20of%20Tb%20by%20ferrets%20in%20the%20northern%20South%20Island%20high%20country.%20Landcare%20Research%20contract%20report%20LC0304146.%20Landcare%20Research,%20Lincoln,%20New%20Zealand,%202004.pdf (accessed 16 September 2014). TBfree New Zealand, Wellington, NZ, 2004
- *Byrom A, Nugent G, McKenzie J, Porphyre T, Pouto N, Shepherd J, Whitford J, Yockney I. Animal Health Board Project No. R-80629 Cost-effective control of Tb in the Northern South Island High Country: Identifying the habitats and vector species requiring control. http://www.tbfree.org.nz/Portalsxxx/0/2014AugResearchPapers/Byrom%20A,%20Nugent%20G,%20Yockney%20I,%20Poutu%20N,%20Whitford%20J,%20McKenzie%20J,%20Shepherd%20J,%20Porphye%20T.%20Cost-effective%20control%20of%20Tb%20in%20the%20Northern%20South%20Island%20High%20Country%20(NSIHC).pdf (accessed 14 September 2014). TBfree New Zealand, Wellington, NZ, 2008
- Caley P, Hone J. Assessing the host disease status of wildlife and the implications for disease control: Mycobacterium bovis infection in feral ferrets. Journal of Applied Ecology 42, 708–19, 2005 doi: 10.1111/j.1365-2664.2005.01053.x
- Caley P, Hone J, Cowan PE. The relationship between prevalence of Mycobacterium bovis infection in feral ferrets and possum abundance. New Zealand Veterinary Journal 49, 195–200, 2001 doi: 10.1080/00480169.2001.36232
- Caley P, McElrea LM, Hone J. Mortality rates of feral ferrets (Mustela furo) in New Zealand. Wildlife Research 29, 323–8, 2002 doi: 10.1071/WR02004
- *Choquenot D, McIlroy JC, Korn T. Managing vertebrate pests: feral pigs. Australian Government Publishing Service, Canberra, Australia, 1996
- *Clapperton BK, Byrom AE. Feral ferret. In: King CM (ed) The handbook of New Zealand mammals. Pp 294–307. Oxford University Press, Melbourne, Australia, 2005
- Craft ME, Hawthorne PL, Packer C, Dobson AP. Dynamics of a multihost pathogen in a carnivore community. Journal of Animal Ecology 77, 1257–64, 2008 doi: 10.1111/j.1365-2656.2008.01410.x
- de Lisle GW, Yates GF, Coleman JD. Isolation of Mycobacterium bovis from brushtail possums with non-visible lesions. New Zealand Veterinary Journal 57, 221–4, 2009 doi: 10.1080/00480169.2009.36905
- Dobson A. Population dynamics of pathogens with multiple host species. The American Naturalist 164, S64–S78, 2004 doi: 10.1086/424681
- Fitzpatrick MC, Hampson K, Cleaveland S, Meyers LA, Townsend JP, Galvani AP. Potential for Rabies Control through Dog Vaccination in Wildlife-Abundant Communities of Tanzania. PLoS Neglected Tropical Diseases 6, e1796, 2012 doi: 10.1371/journal.pntd.0001796
- Forsyth D, Allen RB, Marburg AE, MacKenzie DI, Douglas MJ. Population dynamics and resource use of red deer after release from harvesting in New Zealand. New Zealand Journal of Ecology 34, 277–87, 2010
- Lembo T, Hampson K, Haydon DT, Craft M, Dobson A, Dushoff J, Ernest E, Hoare R, Kaare M, Mlengeya T, Mentzel C, Cleaveland S. Exploring reservoir dynamics: a case study of rabies in the Serengeti ecosystem. Journal of Applied Ecology 45, 1246–57, 2008 doi: 10.1111/j.1365-2664.2008.01468.x
- Livingstone PG, Nugent G, de Lisle GW, Hancox N. Toward eradication: the effect of Mycobacterium bovis infection in wildlife on the evolution and future direction of bovine tuberculosis management in New Zealand. New Zealand Veterinary Journal 63 (Suppl. 1), 4–18, 2015
- Lugton IW, Wobeser G, Morris RS, Caley P. Epidemiology of Mycobacterium bovis infection in feral ferrets (Mustela furo) in New Zealand: II. Routes of infection and excretion. New Zealand Veterinary Journal 45, 151–7, 1997 doi: 10.1080/00480169.1997.36015
- Lugton IW, Wilson PR, Morris RS, Nugent G. Epidemiology and pathogenesis of Mycobacterium bovis infection of red deer (Cervus elaphus) in New Zealand. New Zealand Veterinary Journal 46, 147–56, 1998 doi: 10.1080/00480169.1998.36079
- McIlroy JC. Aspects of the Ecology of Feral Pigs (Sus scrofa) in the Murchison Area, New Zealand. New Zealand Journal of Ecology 12, 11–22, 1989
- *McIlroy JC. Feral pig. In: King CM (ed) The handbook of New Zealand mammals. Pp 334–45. Oxford University Press, Melbourne, Australia, 2005
- *Nugent G. The role of wild deer in the epidemiology and management of bovine tuberculosis in New Zealand. PhD thesis, Lincoln University, Lincoln, New Zealand, 2005
- Nugent G. Maintenance, spillover and spillback transmission of bovine tuberculosis in multi-host wildlife complexes: A New Zealand case study. Veterinary Microbiology 151, 34–42, 2011 doi: 10.1016/j.vetmic.2011.02.023
- *Nugent G, Fraser W. Red deer. In: King CM (ed) The handbook of New Zealand mammals. Pp 401–20. Oxford University Press, Auckland, 2005
- *Nugent G, Coleman J, Fraser W. Optimal buffer widths for control of possums in the Hauhungaroa Range: 1996– Population recovery and Tb prevalence in possums, deer, and pigs two years after control. http://www.tbfree.org.nz/Portals/0/2014AugResearchPapers/NugentEtal1997LC9697124.pdf (accessed 14 September 2014). TBfree New Zealand, Wellington, NZ, 1997
- Nugent G, Whitford J, Young N. Use of released pigs as sentinels for Mycobacterium bovis. Journal of Wildlife Diseases 38, 665–77, 2002 doi: 10.7589/0090-3558-38.4.665
- *Nugent G, Ramsey DSL, Caley P. Animal Health Board Project no. R-10627 Enhanced early detection of Tb through use and integration of wildlife data into the national surveillance model. http://www.tbfree.org.nz/Portals/0/2014xxxAugResearchPapers/Nugent%20G,%20Ramsey%20D,%20Caley%20P.%20Enhanced%20early%20detection%20of%20Tb%20through%20use%20and%20integration%20of%20wildlife%20data%20into%20the%20national%20surveillance%20model.pdf (accessed 14 September 2014). TBfree New Zealand, Wellington, NZ, 2006
- *Nugent G, Yockney I, Whitford J. Eliminating TB from Molesworth Station: II. Persistence of TB on Molesworth Station two years after aerial 1080 baiting. Animal Health Board R-10629_02. http://www.tbfree.org.nz/Portals/0xxx/2014AugResearchPapers/Nugent%20G,%20Yockney%20I,%20Whitford%20J.%20Eliminating%20TB%20from%20Molesworth%20Station%20II.%20Persistence%20of%20TB%20on%20Molesworth%20Station%20two%20years%20after%20aerial%201080%20baiting.pdf (accessed 14 September 2014). TBfree New Zealand, Wellington, NZ, 2011a
- Nugent G, Yockney IJ, Whitford EJ. Intraspecific transmission of Mycobacterium bovis among penned feral pigs in New Zealand. Journal of Wildlife Diseases 47, 364–72, 2011b doi: 10.7589/0090-3558-47.2.364
- *Nugent G, Morriss G, Fitzgerald N, Innes J. Bait aggregation and deer repellent effects on efficacy, and non-target impacts on deer and birds, during aerial 1080 baiting: Hauhungaroa 2011. Animal Health Board R-10710 & R-10743. http://www.tbfree.org.nz/Portalsxxx/0/2014AugResearchPapers/Nugent%20G,%20Morriss%20G,%20Fitzgerald%20N,%20Innes%20J.%20Bait%20aggregation%20and%20deer%20repellent%20effects%20on%20efficacy,%20and%20non-target%20impacts%20on%20deer%20and%20birds,%20during%20aerial%201080%20baiting%20Hauhungaroa%202011.pdf (accessed 14 September 2014). TBfree New Zealand, Wellington, NZ, 2012a
- Nugent G, Whitford J, Yockney IJ, Cross ML. Reduced spillover transmission of Mycobacterium bovis to feral pigs (Sus scrofa) following population control of brushtail possums (Trichosurus vulpecula). Epidemiology and Infection 140, 1036–47, 2012b doi: 10.1017/S0950268811001579
- *Nugent G, Sweetapple P, Yockney I, Barron M, Latham C. Progress toward eradication of TB from wildlife in the Hauhungaroa Ranges. http://www.tbfree.org.nz/Portals/0/2014AugxxxResearchPapers/Nugent%20G,%20Sweetapple%20P,%20Yockney%20I,%20Barron%20M,%20Latham%20C.%20Progress%20toward%20eradication%20of%20TB%20from%20wildlife%20in%20the%20Hauhungaroa%20Ranges.pdf (accessed 14 September 2014). TBfree New Zealand, Wellington, NZ, 2014
- Nugent G, Gortazar C, Knowles GJE, The epidemiology of Mycobacterium bovis in wild deer and feral pigs and their roles in the establishment and spread of bovine tuberculosis in New Zealand wildlife. New Zealand Veterinary Journal 63 (Suppl. 1), 54–67, 2015
- Olmstead AL, Rhode PW. An Impossible Undertaking: The Eradication of Bovine Tuberculosis in the United States. The Journal of Economic History 64, 734–72, 2004
- Pfeiffer DU, Hickling GJ, Morris RS, Patterson KP, Ryan TJ, Crews KB. The epidemiology of Mycobacterium bovis infection in brushtail possums (Trichosurus vulpecula Kerr) in the Hauhungaroa Ranges, New Zealand. New Zealand Veterinary Journal 43, 272–80, 1995 doi: 10.1080/00480169./1995.35906
- Ragg JR, Mackintosh CG, Moller H. The scavenging behaviour of ferrets (Mustela furo), feral cats (Felis domesticus), possums (Trichosurus vulpecula), hedgehogs (Erinaceus europaeus) and harrier hawks (Circus approximans) on pastoral farmland in New Zealand: Implications for bovine tuberculosis transmission. New Zealand Veterinary Journal 48, 166–75, 2000 doi: 10.1080/00480169.2000.36188
- Ramsey DSL, Efford MG. Management of bovine tuberculosis in brushtail possums in New Zealand: predictions from a spatially explicit, individual-based model. Journal of Applied Ecology 47, 911–9, 2010 doi: 10.1111/j.1365-2664.2010.01839.x
- Rasmussen R, Hamilton G. An approximate Bayesian computation approach for estimating parameters of complex environmental processes in a cellular automata. Environmental Modelling & Software 29, 1–10, 2012 doi: 10.1016/j.envsoft.2011.10.005
- Roberts MG. The dynamics of bovine tuberculosis in possum populations, and its eradication or control by culling or vaccination. The Journal of Animal Ecology 65, 451–64, 1996 doi: 10.2307/5780
- Ryan TJ, Livingstone PG, Ramsey DSL, de Lisle GW, Nugent G, Collins DM, Buddle BM. Advances in understanding disease epidemiology and implications for control and eradication of tuberculosis in livestock: The experience from New Zealand. Veterinary Microbiology 112, 211–9, 2006 doi: 10.1016/j.vetmic.2005.11.025
- Tompkins DM, White AR, Boots M. Ecological replacement of native red squirrels by invasive greys driven by disease. Ecology Letters 6, 189–96, 2003 doi: 10.1046/j.1461-0248.2003.00417.x
- Toni T, Welch D, Strelkowa N, Ipsen A, Stumpf MPH. Approximate Bayesian computation scheme for parameter inference and model selection in dynamical systems. Journal of the Royal Society Interface 6, 187–202, 2009 doi: 10.1098/rsif.2008.0172
- Woolhouse MEJ, Taylor LH, Haydon DT. Population Biology of Multihost Pathogens. Science 292, 1109–12, 2001 doi: 10.1126/science.1059026
- *Yockney I, Nugent G. Project No. R-10577: Scavenging of Potentially Tuberculosis Feral Pig Carcasses in the Northern South Island High Country. http://www.tbfree.org.nz/Portals/0/2014xxxAugResearchPapers/Yockney%20I,%20Nugent%20G.%20Scavenging%20of%20potentially%20tuberculous%20feral%20pig%20carcasses%20in%20the%20northern%20South%20Island%20high%20country.pdf (accessed 14 September 2014). TBfree New Zealand, Wellington, NZ, 2003