Abstract
AIMS: To compare ryegrass pastures infected with endophytes producing diverse alkaloids for their ability to cause ryegrass staggers in grazing lambs; to compare respiration rates and rectal temperatures of these lambs after exposure to heat stress, and to compare liveweight gains during the study period.
METHODS: Ryegrass pastures of cultivar Trojan infected with NEA endophytes, branded NEA2 (T-NEA2), endophyte-free Trojan (T-NIL), Samson infected with standard endophyte (S-STD), Samson infected with AR37 endophyte (S-AR37) and endophyte-free Samson (S-NIL), were grazed by lambs (n=30 per cultivar) for up to 48 days in February and March of 2012 and 2013. Pasture samples were analysed for alkaloid concentrations and lambs were scored for ryegrass staggers at intervals during the study period. Liveweight was recorded at the start (Day 0) and end of the study, and rectal temperatures and respiratory rates were measured in lambs exposed to heat stress on Days 23 and 26, in 2012 and 2013, respectively.
RESULTS: Concentrations of alkaloids were lower in 2012 than 2013, associated with warmer and drier conditions in 2013, and the prevalence of ryegrass staggers was low in 2012. In 2013, concentrations of ergovaline were similar in T-NEA2 and S-STD, but concentrations of lolitrem B were lower in T-NEA2 than S-STD. S-AR37 produced epoxy-janthitrems but no lolitrem B or ergovaline. In 2013, by Day 20, 9/30 (30%) sheep grazing S-STD had severe staggers (score ≥4), and by Day 47 all sheep had been removed from this cultivar due to severe staggers. By Day 47, 18/30 (60%), 4/30 (13%) and 0/30 (0%) sheep grazing S-AR37, T-NEA2 and T-NIL pastures, respectively, had severe staggers. There were no differences in mean daily weight gain of lambs between cultivars in either year. In both years, mean rectal temperature and respiration rate following exposure to heat stress were highest in sheep grazing S-STD and T-NEA2, and lowest in sheep grazing T-NIL.
CONCLUSIONS: In lambs grazing different ryegrass pastures infected with endophytes, ryegrass staggers was most severe on S-STD, less severe on S-AR37 and least on T-NEA2. When under heat stress, lambs grazing ergovaline-producing S-STD and T-NEA2 pastures had increased respiration rates and rectal temperatures compared with lambs grazing T-NIL.
CLINICAL RELEVANCE: If ambient temperatures are suitable, NEA2-branded endophytes have the potential to express concentrations of ergovaline sufficient to induce heat stress in grazing sheep.
Introduction
Perennial and long-term hybrid ryegrasses (Lolium perenne and L. boucheanum syn. L. hybridum, respectively) are considered to be highly desirable grasses for feeding livestock in temperate, fertile, moist regions of the world, as these grasses produce high yields of quality feed (Jung et al. Citation1996). Ryegrasses that naturalised in pastures in the 19th century in large areas of southern Australia and New Zealand contain a fungal endophyte. The endophyte that colonises naturalised ryegrass in New Zealand is referred to within the commercial sector as standard or wild-type or common toxic endophyte (Stewart et al. Citation2014). Ryegrasses infected with standard endophyte produce three known classes of alkaloidal secondary metabolites: pyrrolopyrazine alkaloids, primarily peramine; indole diterpenes, primarily lolitrem B, and ergot alkaloids, primarily ergovaline. Peramine is known to have potent activity against Argentine stem weevil (Listronotus bonariensis) which is a major pest of pastures, but it has no adverse effects on livestock (Rowan Citation1993; Pownall et al. Citation1995). In contrast, lolitrem B is the cause of ryegrass staggers and has some minor effects on Argentine stem weevil (Fletcher and Harvey Citation1981; Fletcher Citation1982; di Menna et al. Citation2012). Ergovaline causes heat stress (Klotz Citation2015), as well as giving ryegrass protection against black beetle (Heteronychus arator) (Ball et al. Citation1997; Popay and Hume Citation2011). Due to the beneficial effects of endophytes, the cultivation of endophyte-free ryegrass for permanent pastures is not a cost-effective option under most farming conditions in Australia and New Zealand due to poor productivity and persistence of the ryegrass component (Hume and Sewell Citation2014; Thom et al. Citation2014). To reduce the impact of endophytes on livestock while maintaining their positive effect of pasture protection against insects, endophyte strains have been selected that express no or reduced concentrations of the metabolites detrimental to animals while maintaining expression of the agriculturally-favourable metabolites. The transfer of these selected endophytes into modern, improved ryegrass cultivars has resulted in a number of commercial novel endophytic ryegrass cultivars (Johnson et al. Citation2013).
These commercial endophytic ryegrass products express a range of secondary metabolites which determine their susceptibility or resistance to insect predation and harmful effects on livestock. The AR1 endophyte strain, which produces peramine but not lolitrem B or ergovaline in ryegrass, was the first selected novel ryegrass endophyte to be widely released and adopted. AR1-infected ryegrasses cause no adverse effects on livestock, with liveweight gains and milk production equivalent to grazing endophyte-free ryegrasses (Fletcher Citation1999; Bluett et al. Citation2005). AR1 provides ryegrass with good protection against the insect pests Argentine stem weevil and pasture mealybug (Balanococcus poae), however it has little or no effect on a number of other serious pests (Popay and Hume Citation2011). Cultivars infected with a mixture of the NEA2 and NEA6 endophytes are sold under the NEA2 brand and generally express low or moderate concentrations of peramine, ergovaline and lolitrem B but this may vary depending on the host grass germplasm and the ratio of endophytes in a particular cultivar (van Zijll de Jong et al. Citation2008; Popay and Hume Citation2011). In one study, however, it was shown that ryegrasses infected with NEA-type endophytes had similar or higher concentrations of ergovaline than standard endophyte-infected ryegrass (Logan et al. Citation2015). NEA-type endophytes provide infected cultivars with good protection from insects such as black beetle but varying protection against other insects which can be affected by the cultivar (Popay and Hume Citation2011; Stewart et al. Citation2014). In contrast, the AR37 endophyte expresses none of the metabolites responsible for pest protection associated with AR1, standard and NEA endophytes. AR37, does however, show protection against a wide spectrum of insects including black beetle adults, Argentine stem weevil larvae, pasture mealybug, root aphid (Aploneura lentisci) and porina larvae (Wiseana spp.) (Popay and Hume Citation2011; Stewart et al. Citation2014). It is the only ryegrass-derived endophyte which is known to provide ryegrass with resistance against porina larvae, a major pest in New Zealand. AR37 also does not express lolitrem B but it does produce a group of structurally-related indole-diterpenes called the epoxy-janthitrems (Finch et al. Citation2012, Citation2013) which are thought to be responsible for the ryegrass staggers observed in animals grazing AR37-infected ryegrasses. Comparisons of alkaloid concentrations, and the severity of ryegrass staggers induced in sheep, show the epoxy-janthitrems to be tremorgenic, but at least 10 times less potent than lolitrem B produced by standard endophytes (Finch et al. Citation2012). Furthermore, ryegrass staggers in sheep grazing AR37-infected ryegrass occurs on average at a reduced frequency, severity and duration than in animals grazing ryegrass infected with standard endophyte (Fletcher and Sutherland Citation2009). Ryegrass staggers has not been recorded in dairy cows grazing pastures infected with AR37 endophyte (Thom et al. Citation2007).
The objectives of this study was to compare the severity of ryegrass staggers and response to heat stress in sheep grazing ryegrass infected with commercially available endophyte strains, and to compare liveweight gains during the study period. As adverse effects on animals were the focus of the study, the endophyte strains chosen were standard, AR37 and NEA2 along with appropriate endophyte-free, non-toxic controls.
Materials and methods
Pasture establishment and maintenance
Five ryegrass-endophyte combinations were used in this study: cultivar Trojan infected with NEA endophytes (T-NEA2); endophyte-free cultivar Trojan (T-NIL), generated by treating T-NEA2 seed at 46°C and 45% relative humidity for 4 weeks (Bouton et al. Citation1993); cultivar Grasslands Samson infected with standard endophyte (S-STD); cultivar Grasslands Samson infected with AR37 endophyte (S-AR37); and endophyte-free cultivar Grasslands Samson (S-NIL). All seed was sourced from PGG Wrightson Seeds Limited (Christchurch, NZ). Trojan is a diploid, late-heading ryegrass classified as a long-term hybrid ryegrass, with production and persistence similar to cultivars of perennial ryegrass, while Samson is a diploid, mid-season heading perennial ryegrass (Stewart et al. Citation2014). These ryegrass-endophyte cultivars were established in paddocks (50 m×35 m) in a randomised block design by sowing the ryegrass seed at 18–20 kg/ha in early spring, 28–30 September 2011, into a cultivated seed bed using a coulter seed drill. Three replicates were sown for each ryegrass-endophyte cultivar.
Paddocks were located on the AgResearch Lincoln farm (Canterbury, NZ) where the soil type is a Wakanui silt loam of low to moderate fertility (pH 6.0; Olsen P 17 µg/mL; Mg 62 µg/g; K 98 µg/g; Na 56 µg/g). The paddocks were fertilised, irrigated and managed to provide a cover of 3,000–3,500 kg DM/ha at the start of the experiment. The paddocks were maintained as pure ryegrass.
Weather
Data for minimum and maximum daily temperatures and daily rainfall were collected from the Broadfield weather station, Lincoln (NIWA H32645), located 500 m from the trial site. Historical weather data (1981–2010) were obtained from the NIWA website (https://cliflo.niwa.co.nz/). Using the 10-minute readings from this weather station data, the temperature humidity index (THI) values were calculated using the equation:where T is daily maximum temperature and RH is mean daily percent relative humidity divided by 100 (Bryant et al. Citation2007).
Herbage measurements
In late January 2012, 50 ryegrass tillers were cut at ground level from each paddock (except S-NIL) and percentage endophyte infection determined using a tissue-print immunoblot procedure (Simpson et al. Citation2012). On 16 January 2013, approximately 100 tillers from T-NEA2 were immunoblotted and the endophyte-infected tillers DNA typed by a simple sequence repeat assay for endophyte strain (Card et al. Citation2014).
Between 26 January and 29 March 2012, and on 20 February and 15 March 2013, herbage was cut to ground level from 10 different positions within each paddock, combined to comprise a single sample representative of each paddock, frozen within 1 hour of cutting, freeze-dried and milled (1 mm sieve size). Samples were kept frozen prior to analysis of endophyte alkaloids and pasture quality as described below.
In 2012, due to a low prevalence of ryegrass staggers in sheep grazing endophyte-infected paddocks, only samples of T-NEA2 collected from 19 days before to 44 days after the start of the trial were analysed for ergovaline, lolitrem B and peramine, and samples of S-AR37 were analysed for epoxy-janthitrems, as shown in . To test for possible endophyte contamination in endophyte-free plots, selected samples of T-NIL and S-NIL, as well as S-AR37, were analysed for alkaloids in March 2012, as shown in .
Table 1. Mean (±SEM) concentrations of endophyte alkaloids (mg/kg) in ryegrass herbage measured in 2012 in paddocks sown with different cultivar-endophyte combinations (n=3 per cultivar), in which lambs were grazed from 14 February to 2 April 2012.
In 2013, a greater prevalence of ryegrass staggers was observed in sheep and samples of T-NEA2 and S-STD collected 19 and 42 days after the start of the trial were analysed for ergovaline, lolitrem B and peramine, and samples of S-AR37 were analysed for epoxy-janthitrems, as shown in .
Table 2. Mean (±SEM) concentrations of endophyte alkaloids (mg/kg) in ryegrass herbage measured in 2013 in paddocks sown with different cultivar-endophyte combinations (n=3 per cultivar), in which lambs were grazed from 1 February to 21 March 2013.
Pasture quality was assessed in both years using a sub-sample of that taken for alkaloid analysis. Pasture quality, as determined by metabolisable energy (ME), concentrations of ash, crude protein, neutral detergent fibre, lipid, and water soluble carbohydrates, was estimated using near infrared reflectance spectroscopy (NIRS) at FeedTECH (AgResearch, Palmerston North, NZ) (Corson et al. Citation1999). An MPA near infrared reflectance spectrophotometer (Bruker, Ettlingen, Germany) was used to scan the samples and the resulting NIRS spectra were analysed using Optic user software (OPUS) version 7.0. The NIRS calibrations are based on animal trials and analyses of a range of pasture standards, and were developed using NIRS after scanning in the range of 400–2500 nm.
Endophyte alkaloid analysis
Prior to the analysis of peramine and ergovaline, ground herbage samples were additionally ground with a bead ruptor (FastPrep FP120; Savant Instruments Inc., Farmingdale, NY, USA) with three 3 mm stainless steel beads in a 2 mL polypropylene vial (Sarstedt, Nümbrecht, Germany) for 10 seconds at 5 m/second to increase extraction efficiency.
Peramine was extracted from herbage with 1 mL of 50% (v/v) methanol, with 1.70 ng/mL homoperamine nitrate (BDG Synthesis, Lower Hutt, NZ) as internal standard, using an over-over mixer at 30 rpm for 1 hour. The sample was then centrifuged for 5 minutes at 4000 g (Heraus Megafuge 16; Thermo Scientific, Osterode, Germany), and a 500 µL aliquot of the supernatant transferred to an amber 12×32 mm high performance liquid chromatography vial (Thermo Scientific, Waltham, MA, USA) via a 0.45 µm polyvinyldifluoride syringe filter (Membrane Solutions, Kent, WA, USA). Separation was achieved on a Synergi Polar-RP 100×2.00 mm (2.5 µm) column (Phenomenex, Torrance, CA, USA) using a linear gradient profile (Eluent A=aqueous 0.1% formic acid, eluent B=acetonitrile; T0=5% B, T9=40% B, T11=90% B, T13=90% B, followed by equilibration to initial conditions over the following 8 minutes). Peramine was quantified by mass spectroscopy according to parameters previously described (Moore et al. Citation2015). A 5 µL injection volume gave a limit of quantitation for this technique of 0.1 mg/kg for herbage.
Lolitrem B was analysed using previous methods (Moore et al. Citation2015). Quantification was achieved by comparison with a pure standard (Miles et al. Citation1994). The limit of quantitation for this technique is 0.1 mg/kg.
Ergovaline was analysed using previous methods (Moore et al. Citation2015). The limit of quantitation of this technique was 0.1 mg/kg.
Epoxy-janthitrems were extracted from ground herbage (20 mg) with 1 mL of the extraction solvent (80% acetone) using an over-over mixer at 30 rpm for 1 hour. The extract was then centrifuged for 5 minutes at 5,600 g (Centrifuge 5418, Eppendorf, Hamburg, Germany) and analysed by high performance liquid chromatography. Epoxy-janthitrems were quantified by comparison with a reference standard of 5 µg/mL N-benzyl-1,8-naphthaleneimide (AgResearch, Palmerston North, NZ) which had previously been compared with a pure epoxy-janthitrem I standard. Due to the instability of epoxy-janthitrems the use of an epoxy-janthitrem standard is not practical for routine analysis and samples were protected from light during extraction and analysis. For analysis of herbage extracts a 4.6×250 mm ODS C18 column (Phenomenex) fitted with a 4×3 mm SecurityGuard containing two C18 cartridges (Phenomenex) was used with an eluent of 95% acetonitrile/5% water (1 mL/min). Eluting compounds were detected with an Agilent Series 1100 RF-10A XL fluorescence detector (Shimadzu, Kyoto, Japan), excitation at 333 nm, emission detection at 385 nm. The limit of quantification of this technique was 0.1 mg/kg.
Animals
Approval for all procedures involving the experimental use of animals was granted by the Invermay AgResearch Animal Ethics committee (Dunedin, NZ) prior to commencement of the study (AE12406 and AE12793). In 2012 and 2013, 150 Coopworth ewe lambs aged approximately 6-months-old were obtained with a mean live weight of 30.3 (SEM 2.2) kg in 2012 and 30.4 (SEM 3.1) kg in 2013. In each year, for 2 months prior to the trial all lambs were grazed on non-toxic ryegrass pastures (AR1 or nil endophyte). Immediately prior to the trial, all lambs were administered a combination anthelmintic (80 g/L levamisole hydrochloride and 45.3 g/L oxfendazole; Scanda, Coopers, Wellington, NZ) at 1 mL/10 kg live weight. Lambs were randomly allocated to cultivars and replicates. The paddocks were stocked at 10 lambs per paddock, equivalent to 60 lambs/ha, so that there were 30 per cultivar, but stocking adjustments were made to maintain a similar quantity of herbage on offer across all paddocks as grazing progressed. In 2012, the trial commenced on 14 February (Day 0) and was terminated on 2 April (Day 48). In 2013, the trial commenced on 1 February (Day 0) and was terminated on 21 March (Day 48). The delay in start date in 2012 was due to wet and cold weather in early February not being conducive to the expression of alkaloids.
In both years each lamb was weighed unfasted when entering the trial, after 2 weeks grazing, and at the end of the trial period (48 days) to allow weight gain comparisons to be made between treatment groups.
Individual animals were scored for ryegrass staggers on two occasions in 2012 and four occasions in 2013 using the Keogh scale (0=no clinical signs, 5=severe muscle tremors invariably resulting in staggering and collapse; Keogh Citation1973), beginning when the first signs of staggers became evident (12 March 2012 and 21 February 2013). Individual mobs were moved from their paddocks into a central raceway where they were forced to run 300 m. Those that did not fall (score of <4) were penned at the end of the run and each animal individually scored. They were then moved back into their paddocks and the process repeated for the next mob. Animals scoring ≥4 were removed from the trial to comply with welfare obligations and these animals were replaced with non-trial animals to maintain a similar grazing pressure across all paddocks. Lambs grazing S-NIL were not assessed for staggers in 2013. Lambs were also observed regularly for signs of other known endophyte toxicities such as fescue foot and increased dags and fly-strike.
On a single occasion in each year, 9 March 2012 (Day 23) and 27 February 2013 (Day 26), lambs grazing the T-NEA2, T-NIL and S-STD pastures were moved to a modified tunnel house to assess response to heat stress. S-STD and T-NEA2 pastures were the only ones in the study which expressed ergovaline and so were capable of inducing signs of heat stress. AR37 contains no ergovaline so cannot induce heat stress, this has been confirmed in previous work. T-Nil was included as a non-ergovaline control. Animals were left in the tunnel house while ambient temperature increased to between 35–40°C with a relative humidity >75%. After 4 hours, rectal temperatures and respiration rates were determined for all animals in a randomised order. Rectal temperatures were measured using digital clinical thermometers (Becton Dickinson, Sparks, MD, USA) with a range 32–42°C and resolution 0.05°C, and respirations were measured by counting regular thoracic or flank movements over a 30 second period. On completion, lambs were drafted into their individual mobs and returned to the appropriate paddocks.
Statistical analysis
Metabolisable energy and concentrations of ash, crude protein, lipid and neutral detergent fibre in pasture samples were statistically analysed. Concentrations of water soluble carbohydrates were not analysed as herbage samples were not frozen immediately after harvest so were deemed to be not suitably accurate. For example, mean concentration of water soluble carbohydrate for all measurements reported from the NIRS determination was 12.9%. ANOVA was used to compare the five pasture quality parameters in 2012 between the cultivars T-NEA2 and T-NIL and between measurement days, and included the two factors cultivar and measurement day, as well as their interaction. The same ANOVA was used in 2013 to compare the three cultivars T-NEA2, S-STD and S-AR37 measured on Days 19 and 42. In 2012, pasture quality parameters measured on 29 March (Day 44) were also compared between all five cultivars using a separate ANOVA, which included only cultivar as a factor.
Concentrations of alkaloids in ryegrass herbage were compared between the cultivars (n=3 paddocks for each cultivar) on each sampling date, and between sampling dates for each cultivar using ANOVA. Because not all cultivars were sampled on all sampling dates, the ANOVA consisted of a single factor, which was defined by combinations of sampling dates and cultivars. Only results for cultivars that had non-zero mean concentrations were included in these ANOVA. A one-sample t test was then used to test the null hypothesis that the non-zero mean concentrations were equal to zero.
Liveweight gains in each year were compared between sheep grazing different cultivars (n=3 paddocks per cultivar with 10 lambs per paddock) using ANOVA. Liveweight gains were calculated by subtracting each animal’s weight at the start of the trial from their weight at the end of the trial, then dividing the difference by the number of days of the trial.
Ryegrass staggers scores of the sheep grazing different cultivars were compared on each measurement day using ordinal logistic regression analysis. This analysis was used to compare the ordered categorical response variable of the ryegrass staggers score between cultivars, based on the numbers of animals categorised by the score value. The number of score categories in each regression analysis differed between measuring days because score categories needed to be aggregated on most measuring days so that each category had at least one animal per cultivar.
Respiration rate and rectal temperature measured in the tunnel house were compared between sheep grazing different cultivars in each year using ANOVA (n=3 paddocks per cultivar with 10 lambs per paddock). All statistical analyses were carried out using the Minitab statistical software, version 16 (Minitab Inc., State College, PA, USA).
Results
Weather
Weather conditions in 2012 were moist and cool relative to those in 2013. The total rainfall for the months of January, February and March, when no irrigation was applied, was 139 mm in 2012, which was similar to the long-term mean of 132 mm, while rainfall in 2013 was 93 mm; 30% below the long-term mean. Mean daily air temperatures in 2012 in the 3 months leading up to the study, and for the months of the study, were cool, averaging 14.6 (min 9.2, max 22.0)°C compared with 15.4°C for the long-term mean. In comparison, mean daily air temperatures were warmer in 2013, averaging 15.6 (min 7.3, max 23.1)°C. The mean daily THI during the grazing period for 2012 was 54.5 (min 44, max 67) which was lower than the mean for 2013 of 58.5 (min 44, max 68). The THI was >60 for 8 days in 2012 and for 18 days in 2013.
Endophyte presence and type
Immunoblot analysis of ryegrass tillers to determine endophyte presence in 2012 showed that ryegrass-endophyte cultivars were infected with endophyte at the following rates: T-NEA2 120/150 (80%), T-NIL 6/150 (4%), S-STD 135/150 (90%), and S-AR37 108/150 (72%). Immunoblot analysis of tillers sampled in 2013 from only T-NEA2 pastures revealed that 93/104 (89%) were infected. The infected tillers (n=93) from this cultivar were further tested for endophyte strain using DNA typing. This showed that 53/93 (57%) tillers were infected with the NEA2 strain, 19/93 (20%) the NEA6 strain, and 10/93 (20%) a previously unidentified strain which is referred to in this paper as the NEA variant strain. Also present in T-NEA2 pastures were single AR1 (1/93; 1%) and AR37 (1/93, 1%) infected tillers.
Pasture quality
In 2012, T-NEA2 and T-NIL pasture samples did not differ (p>0.05) on any occasion when analysed over the four measurement dates for the quality parameters reported (data not shown). When only Day 44 was analysed, T-NEA2 and T-NIL differed (p=0.008) in concentrations of lipid (1.8 (SEM 0.1) and 1.5 (SEM 0.1) % of DM, respectively). The combined data for these two cultivars for each of the four different dates showed that over the 2 months of measurement pasture quality declined, for example, ME declined from 10.8 (SEM 0.1) MJ ME/kg DM on Day 0 to 9.0 (SEM 0.1) MJ ME/kg DM on Day 44 (p<0.001). There were no interactions of measurement day with cultivar for any of the parameters (p≥0.218). For samples of all cultivars measured on Day 44, ash (p=0.001) and lipid (p<0.001) concentrations were lowest in S-STD and highest in S-NIL, while concentrations of crude protein, neutral detergent fibre and ME for all cultivars were similar (p≥0.114).
In general pasture quality in 2013 was lower than in 2012, e.g. at the end of the trial the mean ME for all cultivars was 9.4 (SEM 0.1) MJ ME/kg DM in 2012 and 8.4 (SEM 0.1) MJ ME/kg DM in 2013. Pasture quality declined in 2013 as the trial progressed, e.g. mean ME was 8.8 (SEM 0.1) MJ ME/kg DM on Day 19 and 8.4 (SEM 0.1) MJ ME/kg DM on Day 42 (p=0.012). S-STD pastures had the highest concentrations of crude protein (p=0.039) and lipid (p=0.002), while T-NEA2 and T-NIL were similar for these parameters (data not shown). Pastures did not differ (p≥0.05) for concentrations of ash, neutral detergent fibre and ME and there were no interactions of pasture cultivars with day of sampling for any parameters (p≥0.112).
Alkaloids in ryegrass herbage
None of the T-NIL pasture samples collected in 2012 had detectable concentrations of ergovaline, lolitrem B or peramine, and S-NIL pasture samples had no detectable concentrations of ergovaline, lolitrem B, peramine or epoxy-janthitrems (). Furthermore, S-AR37 pasture samples had no detectable concentrations of ergovaline, lolitrem B or peramine ().
In T-NEA2 pastures in 2012, mean concentrations of ergovaline and lolitrem B increased over the experimental period (). Mean concentrations of peramine in T-NEA2 had a large range, and while varying between consecutive dates, showed no consistent upward trend over the period of measurement. Mean epoxy-janthitrem concentrations in S-AR37 were found to vary between dates ().
Mean alkaloid concentrations in 2013 were similar to or greater than in 2012 for T-NEA2 and S-AR37 (). For ergovaline, mean concentrations were numerically higher in March than February for T-NEA2 and S-STD pastures (p=0.080 and p=0.098, respectively), and concentrations were higher in T-NEA2 than S-STD pastures in February and in March (p=0.153 and p=0.226, respectively). For lolitrem B there was a month by cultivar interaction (p=0.009); mean concentrations were higher in March than February for S-STD (p<0.001) but not for T-NEA2 (p=0.563) pastures. Mean concentrations of lolitrem B were higher in S-STD than T-NEA2 pastures in both February and March (both p<0.001). There was also a month by cultivar interaction for peramine (p=0.034); mean concentrations were lower in March than February for S-STD (p=0.007) and similar in both months for T-NEA2 (p=0.805). In both months, mean concentrations of peramine were considerably higher in S-STD than T-NEA2 pastures (both p<0.001). Mean concentrations of epoxy-janthitrem in S-AR37 were nearly twice as high in March than February (p=0.022).
Live weight and mean daily weight gains
At the start of the experiment (Day 0) in 2012 mean liveweight (30.2, min 29.9, max 31.1 kg) was similar for lambs grazing all cultivars (p=0.292). Mean daily weight gain over the 48-day trial period (136 (SEM 4); min 126, max 149 g/day) did not differ between cultivars (p>0.28; a).
Figure 1. Mean (±SEM) liveweight gain (g/day) of lambs grazing pastures sown with different cultivar-endophyte combinations (n=30 per cultivar) for 48 days in February and March in (a) 2012 (Year 1) and (b) 2013 (Year 2). The cultivars were endophyte-free Trojan ryegrass (grey bars), Trojan ryegrass infected with NEA endophytes (black bars), endophyte-free Samson ryegrass (coarsely striped bars), Samson ryegrass infected with standard endophyte (S-STD, white bars) and Samson ryegrass infected with AR37 endophyte (finely striped bars). Note that in 2013 liveweight gain was not determined for the S-STD group because all animals were removed before Day 48 due to severe staggers.
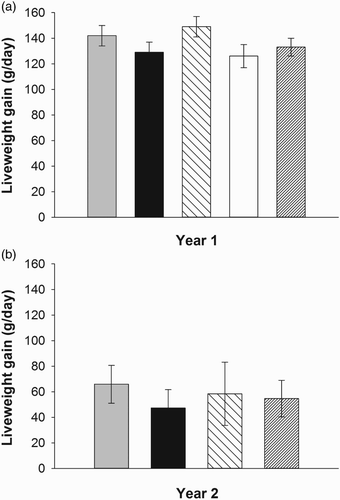
In 2013, mean liveweight on Day 0 (30.4, min 29.6 max 31.1 kg) was similar between lambs grazing all cultivars (p=0.545). It was not possible to determine liveweight gains for lambs on S-STD pastures due to the early onset of severe staggers and subsequent removal of animals from toxic pasture, however mean daily liveweight gain was poor for lambs grazing all cultivars (79 (SEM 9); min 47, max 66 g/d) with no differences among cultivars (p=0.842; b).
Ryegrass staggers and general health
In 2012, no ryegrass staggers was observed in lambs grazing S-NIL. For lambs grazing T-NIL the highest score was 1 (low staggers), which was observed only on 12 March (Day 27) in 3/10 lambs grazing one paddock.
Ryegrass staggers was recorded in 2012 in lambs grazing T-NEA2, S-STD and S-AR37 (). In the T-NEA2 and S-AR37 groups there were no sheep with severe staggers (score ≥4), while in the S-STD group, severe staggers was observed for 5/30 (17%) on Day 27 and by Day 48 a cumulative total of 7/30 (23%) lambs. When analysed over the full range of staggers scores, lambs grazing T-NEA2 and S-AR37 had a similar distribution of scores on Days 27 and 48 (), whereas the distribution differed between lambs grazing S-STD and T-NEA2 on both Days 27 and 48 (p≤0.002). For lambs grazing S-AR37, the distribution of scores differed compared with S-STD only on Day 27 (p=0.001; ).
Table 3. Number of lambs categorised by ryegrass staggers score (Keogh Citation1973) on two occasions in 2012, that were grazing different cultivar-endophyte combinations between 14 February and 2 April 2012.
In 2013, no ryegrass staggers was observed in lambs grazing the T-NIL pastures, but was observed in lambs grazing T-NEA2, S-STD and S-AR37 (). In the T-NEA2 and S-AR37 groups severe staggers was observed from Day 38 onwards, whereas in the S-STD group severe staggers was first observed on Day 20. Due to the high prevalence of severe staggers on Day 32 (22/30; 73%) all lambs grazing S-STD were removed from the trial after the staggers assessment had been completed. During the 48-day trial period, >50% of lambs showed signs of severe staggers in the S-STD and S-AR37 groups. When analysed over the full range of staggers scores, the distribution of scores for lambs grazing S-STD differed compared with lambs grazing T-NEA2 or S-AR37 on Days 20 and 32 (p<0.001). The distribution of scores were similar for the T-NEA2 and S-AR37 groups on Days 20 and 38 but differed on Days 32 and 47 ().
Table 4. Number of lambs categorised by ryegrass staggers score (Keogh Citation1973) on four occasions in 2013, that were grazing different cultivar-endophyte combinations between 1 February and 21 March 2013.
No signs of other known endophyte toxicities, such as fescue foot and increased dags and fly-strike, were observed in either year.
Rectal temperatures and respiration
In 2012, when subject to heat stress in the tunnel house, lambs grazing S-STD had similar mean rectal temperatures to those grazing T-NEA2 (p=0.102), but higher mean rectal temperatures than T-NIL (p=0.025; a). Mean rectal temperatures were similar for lambs grazing T-NEA2 and T-NIL (p=0.528). In 2013, mean rectal temperatures of lambs grazing T-NIL were lower than for lambs grazing either S-STD (p=0.033) or T-NEA2 (p=0.008). Temperatures were similar for lambs grazing S-STD and T-NEA2 (p=0.715).
Figure 2. Mean (±SEM) (a) rectal temperature (°C) and (b) respiration rate (breaths/minute) of lambs when subject to heat stress after grazing pastures sown with different cultivar-endophyte combinations (n=30 per cultivar) for 24 days in 2012 (Year 1) and 26 days in 2013 (Year 2). The cultivars were endophyte-free Trojan ryegrass (grey bars), Trojan ryegrass infected with NEA endophytes (black bars) and Samson ryegrass infected with standard endophyte (white bars).
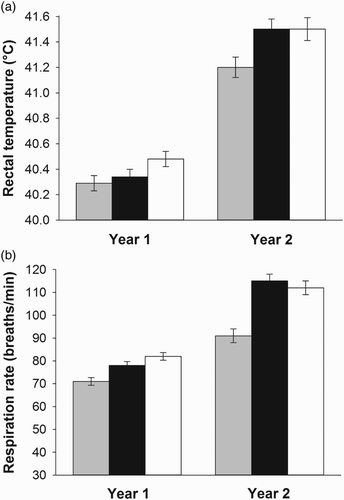
Mean respiration rates in 2012, when subject to heat stress, were lower for lambs grazing T-NIL than those grazing S-STD (p<0.001) or T-NEA2 (p = 0.009; b). Lambs grazing S-STD and T-NEA2 had similar mean respiration rates (p=0.079). Similarly, in 2013, mean respiration rates for lambs grazing T-NIL were lower than lambs grazing S-STD (p<0.001) or T-NEA2 (p<0.001), while mean respiration rates were similar for lambs grazing S-STD and T-NEA2 (p=0.519).
Discussion
This study has for the first time compared the severity of ryegrass staggers in sheep grazing T-NEA2 with that observed in sheep grazing other commercially-available cultivars with appropriate endophyte-free controls. Rectal temperatures and respiration rates were also compared in sheep subject to heat stress that were grazing T-NEA2, S-STD and T-NIL pastures. The grazing of the five cultivar pastures in one experiment is informative as the comparison of performance across multiple experiments is difficult due to different environmental conditions, pasture management and experimental protocols.
This study has shown that the severity of ryegrass staggers and response to heat stress (body temperature and respiration) differed in animals grazing the different endophyte-ryegrass cultivar combinations. Animals grazing any of the endophyte-infected cultivars had similar liveweight gains when compared to animals grazing endophyte-free controls. Effects differed between years, being greater in 2013 which was drier than normal for the Lincoln site. Alkaloid analysis of the cultivars used in the study showed them all to be of high purity and contain high frequencies of the appropriate endophytes. Immunoblotting and alkaloid analysis showed the endophyte-free controls to contain minimal contamination.
The occurrence of ryegrass staggers in lambs grazing S-STD pastures, and absence or very low prevalence of staggers in lambs grazing endophyte-free cultivars, is analogous to that reported on numerous occasions at this location using similar trial protocols (Fletcher Citation1999; Fletcher and Sutherland Citation2009). The threshold concentration of lolitrem B required to induce ryegrass staggers is accepted to be 1.8–2.5 mg/kg in herbage (di Menna et al. Citation2012). In 2013, concentrations of lolitrem B concentrations exceeded this threshold in S-STD pastures (4.1 mg/kg) and severe staggers was observed.
Mean concentrations of lolitrem B were lower in T-NEA2 pastures in 2012 in comparison with 2013, as expected in a year with cooler temperatures and higher rainfall. However, given the concentrations of lolitrem B in T-NEA2 pastures were up to 1.1 mg/kg, which is well below the accepted threshold concentration for the generation of ryegrass staggers, the severity of staggers observed in lambs grazing this cultivar was surprising. This result highlights the difficulty in predicting the toxicity of pastures based solely on alkaloid concentrations (Easton et al. Citation1996; Nicol and Klotz Citation2015) and may also suggest that other unidentified endophyte tremorgens may have been present in herbage. Ryegrass staggers when grazing NEA2-infected ryegrass has previously been reported in elk (Cervus canadensis), a deer species particularly susceptible to ryegrass staggers (Stewart et al. Citation2014). Furthermore, for Trojan and Rohan cultivars, Logan et al. (Citation2015) reported ryegrass staggers in sheep grazing some NEA-infected ryegrass combinations, the severity of which was in part driven by which endophyte strain or strains were present. The presence of different NEA-type endophyte strains (NEA2, NEA3, NEA6 and NEA variant) in different products makes the prediction of ryegrass staggers complicated. Of these strains only the NEA2 strain (also referred to as NEA2 A) produces lolitrem B (van Zijll de Jong et al. Citation2008). In the current study, tillers of T-NEA2 were infected with a mixture of the NEA2, NEA6 and NEA variant strains, which in combination produced low concentrations of lolitrem B which induced ryegrass staggers in grazing sheep. The severity of ryegrass staggers which could be anticipated from grazing NEA-type endophytic ryegrass products will depend on the proportion of the NEA2 strain present. It is also possible that the ratios of the different NEA strains may change over time in the field which would influence the concentrations of lolitrem B produced and the severity of ryegrass staggers observed. Currently, industry tables rate ryegrasses infected with the NEA2-branded endophytes as being equivalent to endophyte-free ryegrass with respect to generating ryegrass staggers (Stewart et al. Citation2014), which is not consistent with the results of this study or with those of Logan et al. (Citation2015).
Ryegrass infected with AR37 can cause ryegrass staggers in lambs, usually less severe and less frequent than for standard endophyte-infected ryegrass. This was recorded for both years of this study, confirming previous research with sheep grazing AR37-infected ryegrass at this location (Fletcher and Sutherland Citation2009). AR37-infected ryegrasses do not produce the mammalian toxins ergovaline and lolitrem B and it is the presence of epoxy-janthitrems which is thought to be the cause of ryegrass staggers for AR37-ryegrass associations (Finch et al. Citation2012). The production of high concentrations of epoxy-janthitrems by AR37-infected ryegrass compared with low concentrations of lolitrem B by standard endophyte-infected ryegrass, and the lower severity of ryegrass staggers on AR37-infected ryegrass pastures, indicates that the epoxy-janthitrems are low potency tremorgens compared with lolitrem B, as described by Finch et al. (Citation2012).
Ingestion of ergovaline, and ergot alkaloids in general, are known to exacerbate heat stress in livestock due to constricting blood flow and therefore reducing the ability of the animal to dissipate heat through the skin (Rhodes et al. Citation1991). Fletcher et al. (Citation1999) reported that differences in animals’ ability to handle heat stress could be detected without the use of the tunnel house for sheep grazing standard endophyte ryegrass at Lincoln when measured on hot days in the field in summer and autumn. However, the tunnel house was used in this experiment to expose lambs to a consistent heat stress and to ensure the thermoregulatory system of the animals was challenged. Lambs grazing S-AR37 were not included in the testing as this cultivar did not contain any detectable ergovaline, AR37 does not produce ergot alkaloids, and previous research has shown that heat stress does not occur in lambs grazing AR37-infected ryegrass (Fletcher and Sutherland Citation2009).
When exposed to heat stress, lambs grazing pastures of S-STD and T-NEA2 had higher body temperatures and respiration rates than lambs grazing T-NIL pastures. Lambs grazing S-STD and T-NEA2 had similar temperature and respiration rates, which is consistent with both cultivars producing similar concentrations of ergovaline. Greater differences were measured between lambs grazing S-STD and T-NEA2 and T-NIL in 2013 compared to 2012. This greater effect in 2013 can be attributed to both higher concentrations of ergovaline in pastures and warmer, more humid ambient conditions (higher THI). On the basis of the results of the current study, we conclude that T-NEA2 has a similar potential to cause heat stress as ryegrasses infected with standard endophyte. Consistent with these observations, concentrations of ergovaline in Trojan herbage harvested from a field site near the Lincoln location were similar to, or greater than, concentrations in the cultivar Arrow infected with standard endophyte (Logan et al. Citation2015).
Concentrations of ergovaline reported in New Zealand pastures vary between studies, with our results being similar to those previously reported at Lincoln for standard endophyte infected ryegrass (Logan et al. Citation2015). For ryegrass samples taken throughout the North Island, along with samples from Canterbury, Easton et al. (Citation1996) found no major regional differences, with concentrations of ergovaline in summer and autumn being 0.5–1.0 mg/kg, with mean maximum values of 1.5 mg/kg. Those authors concluded that while ergovaline concentrations in ryegrass were sufficient to cause heat stress in livestock, the occurrence of clinical heat stress would be dependent on the length of exposure to elevated concentrations of ergovaline, along with the ambient air temperature and relative humidity, as these climatic factors increase the heat stress on animals. Due to the increased solar radiation and temperatures experienced in Australia, animals there are likely to experience more severe heat stress which will yield greater detrimental effects of ergovaline-expressing endophytes (Reed et al. Citation2016).
In addition to heat stress, the ergovaline produced by standard endophyte has been seen as contributing towards the “summer/autumn ill thrift” syndrome in sheep in New Zealand (Fletcher et al. Citation1999), and it has been suggested that the subclinical impact of ergot-alkaloids on livestock production in Australia is likely to be greater than that in New Zealand due to environmental conditions (Reed et al. Citation2016). Easton et al. (Citation1996) concluded that in New Zealand, if ambient temperatures are suitable, ergovaline in standard endophyte-infected pastures can reach concentrations sufficient to cause fescue toxicoses. Therefore, on the basis of ergovaline concentrations, it is likely that this is also the case for T-NEA2. Unlike Nicol and Klotz (Citation2016) we believe that many farmers do not appreciate the potential for ergovaline to induce animal production problems. However, currently, the impact of ergovaline in New Zealand grazing systems is not well understood and work should be undertaken to better clarify what concentrations are associated with detrimental animal performance.
Fescue foot (lameness, necrosis of hooves, tails or ear tips) is also associated with ingestion of ergot alkaloids due to constriction of blood flow to the extremities (Waller Citation2009). However, this is typically observed during the winter and is associated with low ambient temperatures (<10°C). It is therefore not surprising that fescue foot was not observed in the current study.
In conclusion, this 2-year study has shown ryegrass staggers to be induced in sheep grazing S-STD, S-AR37 and T-NEA2 pastures. S-STD induced the highest severity of ryegrass staggers and had the highest concentrations of lolitrem B. S-AR37 induced a much lower severity of ryegrass staggers, despite no lolitrem B but high epoxy-janthitrem concentrations being expressed, which is consistent with the epoxy-janthitrems being low potency tremorgens. T-NEA2 showed the lowest severity of ryegrass staggers and had low concentrations of lolitrem B. It may be anticipated that ryegrass staggers would be worse under these trial conditions, in comparison to normal farming practice, due to increased grazing intensities and the use of pure swards of ryegrass. However the expression of lolitrem B and the observation of ryegrass staggers in sheep grazing T-NEA2 in this experiment, along with the observations of staggers mentioned in other studies, means that the industry tables should be revised that show NEA2-branded endophytes to have an equivalent ryegrass staggers safety rating as endophyte-free ryegrass. This study also showed that the concentrations of ergovaline expressed in S-STD and T-NEA2 cultivars were very similar and that both were associated with increased body temperature and respiration rates on exposure to heat stress in the tunnel house.
| NIRS | = | Near infrared reflectance spectroscopy |
| ME | = | Metabolisable energy |
| S-AR37 | = | Samson ryegrass infected with AR37 endophyte |
| S-NIL | = | Endophyte-free Samson ryegrass |
| S-STD | = | Samson ryegrass infected with standard endophyte |
| THI | = | Temperature humidity index |
| T-NEA2 | = | Trojan ryegrass infected with NEA endophytes |
| T-NIL | = | Endophyte-free Trojan ryegrass |
Acknowledgements
We thank Allan Hawkes, Xinqi Liu, Navjot Kaur and Tomoko Maruyama of AgResearch for alkaloid analyses and Marty Faville of AgResearch for DNA typing.
ORCID
SC Finch http://orcid.org/0000-0003-2765-5843
G deNicolo http://orcid.org/0000-0002-5021-0386
WJ Mace http://orcid.org/0000-0002-3529-7700
C van Koten http://orcid.org/0000-0002-0630-6807
Additional information
Funding
References
- Ball OJ-P, Miles CO, Prestidge RA. Ergopeptine alkaloids and Neotyphodium lolii-mediated resistance in perennial ryegrass against adult Heteronychus arator (Coleoptera: Scarabaeidae). Journal of Economic Entomology 90, 1382–91, 1997 doi: 10.1093/jee/90.5.1382
- Bluett SJ, Thom ER, Clark DA, Macdonald KA, Minneé EMK. Effects of perennial ryegrass infected with either AR1 or wild endophyte on dairy production in the Waikato. New Zealand Journal of Agricultural Research 48, 197–212, 2005 doi: 10.1080/00288233.2005.9513650
- Bouton JH, Gates RN, Belesky DP, Owsley M. Yield and persistence of tall fescue in the southeastern coastal plain after removal of its endophyte. Agronomy Journal 85, 52–5, 1993 doi: 10.2134/agronj1993.00021962008500010011x
- Bryant JR, López-Villalobos N, Pryce JE, Holmes CW, Johnson DL. Quantifying the effect of thermal environment on production traits in three breeds of dairy cattle in New Zealand. New Zealand Journal of Agricultural Research 50, 327–38, 2007 doi: 10.1080/00288230709510301
- Card SD, Faville MJ, Simpson WR, Johnson RD, Voisey CR, De Bonth ACM, Hume DE. Mutualistic fungal endophytes in the Triticeae – survey and description. FEMS Microbiology Ecology 88, 94–106, 2014 doi: 10.1111/1574-6941.12273
- Corson DC, Waghorn GC, Ulyatt MJ, Lee J. NIRS: forage analysis and livestock feeding. Proceedings of the New Zealand Grassland Association 61, 127–32, 1999
- di Menna ME, Finch SC, Popay AJ, Smith BL. A review of the Neotyphodium lolii/Lolium perenne symbiosis and its associated effects on animal and plant health, with particular emphasis on ryegrass staggers. New Zealand Veterinary Journal 60, 315–28, 2012 doi: 10.1080/00480169.2012.697429
- Easton HS, Lane GA, Tapper BA, Keogh RG, Cooper BM, Blackwell M, Anderson M, Fletcher LR. Ryegrass endophyte-related heat stress in cattle. Proceedings of the New Zealand Grassland Association 57, 37–41, 1996
- Finch SC, Fletcher LR, Babu JV. The evaluation of endophyte toxin residues in sheep fat. New Zealand Veterinary Journal 60, 56–60, 2012 doi: 10.1080/00480169.2011.634746
- Finch SC, Thom ER, Babu JV, Hawkes AD, Waugh CD. The evaluation of fungal endophyte toxin residues in milk. New Zealand Veterinary Journal 61, 11–7, 2013 doi: 10.1080/00480169.2012.704626
- Fletcher LR. Observations of ryegrass staggers in weaned lambs grazing different ryegrass pastures. New Zealand Journal of Experimental Agriculture 10, 203–7, 1982 doi: 10.1080/03015521.1982.10427871
- Fletcher LR. “Non-toxic” endophytes in ryegrass and their effect on livestock health and production. In: Woodfield DR, Matthew C (eds). Ryegrass Endophyte – An Essential New Zealand Symbiosis. Grassland Research and Practice Series No. 7. Pp 133–9. New Zealand Grassland Association, Dunedin, NZ, 1999
- Fletcher LR, Harvey IC. An association of a Lolium endophyte with ryegrass staggers. New Zealand Veterinary Journal 29, 185–6, 1981 doi: 10.1080/00480169.1981.34839
- Fletcher LR, Sutherland BL. Sheep responses to grazing ryegrass with AR37. Proceedings of the New Zealand Grassland Association 71, 127–32, 2009
- Fletcher LR, Sutherland BL, Fletcher CG. The impact of endophyte on the health and productivity of sheep grazing ryegrass based pastures. In: Woodfield DR, Matthew C (eds). Ryegrass Endophyte – An Essential New Zealand Symbiosis. Grassland Research and Practice Series No. 7. Pp 11–7. New Zealand Grassland Association, Dunedin, NZ, 1999
- Hume DE, Sewell JC. Agronomic advantages conferred by endophyte infection of perennial ryegrass (Lolium perenne L.) and tall fescue (Festuca arundinacea Schreb.) in Australia. Crop and Pasture Science 65, 747–57, 2014 doi: 10.1071/CP13383
- Johnson LJ, de Bonth ACM, Briggs LR, Caradus JR, Finch SC, Fleetwood DJ, Fletcher LR, Hume DE, Johnson RD, Popay AJ, et al. The exploitation of epichloae endophytes for agricultural benefit. Fungal Diversity 60, 171–88, 2013 doi: 10.1007/s13225-013-0239-4
- *Jung GA, Van Wijk AJP, Hunt WF, Watson CE. Ryegrasses. In: Moser LE, Buxton DR, Casler MD (eds). Cool-Season Forage Grasses. Agronomy Monograph 34. Pp 605–41. ASA, CSSA, SSSA, Madison, WI, USA, 1996
- Keogh RG. Induction and prevention of ryegrass staggers in grazing sheep. New Zealand Journal of Experimental Agriculture 1, 55–7, 1973 doi: 10.1080/03015521.1973.10427616
- Klotz JL. Activities and effects of ergot alkaloids on livestock physiology and production. Toxins 7, 2801–21, 2015 doi: 10.3390/toxins7082801
- Logan CM, Edwards GR, Kerr GA, Williams S. Ryegrass staggers and liveweight gain of ewe lambs and hoggets grazing four combinations of perennial ryegrass and strains of endophyte. Proceedings of the New Zealand Society of Animal Production 75, 175–8, 2015
- Miles CO, Munday SC, Wilkins AL, Ede RM, Towers NR. Large-scale isolation of lolitrem B and structure determination of lolitrem E. Journal of Agricultural and Food Chemistry 42, 1488–92, 1994 doi: 10.1021/jf00043a018
- Moore JR, Pratley JE, Mace WJ, Weston LA. Variation in alkaloid production from genetically diverse Lolium accessions infected with Epichloë species. Journal of Agricultural and Food Chemistry 63, 10355–65, 2015 doi: 10.1021/acs.jafc.5b03089
- Nicol AM, Klotz JL. The difficulties in reviewing ergovaline. Proceedings of the New Zealand Society of Animal Production 75, 179–83, 2015
- Nicol AM, Klotz JL. Ergovaline, an endophytic alkaloid. 2. Intake and impact on animal production, with reference to New Zealand. Animal Production Science 56, 1775–86, 2016 doi: 10.1071/AN14963
- Popay AJ, Hume DE. Endophytes improve ryegrass persistence by controlling insects. In: Mercer CF (ed). Pasture Persistence. Grassland Research and Practice Series No. 15. Pp 149–56. New Zealand Grassland Association, Dunedin, NZ, 2011
- Pownall DB, Familton AS, Field RJ, Fletcher LR, Lane GA. The effect of peramine ingestion in pen-fed lambs. Proceedings of the New Zealand Society of Animal Production 55, 186, 1995
- Reed KFM, Mace WJ, Walker LV, Fletcher LR. Endophyte metabolites associated with perennial ryegrass toxicosis. Animal Production Science 56, 895–907, 2016 doi: 10.1071/AN14495
- Rhodes MT, Paterson JA, Kerley MS, Garner HE, Laughlin MH. Reduced blood flow to peripheral and core body tissues in sheep and cattle induced by endophyte-infected tall fescue. Journal of Animal Science 69, 2033–43, 1991 doi: 10.2527/1991.6952033x
- Rowan DD. Lolitrems, peramine and paxilline: mycotoxins of the ryegrass/endophyte interaction. Agriculture, Ecosystems and Environment 44, 103–22, 1993 doi: 10.1016/0167-8809(93)90041-M
- Simpson WR, Schmid J, Singh J, Faville MJ, Johnson RD. A morphological change in the fungal symbiont Neotyphodium lolii induces dwarfing in its host plant Lolium perenne. Fungal Biology 116, 234–40, 2012 doi: 10.1016/j.funbio.2011.11.006
- Stewart A, Kerr G, Lissaman W, Rowarth J. Endophyte in ryegrass and tall fescue. In: Davies K, Casey M (eds). Pasture and Forage Plants for New Zealand. Grassland Research and Practice Series No. 8. Pp 66–77. New Zealand Grassland Association, Dunedin, NZ, 2014
- Thom ER, Waugh CD, Minneé EMK, Waghorn GC. A new generation ryegrass endophyte – the first results from dairy cows fed AR37. In: Popay AJ, Thom ER (eds). Proceedings of the 6th International Symposium on Fungal Endophytes of Grasses. Pp 293–6, 2007
- Thom ER, Popay AJ, Waugh CD, Minneé EMK. Impact of novel endophytes in perennial ryegrass on herbage production and insect pests from pastures under dairy cow grazing in northern New Zealand. Grass and Forage Science 69, 191–204, 2014 doi: 10.1111/gfs.12040
- van Zijll de Jong E, Dobrowolski MP, Sandford A, Smith KF, Willocks MJ, Spangenberg GC, Forster JW. Detection and characterisation of novel fungal endophyte genotypic variation in cultivars of perennial ryegrass (Lolium perenne L.). Australian Journal of Agricultural Research 59, 214–21, 2008 doi: 10.1071/AR07270
- Waller JC. Endophyte effects on cattle. In: Fribourg HA, Hannaway DB, West CP (eds). Tall Fescue for the Twenty-first Century. Agronomy Monograph 53. Pp 289–310, ASA, CSSA, SSSA, Madison, WI, USA, 2009
