ABSTRACT
Aims
To assess the prevalence of carriage of Salmonella spp. in wild reptiles translocated from multiple locations to a single island, and determine changes in their body condition (BC) during quarantine.
Methods
Between 2007 and 2009, six endemic reptile species (Oligosoma aeneum, O. moco, O. ornatum, O. smithi, Dactylocnemis pacificus, and Woodworthia maculata) were caught from several locations in the northern North Island of New Zealand. Reptiles were held in quarantine for 14–41 days while being tested for carriage of Salmonella spp. Morphometric data were collected, and scaled body mass index for each species was calculated to determine changes in BC during the quarantine.
Results
Of 221 individuals tested 12 (5%) were positive for Salmonella spp. All 12 were shore skinks (O. smithi; n = 30), with a test prevalence of 0.4 (95% CI = 0.25–0.58). Eleven were carrying Salmonella enterica Warragul and one S. enterica Mississipi. There was no difference in BC at the start of quarantine of shore skinks between those that tested negative and those that tested positive for Salmonella spp. (p = 0.184). Reptiles that were quarantined for 15–20 days (three species) lost 3–5% of BC (mean proportional change 0.03–0.05), while those quarantined for >30 days increased BC by 3–13% (mean proportional change 0.03–0.13). All animals except the one individual positive for S. Mississippi were translocated to the recipient island, while the latter was returned to the source site.
Conclusions and clinical relevance
The prevalence of Salmonella spp. carriage in the translocated reptiles was low overall and consistent with other records of Salmonella spp. in wild New Zealand reptiles. However, the prevalence of 0.4 in shore skinks is the highest recorded in this species. In addition to time required for health-screening, we recommend that duration of quarantine should include time to allow animals to recover from captive stress and to provide an opportunity to increase their BC before release.
Introduction
Conservation translocation (Anonymous Citation2013), the intentional movement of wildlife for conservation purposes, is increasingly common in ecological restoration projects. Many of these include plans for translocation of multiple species to the restored site (e.g. Ritchie Citation2000; Gardner-Gee et al. Citation2007; Baling et al. Citation2013b). This intentional movement of many species to one area, even at different time intervals, can increase the likelihood of parasite or disease transmission (Van Andel et al. Citation2015b). The introduction of a novel parasite to a naïve population can have devastating effects on either the translocated or resident populations at the release area (see examples in Ewen et al. Citation2012). Although many studies have highlighted this potential effect, they acknowledge that the full implication of parasite transmission is still unknown because the natural frequency and the level of transmission of parasites within a wild ecosystem are not fully understood (Parker et al. Citation2006; Ewen et al. Citation2012).
There is a lack of records of prevalence for pathogens in wild reptiles in New Zealand, even for generalist pathogens such as Salmonella spp. The few significant published data are either from studies at single time points (e.g. Middleton et al. Citation2010; Middleton et al. Citation2014) or were collected during unusual events such as disease outbreaks (e.g. McInnes Citation1971; van Andel et al. Citation2015a). Unfortunately, prevalence data from outbreaks are likely to be higher than background prevalence. The limited knowledge of Salmonella spp. carriage in wild populations will affect interpretation of any potential disease threat and the ability to assess the risk of pathogen transmission due to translocation activities (i.e. the effect on resident wildlife populations, founder populations, and humans). Multiple conservation translocations can provide opportunities to collect data on the occurrences of natural pathogens from capture and release sites (Gartrell et al. Citation2006; Gartrell et al. Citation2007; Baling et al. Citation2013a) providing a better picture of pathogen frequency over time.
Collecting health assessment data during wildlife translocations generally requires some degree of temporary captivity for animals for quarantine purposes (Jakob-Hoff et al. Citation2004). The time spent in quarantine for translocated animals is often dictated by the length of time required to obtain and interpret test results (Munson and Cook Citation1993; Ewen et al. Citation2012). Tests for Salmonella spp. in New Zealand reptiles may require 2 weeks to several months (van Winkel et al. Citation2010). This large difference in quarantine time may affect the overall condition of the animals before release (Teixeira et al. Citation2007; Ewen et al. Citation2012). To our knowledge there is currently no published report of records of time in quarantine and the overall condition of wild-born reptiles.
This study aims to assess test prevalence for carriage of Salmonella spp. in wild reptiles translocated from different locations in New Zealand to a single island, and to document the change in body condition of translocated animals during the quarantine. Data were collected from six endemic reptile species, five of which were quarantined before being translocated to Matakohe-Limestone Island Scenic Reserve (MLI) in Whangarei, as part of the island’s restoration plan (Ritchie Citation2000).
Materials and methods
All sites were located in Northland, New Zealand (). Study sites comprised the release site (MLI) and source sites on the mainland (Mimiwhangata Scenic Reserve) and offshore islands within the Hen and Chickens Island Group Nature Reserve (Pupuha, Muriwhenua, Middlestack, Wareware, Lady Alice, Whatupuke, and Coppermine Islands). MLI has undergone much habitat restoration, and its restoration plan includes translocations of several reptile species to the island (Ritchie Citation2000).
Figure 1. Location of sites within Northland, New Zealand where reptiles were captured and translocated to Matakohe-Limestone Island.
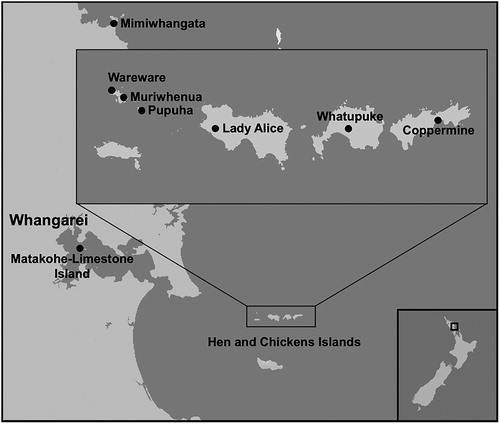
All procedures were conducted under the approval of translocation permits (NO-229996-FAU, NO-21545-FAU, and NO-25872-FAU) and a holding permit (AK-21486-CAP) from the Department of Conservation (Wellington, NZ). A pre-translocation survey was conducted on the only resident reptile species, copper skink (Oligosoma aeneum), at MLI. Following this, four translocations of five reptile species were carried out from source locations to MLI during 2007–2009. Translocated species were Pacific gecko (Dactylocnemis pacificus); moko skink (O. moco); ornate skink (O. ornatum); shore skink (O. smithi), and raukawa gecko (Woodworthia maculata). Samples and data were collected either onsite during the pre-translocation survey (resident species at MLI), or during the quarantine period at Massey University (Auckland, NZ). A mix of cloacal and faecal swabs (urogenital transport swab containing Amies with charcoal media; Sterilin, South Wales, UK) were collected once from each individual, within 1–3 days of the start of quarantine. All samples (from both resident and quarantined reptiles) were transported in chilled boxes to the laboratory within 48 hours. Blood samples were not taken due to the small size of all species. The following data were collected from all except one species (moko skinks): sex, general morphological data (body size as snout-to-vent length, body mass at start and end of quarantine), and the number of days the animals were held in captivity. Morphological data were only collected from a subset (n = 8) of moko skinks.
Data analysis
A total of 221 individual samples were cultured for Salmonella spp. at New Zealand Veterinary Pathology (Hamilton, NZ), and at the Institute of Veterinary, Animal and Biomedical Sciences (IVABS; Massey University, Palmerston North, NZ).
For detection of Salmonella spp., all swabs were inoculated onto xylose lysine deoxycholate with novobiocin and MacConkey plates (used by New Zealand Veterinary Pathology only), and also two selective enrichment broths (tetrathionate and selenite). All plates and broths were then incubated aerobically at 37°C for 18–24 hours, and plates were examined for suspect colonies. Selective enrichment broths were sub-cultured onto xylose lysine deoxycholate agar with novobiocin and MacConkey plates (IVABS only) and incubated at 37°C for 18–24 hours. Suspect colonies were then inoculated onto Columbia sheep blood agar (IVABS only), MacConkey agar (IVABS only), lysine iron agar, urea slants (IVABS only), lysine decarboxylase tubes (IVABS only), triple sugar iron agar, and a nutrient agar slope. These were incubated overnight, examined for typical reactions, and further tests were conducted, including agglutination. Colonies suspected to be Salmonella spp. were also sent to Environmental Science and Research (Wellington, NZ) for confirmation and serovar typing.
Statistical analyses
Test prevalence for detection of Salmonella spp. in each reptile species was determined, with Wilson’s 95% CI, using an online tool (Epitools; Sergeant Citation2013). True prevalence could not be calculated because of the low number of positive samples. CI, where test prevalence was zero, was calculated using the Rule of Three (Jovanovic and Levy Citation1997).
To assess the effect of quarantine on body condition (BC), BC was calculated as scaled mass index (Peig and Green Citation2009), which eliminates the effect of body length on mass. Here, the body mass is standardised at a fixed value based on the scaling relationship between mass and snout-to-vent length (see methods in Peig and Green Citation2009). As BC was calculated for non-gravid individuals only, a subset of each species was used for determining changes in BC. One-way ANOVA was used to compare the difference in BC (at the start of quarantine) between individuals that tested positive for Salmonella spp. and those of the same species that tested negative. As there was no association, we pooled all available BC scores to determine the mean (and 95% CI) change in BC during quarantine for each species. Change in BC for each individual was calculated as the difference between BC at the start and end of quarantine, divided by BC at the start of quarantine. Positive values indicate a gain in BC, and negative values a loss.
Results
A total of 12/221 (5%) individuals tested positive for Salmonella spp. (). Eleven shore skinks were positive for S. enterica serovar Warragul (four males, seven females) and one female for S. enterica serovar Mississippi, with a total test prevalence of infection with Salmonella spp. of 0.4 (95% CI = 0.25–0.58) for this sampled population species. There was no evidence for a difference (p = 0.184) in mean BC between shore skinks that tested positive (n = 11) for Salmonella spp. infection and those that did not (n = 20).
Table 1. Location of origin and days in quarantine (DIQ) of five endemic reptile species translocated to Matakohe-Limestone Island (MLI), New Zealand between 2007 and 2009. Prevalence of carriage of Salmonella spp. in these reptiles and in copper skinks (Oligosoma aeneum) resident on MLI is shown along with a translocation and sampling summary.
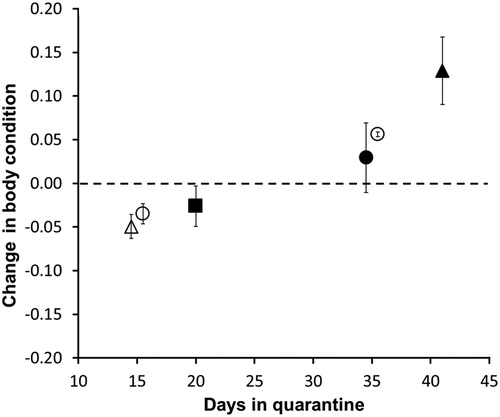
Body condition varied across the different length of time in quarantine (). Animals kept for shorter times (15 and 20 days) had 2–5% loss in BC (mean proportional change in BC: raukawa gecko −0.05 (95% CI = −0.035 to −0.063); Pacific gecko −0.03 (95% CI = −0.023 to −0.046); ornate skink: −0.03 (95% CI = −0.003 to −0.049)). Conversely, animals quarantined for >30 days increased BC by 3–13% (mean proportional change in BC: moko skink 0.03 (95% CI = −0.011 to 0.069); Pacific gecko 0.06 (95% CI = 0.053–0.058); shore skinks 0.13 (95% CI = 0.090–0.168)).
Figure 2. Mean (±95% CI) change in body condition (BC; calculated as scaled mass index) during quarantine as a proportion of BC at start of quarantine, of five endemic reptile species (○ = Dactylocnemis pacificus, n = 50; ▵ = Woodworthia maculata, n = 15; ▪ = Oligosoma ornatum, n = 30; • = O. moco, n = 8; ▴ = O. smithi, n = 14) which were translocated to Matakohe-Limestone Island during 2007–2009.
Discussion
Of the six wild reptile species tested in 2007–2009 for translocation, only one species, shore skink, was positive for Salmonella spp. with a test prevalence of 0.4. The presence of Salmonella spp. in shore skinks is not new, and was generally expected for this coastal species (Middleton et al. Citation2010; Baling et al. Citation2013a). However, this is the highest prevalence reported for carriage of Salmonella spp. by shore skinks. In comparison, prevalence of 0.167 (n = 72) and 0.01 (n = 120) were reported by Middleton et al. (Citation2010) and Baling, Gartrell, et al. (Citation2013), respectively. Prevalence in other New Zealand reptile species in the wild ranges from 0 to 0.18 (Gartrell et al. Citation2007; Middleton et al. Citation2010; Baling et al. Citation2013a). The majority of our results are consistent with previous New Zealand studies, where similarly low prevalence of Salmonella spp. was detected in wild native reptile populations (Middleton et al. Citation2010; Baling et al. Citation2013a; Grange et al. Citation2017).
Many factors may influence infection rates, including climate, environmental conditions, species (both host and pathogen), diet, and the composition of species within a habitat (Mitchell and Shane Citation2001; Chambers Citation2011; Gupta et al. Citation2020). It may also be affected by different sampling and testing approaches used in the studies. Detection of Salmonella spp. using microbiological methods is generally assumed to have a sensitivity of 50% and a specificity of 98% (Middleton et al. Citation2010). Our study was limited in that individuals were only sampled once. Accurate estimation of true prevalence for low-prevalence pathogens in wildlife populations requires systematic, multiple sampling within a population or the use of a test with high sensitivity (Bager and Petersen Citation1991).
The most common of the two serovars isolated in our sampled populations was S. Warragul, which has currently been observed only in reptiles in New Zealand (Anonymous Citation2019). This serotype was found in one other island, Korapuki Island, approximately 150 km southeast of our source site, Mimiwhangata (Middleton et al. Citation2010). In contrast, S. Mississippi has been infrequently isolated in New Zealand from reptiles (median of twice per year), birds (once in 2007), other mammals (median of once per year), in the environment (once in 2006) and humans (median of 13 times per year) between 2003 and 2009 (Anonymous Citation2019). Therefore, to avoid the risk of infection to residents of the island, the single shore skink that was positive for S. Mississippi was returned to the source population (Grange et al. Citation2017).
Among the subsample of reptiles which were measured, body condition varied with the length of time spent in captivity; those that experienced a shorter duration in captivity (3–13 days) lost BC, whereas those that were in captivity for >30 days mostly gained BC. We presume that any loss of BC, particularly for animals already in a low condition, is indicative of stress (MacDonald et al. Citation2007; Waye and Mason Citation2008), and that stress could be particularly high in early stages of captivity (e.g. Coddington and Cree Citation1995; Gregory et al. Citation1996). Additionally, releasing individuals into an unfamiliar site (i.e. refuge and food source locations are unknown) imposes chronic stress to the translocated animals. A lower BC resulting from both quarantine stress and stress from release may potentially affect foraging, dispersal, and breeding behaviour, increase exposure to predators, reduce individual health (e.g. increase parasite load), and consequently, overall population survival at the release site (Amo et al. Citation2007; Gelling et al. Citation2012).
Extended time in quarantine may provide the opportunity not only for individuals to recover from acute stress of capture and chronic stress (as defined in Teixeira et al. Citation2007) of captivity, but also for individuals with low BC to build up reserves before being released. Veterinarians often recommend that animals are held in quarantine for 30–90 days to allow time for diseases with long incubation periods to become apparent (Woodford Citation2000; Ewen et al. Citation2012). Our results indicate that for native lizards, captivity for at least 30 days (i.e. the minimum recommended by veterinarians) may also be sufficient to improve BC. However, the gain or loss of BC (as a measure of stress) is also dependent on species, however this cannot be confirmed from our data. More controlled experiments will be required to determine species effect on body condition during short-term captivity. Future studies into cost-effective quarantine timelines will be valuable for translocation managers to consider the cost of money, labour, and time constraints for quarantine in translocation projects.
In conclusion, only one of six endemic reptile species collected for translocation, tested positive for Salmonella spp., and this sampled species (shore skink) had the highest Salmonella spp. prevalence recorded to date in New Zealand. Overall prevalence of Salmonella spp. in the translocated reptiles was low and in agreement with that found in other populations of wild reptiles in New Zealand. BC of reptiles may vary across the different length of time in quarantine. When holding lizards in quarantine, we recommend taking into account the time required for animals to recover from captive stress and to allow an increase in BC before release.
Acknowledgements
We thank Friends Of Matakohe-Limestone Island, especially P. Mitchell, G. Brackenbury, L. Mead, and P. Anderson; Massey University (B. Gartrell, M. Barry, D. van Winkel, M. Delaney, M. Van Rensburg, C. Wong, J. Peace, D. Brunton), R. Parrish, and Department of Conservation. We also thank Northland Regional Council – Harbour Restoration Fund, and Golden Bay Cement for the funding provided.
References
- Amo L, López P, Martín J. Refuge use: a conflict between avoiding predation and losing mass in lizards. Physiology & Behavior 90, 334–43, 2007
- *Anonymous. IUCN guidelines for reintroductions and other conservation translocations. IUCN Species Survival Commission, Gland, Switzerland, 2013
- *Anonymous. Public Health Surveillance: Non-human Salmonella Isolates. https://surv.esr.cri.nz/enteric_reference/enteric_reference.php (accessed 22 August 2019). Institute of Environmental Science & Research, Wellington, NZ, 2019
- Bager F, Petersen J. Sensitivity and specificity of different methods for the isolation of Salmonella from pigs. Acta Veterinaria Scandinavica 32, 473–81, 1991
- Baling M, Gartrell B, Ji W, Brunton DH. Detection of Salmonella during the translocation of two endemic New Zealand lizard species within the Hauraki Gulf. New Zealand Journal of Zoology 40, 249–54, 2013a
- Baling M, van Winkel D, Rixon (née Habgood) M, Ruffell J, Ji W, Ussher G. A review of reptile research and conservation management on Tiritiri Matangi Island, New Zealand. New Zealand Journal of Ecology 37, 272–81, 2013b
- *Chambers DL. Salmonella infections in reptiles and amphibians in a changing world. In: Baker KJ (ed). Reptiles: Biology, behavior and conservation. Pp 163–71. Nova Science, Hauppauge, NY, 2011
- Coddington EJ, Cree A. Effect of acute captivity stress on plasma concentrations of corticosterone and sex steroids in female whistling frogs Litoria ewingi. General and Comparative Endocrinology 100, 33–8, 1995
- *Ewen JG, Acevedo-Whitehouse K, Alley MR, Carraro C, Sainbury AW, Swinnerton K, Woodroffe R. Empirical consideration of parasite and health in reintroductions. In: Ewen JG, Armstrong DP, Parker KA, Seddon PJ (eds), Reintroduction Biology: Integrating Science and Management. Pp 290–35. Wiley-Blackwell, Oxford, UK, 2012
- *Gardner-Gee R, Graham S, Griffiths R, Habgood MJ, Dunlop Heis S, Lindsay H. Motuora native species restoration plan. Motuora Restoration Society and Department of Conservation, Auckland, NZ, 2007
- Gartrell BD, Jillings E, Adlington BA, Mack H, Nelson NJ. Health screening for a translocation of captive-reared tuatara (Sphenodon punctatus) to an island refuge. New Zealand Veterinary Journal 54, 344–9, 2006
- Gartrell BD, Youl JM, King CM, Bolotovski I, McDonald WL, Nelson NJ. Failure to detect Salmonella species in a population of wild tuatara (Sphenodon punctatus). New Zealand Veterinary Journal 55, 134–6, 2007
- Gelling M, Johnson PJ, Moorhouse TP, Macdonald DW. Measuring animal welfare within a reintroduction: An assessment of different indices of stress in water voles Arvicola amphibius. PLoS One 7, e41081, 2012
- Grange ZL, Biggs PJ, Rose SP, Gartrell B, Nelson NJ, French NP. Genomic epidemiology and management of Salmonella in island ecosystems used for takahe conservation. Microbial Ecology 74, 735–44, 2017
- Gregory LF, Gross TS, Bolten AB, Bjorndal KA, Guillette LJ. Plasma corticosterone concentrations associated with acute captivity stress in wild loggerhead sea turtles (Caretta caretta). General and Comparative Endocrinology 104, 312–20, 1996
- Gupta P, Robin VV, Dharmarajan G. Towards a more healthy conservation paradigm: Integrating disease and molecular ecology to aid biological conservation. Journal of Genetics 99, 65, 2020
- *Jakob-Hoff R, McInnes K, Frank M, Cromarty P. Translocation Health Management Process. Part B: Translocation Health Management Plan. Department of Conservation, Auckland, NZ, 2004
- Jovanovic BD, Levy PS. A look at the rule of three. The American Statistician 51, 137–9, 1997
- MacDonald EA, Czekala NM, Gerber GP, Alberts AC. Diurnal and seasonal patterns in corticosterone in the Turks and Caicos iguana (Cyclura carinata carinata). Caribbean Journal of Science 43, 266–72, 2007
- McInnes HM. Salmonella saintpaul infection of sheep with lizards as possible reservoirs. New Zealand Veterinary Journal 19, 163–4, 1971.
- Middleton DMRL, Minot EO, Gartrell BD. Salmonella enterica serovars in lizards of New Zealand’s offshore islands. New Zealand Journal of Ecology 34, 247–52, 2010
- Middleton DMRL, La Flamme AC, Gartrell B, Nelson NJ. Reptile reservoir and seasonal variation in the environmental presence of Salmonella in an island ecosystem, Stephens Island, New Zealand. Journal of Wildlife Diseases 50, 655–9, 2014
- Mitchell MA, Shane SM. Salmonella in reptiles. Seminars in Avian and Exotic Pet Medicine 10, 25–35, 2001
- Munson L, Cook RA. Monitoring, investigation, and surveillance of diseases in captive wildlife. Journal of Zoo and Wildlife Medicine 24, 281–90, 1993
- Parker KA, Brunton DH, Jakob-Hoff R. Avian translocations and disease; implications for New Zealand conservation. Pacific Conservation Biology 12, 155–62, 2006
- Peig J, Green J. New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118, 1883–91, 2009
- *Ritchie J. Matakohe/Limestone Scenic Reserve restoration plan. Friends of Matakohe/Limestone Incorporated Society, Whangarei, NZ, 2000
- *Sergeant E. Estimated True Prevalence and Predictive Values from Survey Testing. (http://epitools.ausvet.com.au/content.php?page=TruePrevalence). (accessed 3 February 2021), Ausvet, Bruce, ACT, Australia, 2013
- Teixeira CP, De Azevedo CS, Mendl M, Cipreste CF, Young RJ. Revisiting translocation and reintroduction programmes: The importance of considering stress. Animal Behaviour 73, 1–13, 2007
- van Andel M, Jackson BH, Midwinter AC, Alley MR, Ewen JG, Mcinnes K, Jakob-Hoff R, Reynolds AD, French N. Investigation of mortalities associated with Salmonella spp. infection in wildlife on Tiritiri Matangi Island in the Hauraki Gulf of New Zealand. New Zealand Veterinary Journal 63, 235–9, 2015a
- Van Andel M, Mcinnes K, Tana T, French NP. Network analysis of wildlife translocations in New Zealand. New Zealand Veterinary Journal 64, 169–73, 2015b
- *van Winkel D, Baling M, Barry M, Ji W, Brunton D. Translocation of Duvaucel’s geckos to Tiritiri Matangi and Motuora Islands, Hauraki Gulf, as part of island ecological restoration initiatives. In: Soore P (ed), Global Re-introduction Perspectives: Additional Case-studies from Around the Globe. Pp. 113–5. IUCN/SSC Re-introduction Specialist Group, Abu Dhabi, UAE 2010
- Waye HL, Mason RT. A combination of body condition measurements is more informative than conventional condition indices: Temporal variation in body condition and corticosterone in brown tree snakes (Boiga irregularis). General and Comparative Endocrinology 155, 607–12, 2008
- *Woodford MH. Quarantine and health screening protocols for wildlife prior to translocation and release into the wild. University of Nebraska, Lincoln, NE, USA, 2000
- *Non-peer-reviewed
