ABSTRACT
In 2019 a novel coronavirus termed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged from an unidentified source and spread rapidly among humans worldwide. While many human infections are mild, some result in severe clinical disease that in a small proportion of infected people is fatal. The pandemic spread of SARS-CoV-2 has been facilitated by efficient human-to-human transmission of the virus, with no data to indicate that animals contributed to this global health crisis. However, a range of domesticated and wild animals are also susceptible to SARS-CoV-2 infection under both experimental and natural conditions. Humans are presumed to be the source of most animal infections thus far, although natural transmission between mink and between free-ranging deer has occurred, and occasional natural transmission between cats cannot be fully excluded. Considering the ongoing circulation of the virus among people, together with its capacity to evolve through mutation and recombination, the risk of the emergence of animal-adapted variants is not negligible. If such variants remain infectious to humans, this could lead to the establishment of an animal reservoir for the virus, which would complicate control efforts. As such, minimising human-to-animal transmission of SARS-CoV-2 should be considered as part of infection control efforts. The aim of this review is to summarise what is currently known about the species specificity of animal coronaviruses, with an emphasis on SARS-CoV-2, in the broader context of factors that facilitate cross-species transmission of viruses.
Introduction
In January 2020, the World Health Organisation announced the identification of a novel coronavirus (CoV) associated with a cluster of cases of viral pneumonia in Wuhan, China (Huang et al. Citation2020b; WHO Citation2020; Zhu et al. Citation2020). The disease was named coronavirus disease 2019 (COVID-2019) and was shown to be caused by a novel coronavirus, named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Since then, the virus has spread among people across the globe causing an unprecedented pandemic with over 680 million infections including 6.8 million deaths (as of 2 March 2022, https://www.worldometers.info/coronavirus/). The origin of SARS-CoV-2 remains obscure, but most researchers agree that the virus crossed into a human population from animals (Holmes et al. Citation2021; Lytras et al. Citation2021). The aim of this review is to summarise what is currently known about the species specificity of SARS-CoV-2 in the broader context of factors that facilitate cross-species transmission of viruses.
General characteristics of coronaviruses
Coronaviruses are enveloped viruses with a positive sense, non-segmented RNA genome. There are over 2,500 coronavirus sequences available in public databases, which represent coronaviruses from a variety of vertebrate hosts. Based on the similarities in the conserved regions of coronavirus genomes, these viruses are currently classified into 54 species, 28 subgenera, six genera, and three subfamilies within the family Coronaviridae, suborder Cornidovirineae, and order Nidovirales (Woo Citation2023). SARS-CoV-2 is classified in the species Severe acute respiratory syndrome-related coronavirus, subgenus Sarbecovirus and genus Betacoronavirus (Coronaviridae Study Group of the International Committee on Taxonomy of Viruses Citation2020). In general, coronaviruses have a predilection for the respiratory or gastrointestinal tract and are usually associated with gastrointestinal or respiratory disease, which is often mild or subclinical (Zappulli et al. Citation2020; Kayode et al. Citation2021). However, selected human and animal coronaviruses can cause severe systemic disease, sometimes with a neurological component. Examples include severe acute respiratory syndrome (SARS) and COVID-19 in people, feline infectious peritonitis, ferret systemic coronavirus disease, or the cardiovascular and central nervous system presentations that can accompany gastrointestinal disease caused by coronaviruses of swine (Murray et al. Citation2010; Pedersen Citation2014; Thakor et al. Citation2022). One animal species can serve as a host for several different coronaviruses ().
Table 1. Host and disease characteristics of selected coronaviruses that can infect animals that are present in New Zealand, including whether each virus has been identified in New Zealand.
All coronaviruses have similar genome organisation and replication strategies (Gorbalenya et al. Citation2006; Pasternak et al. Citation2006). Approximately two-thirds of the genomic RNA encodes two large polyproteins, 1a and 1ab, which are expressed from two overlapping open reading frames located at the 5’ end of the genome. These viral polyproteins are further cleaved into 16 non-structural proteins (nsp1 to nsp16) that are involved in virus replication and interactions with the host’s immune system (Fang et al. Citation2021). The downstream open reading frame encodes structural viral proteins, which comprise spike (S), envelope, membrane, and nucleocapsid proteins, and several accessory proteins that differ between various coronaviruses (Gorbalenya et al. Citation2006; Pasternak et al. Citation2006).
Coronavirus entry into the cell
Coronavirus entry into cells is mediated by interactions between the S protein and host receptors, which vary between different coronaviruses (Cheng et al. Citation2021). The receptor used by SARS-CoV-2 is angiotensin-converting enzyme 2 (ACE2) (Jackson et al. Citation2022). Each spike in the “crown” of coronaviruses consists of three copies of the S protein. The S protein of SARS-CoV-2 is cleaved during the assembly within infected cells into two subunits, S1 and S2 (). These two subunits remain non-covalently bonded on the surface of the virion and play different roles during SARS-CoV-2 entry into the cell: subunit 1 binds ACE2 through its receptor binding domain (RBD), while subunit 2 anchors the virion to the host cell and mediates membrane fusion (Jackson et al. Citation2022). The changes in the conformation of the S trimer before and after the binding to ACE2 are crucial for the successful entry of the virus into the cell (Jackson et al. Citation2022).
Figure 1. Schematic representation of the structure of a coronavirus including details of the structure of the spike (S) protein of severe acute respiratory coronavirus type 2 (SARS-CoV-2). Each viral spike comprises three S proteins. A single S protein consists of two subunits (S1 and S2) which are separated by a furin cleavage site. These subunits contain different domains and play different roles during virus entry into the cell: S1 binds angiotensin-converting enzyme 2 (ACE2) receptor on the host cell through its receptor binding domain (RBD), while S2 anchors the virion to the host cell and mediates membrane fusion (Jackson et al. Citation2022). Adapted from “An in-depth look into the structure of SARS-CoV-2 spike glycoprotein” by BioRender.com (2023). Retrieved from https://app.biorender.com/biorender-templates.
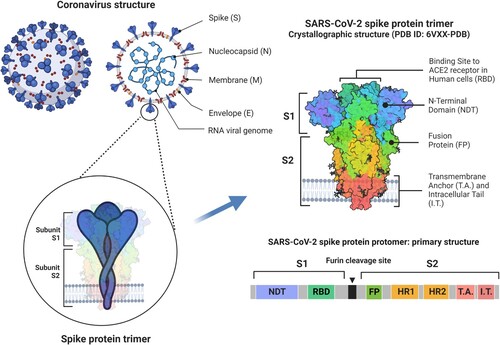
In addition to its role in the virus entry into the host cell, the RBD on the S protein is also the main target for neutralising antibodies during SARS-CoV-2 infection. Although correlates of protective immunity against SARS-CoV-2 are not yet fully elucidated, the levels and specificity of anti-S neutralising antibodies have been used as a proxy for the assessment of levels of protective immunity following natural infection or vaccination (Huang et al. Citation2020a; Gilbert et al. Citation2022).
The sequence of the S protein is one of the crucial determinants for the species- and tissue-specificity of SARS-CoV-2, as well as the virulence of the virus (Gussow et al. Citation2020).
Human coronaviruses
Four coronaviruses are endemic in human populations worldwide, including New Zealand (CDC Citation2020). These include two alphacoronaviruses (human coronavirus (HCoV-229E and HCoV-NL63) and two betacoronaviruses (HCoV-HKU1 and HCoV-OC43). Infections with these endemic viruses are typically associated with mild-to-moderate respiratory disease referred to as “common colds” (Kayode et al. Citation2021). Coronaviruses had not attracted much scientific attention until 2002 when the first worldwide outbreak of SARS occurred (Song et al. Citation2019). This was followed by the emergence of Middle East respiratory syndrome (MERS) in 2012 (Guan et al. Citation2003) and finally the current COVID-19 pandemic. Unlike upper respiratory tract infections associated with endemic human coronaviruses, which are typically self-limiting, a comparatively high proportion of people infected with these three emerging coronaviruses go on to develop severe pneumonia. Case fatality rates are estimated to range from < 5% for SARS-CoV-2 (Sreedharan et al. Citation2021) to 35% for MERS-CoV (WHO Citation2016). Unlike the global spread of SARS-CoV-2, SARS-CoV-1 (the virus responsible for SARS) has not been circulating among humans since 2004, and transmission of MERS-CoV has been limited to the Middle East.
All three recently emerged human coronaviruses are believed to have a zoonotic origin. An overall prevalence of coronavirus infection of 6.8% was found in a recent study of bats (n = 1,067, from 21 species) from three provinces of China (Lin et al. Citation2017). The researchers indentified 73 different coronaviruses among which were 41 betacoronaviruses closely related to the known members of the species Severe acute respiratory syndrome related viruses, including SARS-CoV-1 and SARS-CoV-2. This suggests that bats probably had a role as direct or indirect sources of SARS-CoV-1 (Guan et al. Citation2003), MERS-CoV (Drosten et al. Citation2014), and possibly SARS-CoV-2 (Zhou et al. Citation2020), and may also harbour other coronaviruses that may threaten human health. In fact, it has been recently suggested that the “Russian flu” pandemic, which killed an estimated 1 million people (approximately 7% of the human population at the time) between 1889 and 1891, may have been caused by a coronavirus rather than by influenza virus, as has been presumed until now (Brussow and Brussow Citation2021; Ramassy et al. Citation2022). Coincidently, results of a phylogenetic analysis suggested that HCoV-OC43 may have originated from a relatively recent zoonotic transmission of bovine coronavirus. The most recent common ancestor of these two viruses was estimated to exist around 1890 (Vijgen et al. Citation2005). Taken together, those findings suggest a possibility that the now endemic HCoV-OC43 originated from cross-species transmission of a bovine coronavirus that coincided with a global pandemic that clinically resembled the current COVID-19 disease, although more research is needed to confirm or refute this theory.
Animal coronaviruses
Many coronaviruses have been detected from various domestic and wild animals (Haake et al. Citation2020; Stout et al. Citation2020; Zhang et al. Citation2021). Coronaviruses that can infect animals living in New Zealand are listed in . Only some of those viruses are known to be present in New Zealand, including canine, feline, equine, bovine and avian coronaviruses. Other coronaviruses have never been detected here. However, only two porcine diseases (transmissible gastroenteritis virus (TGEV) and porcine epidemic diarrhoea) are specified as “absent from New Zealand” by the Ministry for Primary Industries (MPI Citation2022). As there is no active surveillance for any of the animal coronaviruses in New Zealand, the lack of reports of a specific virus does not equate to absence of that virus in the country.
Several animal coronaviruses evolved through mutations and recombinations (Focosi and Maggi Citation2022). Those events can lead to changes in hosts or the virulence of the virus. Examples include the emergence of TGEV in pigs and feline coronavirus in cats, which are believed to represent cross-species transfer of canine coronavirus to pigs and cats, respectively (Licitra et al. Citation2014).
SARS-CoV-2 infection of animals
Direct transmission of SARS-CoV-2 between people occurs via respiratory droplets produced during coughing, sneezing or talking, or through contact with body fluids and secretions, such as faeces or saliva (Chan et al. Citation2020a; Wang et al. Citation2020; Bahl et al. Citation2022). The contribution of airborne transmission by aerosols has been suggested, although views on the importance of that route in the global spread of the virus vary (Bushmaker et al. Citation2022; Tellier Citation2022). Indirect transmission may occur through contact with fomites such as contaminated objects or common-use surfaces. Viable SARS-CoV-2 virus can survive for up to 4 days on various surfaces, depending on the environmental conditions and type of surface (Guo et al. Citation2020; Pastorino et al. Citation2020; van Doremalen et al. Citation2020).
Similar routes of infection would be expected to exist between humans and domesticated animals, especially those in close contact with people (pets or livestock). There is no indication that domesticated animals have played a role in the current pandemic spread of SARS-CoV-2 among people. However, SARS-CoV-2 can experimentally infect a wide range of animals including cats (Bosco-Lauth et al. Citation2020; Gaudreault et al. Citation2020; Halfmann et al. Citation2020), ferrets (Shi et al. Citation2020; Kutter et al. Citation2021), mink (Virtanen et al. Citation2022), hamsters (Chan et al. Citation2020b; Osterrieder et al. Citation2020), rabbits (Mykytyn et al. Citation2021), raccoon dogs (Nyctereutes procyonoides; Freuling et al. Citation2020), tree shrews (Tupaia belangeris; Zhao et al. Citation2020), white-tailed deer (Odocoileus virginianus; Martins et al. Citation2022) and non-human primates (Trichel Citation2021). Dogs appeared partially refractory to experimental infection as only some, but not all, became infected with the virus, and the infection was not passed on further to susceptible dogs (Shi et al. Citation2020). Pigs, chickens and ducks were not susceptible to experimental infection with SARS-CoV-2 (Schlottau et al. Citation2020; Suarez et al. Citation2020). There are currently no data available on the susceptibility of horses to experimental infection with SARS-CoV-2, but horses were refractory to experimental infection with MERS-CoV-1 (Vergara-Alert et al. Citation2017).
The susceptibility to experimental infection cannot be used to directly extrapolate the likelihood of natural infection in the same species. To exemplify this, cats experimentally infected with SARS-CoV-2 transmitted the virus to one of three uninfected cats housed in adjacent cages (Shi et al. Citation2020). The experimental conditions (high load of the inoculated virus and prolonged indirect exposure of naïve cats to infected cats) would be unlikely to be replicated in real life and hence, the ability of SARS-CoV-2 to be transmitted from naturally infected cats to other cats, or to humans, needs to be further investigated.
Natural infection with SARS-CoV-2 has, however, been reported to the World Organisation for Animal Health for 26 different animal species (WOAH Citation2022) including domestic cats and several species of wild felids, dogs and red foxes (Vulpes vulpes), several mustelid spp., non-human primates including both apes and monkeys, several species of deer, and a variety of other mammals across widely diverse orders. As of 31 December 2022, a total of 699 outbreaks (one or more epidemiologically linked cases) of SARS-CoV-2 infection in animals have been reported from 36 countries in the Americas, Africa, Asia and Europe. There is also serological evidence suggesting that a pet rabbit (Fritz et al. Citation2022) and a horse (Pusterla et al. Citation2022) may have become infected with the virus. However, nasal secretions from 667 horses with acute onset of fever and respiratory signs that were submitted to a diagnostic laboratory in California (USA) from January to December 2020 were all negative for SARS-CoV-2 by quantitative PCR (Lawton et al. Citation2022). In addition, 633 serum samples from 587 healthy racing Thoroughbred horses from California, collected between July and September 2020 during the time of a confirmed circulation of SARS-CoV-2 among the racetrack personnel, were also negative for SARS-CoV-2 antibodies. Altogether, these data suggest that SARS-CoV-2 infection of the horse is rare. Similarly, there are no reports of SARS-CoV-1 infection of horses, even though both the SARS-CoV-1 and SARS-CoV-2 spike was shown to bind to the equine ACE2 receptor in vitro, albeit with lower affinity than to human ACE2 (Lan et al. Citation2022).
This underscores the ability of the virus to infect a variety of hosts, despite its clear preference for humans. Most of the natural SARS-CoV-2 infections in animals were linked to contact with infected people and hence most likely represented “reverse zoonosis,” defined as human-to-animal spread (Barrs et al. Citation2020; Musso et al. Citation2020; Segalés et al. Citation2020). The frequency of such reverse zoonotic transmissions is not well understood but has likely been low (Kannekens-Jager et al. Citation2022). However, the ability of the virus to evolve and adjust to the new hosts may occasionally lead to sustained animal-to-animal transmission, as presumably occurred among farmed mink in Europe (Rasmussen et al. Citation2021; Wolters et al. Citation2022) and free-ranging deer in the USA (Hale et al. Citation2022; Kuchipudi et al. Citation2022; Roundy et al. Citation2022).
SARS-CoV-2 infection of dogs and cats
Among domesticated animals that have close contact with people, cats have been most frequently reported to be naturally infected with SARS-CoV-2 based on both virological and serological data. The frequency of detection of SARS-CoV-2 antibodies in cats differed between different studies (). Those discrepancies most likely reflect differences in the sampled populations, sampling strategies, the type of serological tests employed, and interpretation of the results obtained.
Table 2. Seroprevalence of SARS-CoV-2 antibody among cats.
The most likely source of SARS-CoV-2 infection in cats was people, although other sources cannot be fully excluded (e.g. cat-to-cat or environmental contamination). Blood samples for various studies were collected from cats during the pandemic spread of SARS-CoV-2 among people in the same geographical area, but the level of contact between cats and SARS-CoV-2 infected people was unknown in most studies (). When such data for individual animals were available, the frequency of infection among cats with known exposure to SARS-CoV-2 was higher than the frequency of infection among cats with unknown exposure to the virus. This can be exemplified by a French study where 10/47 (21%) cats from COVID-19 households were positive for SARS-CoV-2 antibodies as compared to 1/16 (6%) cats from households with unknown COVID-19 status (Fritz et al. Citation2021).
The serological tests employed differed between studies. Several authors employed more than one test, and only sera that tested positive for SARS-CoV-2 antibodies in all tests used were considered positive (Dileepan et al. Citation2021; Fritz et al. Citation2021). While such an approach increased the specificity of results, it also decreased sensitivity, so the number of seropositive cats was likely to be underestimated in some of the studies. Similar issues have been described for serological testing for SARS-CoV-2 exposure in people. In one study, antibodies to S protein were detectable for longer than neutralising antibodies to the nucleocapsid protein after SARS-CoV-2 infection (Fenwick et al. Citation2021). As a result, serological tests based on detection of the S and nucleocapsid proteins produced similar results during the acute phase of infection, but the latter considerably underestimated the level of seropositive individuals in the general population with various histories of SARS-CoV-2 exposure.
Several studies demonstrated that dogs can also be naturally infected with SARS-CoV-2. In most of those studies, the prevalence of SARS-CoV-2 antibodies was lower in dogs than in cats from the same geographical area (Dileepan et al. Citation2021; Fritz et al. Citation2021; Hamer et al. Citation2021), in agreement with results of experimental transmission studies (Bosco-Lauth et al. Citation2020; Shi et al. Citation2020).
Overall, the available data indicate that both dogs and cats can be naturally infected with SARS-CoV-2 and that the most likely source of infection is SARS-CoV-2-infected people. The reported frequency of infection with SARS-CoV-2 in pets owned by COVID-19-affected people varied between studies, which most likely reflects factors such as the variant of the virus involved, the level of close contact between infected people and their pets, and individual differences between pets (e.g. breed, age, presence of concurrent infections, etc).
Clinical signs in animals infected with SARS-CoV-2
Little is known about clinical disease associated with SARS-CoV-2 infection in animals. Experimental infection with SARS-CoV-2 produced no clinical signs in dogs and adult cats (Bosco-Lauth et al. Citation2020). Juvenile cats were more likely to develop disease than adult cats based on severity of pathological lesions described (Shi et al. Citation2020). In agreement with those studies, SARS-CoV-2 RNA has been detected in samples from naturally infected, clinically healthy cats and dogs (Sit et al. Citation2020; Calvet et al. Citation2021; Hamer et al. Citation2021). The clinical signs observed in mink on SARS-CoV-2-infected farms were mostly restricted to watery nasal discharge, although some animals developed severe respiratory disease (Oreshkova et al. Citation2020). There are, however, some reports of SARS-CoV-2 detection in pets that displayed clinical signs resembling those of COVID-19 infection in people, including lethargy, anorexia, diarrhoea, vomiting, breathing difficulties, and cough (Sailleau et al. Citation2020; Calvet et al. Citation2021; Hamer et al. Citation2021). Similar clinical signs were reported in SARS-CoV-2-infected large cats (tigers and lions) in zoos (McAloose et al. Citation2020; Grome et al. Citation2022). These clinical signs are non-specific and can be induced by several infectious or non-infectious causes. Hence, such reports should be evaluated with caution, as it is difficult to conclusively establish a causal link between SARS-CoV-2 infection and disease for an individual naturally infected animal.
Factors affecting cross-species transmission of viruses
Successful cross-species transmission of a virus from an animal to a person, with subsequent person-to-person spread, as has occurred with SARS-CoV-2, is fortunately a rare event. Several well-timed coincidences need to happen for this to occur (Parrish et al. Citation2008; Plowright et al. Citation2017). These are briefly outlined below.
Vicinity of the infected host animal to a potential new host
To start with, the infected animal needs to find itself in close vicinity to the prospective new host. A major source of new emerging infections for humans are viruses that circulate in wildlife among animals that rarely, if ever, find themselves near people (Epstein and Anthony Citation2017). Human expansion coupled with activities such as deforestation, urbanisation, illegal wildlife, or bushmeat trade all tip this balance in favour of potential “spillover” events to occur (Parrish et al. Citation2008; Hassell et al. Citation2017; Plowright et al. Citation2017). It is probably not a coincidence that the emergence of both SARS-CoV-1 and SARS-CoV-2 has strong epidemiological links to live-animal markets (Lytras et al. Citation2021). A variety of animals, both farmed and caught locally from the wild, are sold at such markets. In addition, carcasses of wildlife that were either caught or farmed elsewhere can be transported to the markets using established cold chains, both legal and illegal (Lytras et al. Citation2021). High levels of stress among animals at the market, coupled with crowded conditions, create a perfect opportunity for a cross-species transfer of viruses between animals or from animals to humans. During the 2003 SARS-CoV-1 outbreak, 66/508 (13%) animal traders from three markets in the Guangdong province of China were positive for SARS-CoV-1 antibodies with the seroprevalence reaching 16/22 (73%) among traders of palm civets (Paradoxurus hermaphroditus; CDC Citation2003). Only 4/137 (3%) hospital workers sampled at the same time had SARS-CoV-1 antibodies, supporting the view that close contact with live animals at the market was a significant risk factor for SARS-CoV-1 infection. While the first cases of SARS-CoV-2 infection were also epidemiologically linked to a live-animal market in China, similar serological data to support this connection are not available, most likely due to the rapid spread of SARS-CoV-2 and presumed lack of appropriate samples from the early phases of the outbreak.
Intermediate and reservoir hosts
Transmission of viruses from wildlife to humans can occur directly or indirectly via domesticated animals. Livestock and some pet animals (e.g. cats or hunting dogs) have more opportunities to come into close contact with potentially infected wildlife than do humans. At the same time, domesticated animals are frequently handled by people, and can therefore act as intermediate hosts for human infection. If the virus establishes itself in the population of domesticated animals, they can also become new reservoir hosts for that virus and be a source of future infections for people. The two terms (reservoir and intermediate host) are not synonymous. This may be exemplified by the role of civet cats in the transmission of SARS-CoV-1 to people during the 2003 outbreak, which was believed to initially occur through contact with infected civet cats at the market (Wang and Eaton Citation2007). Although the rates of SARS-CoV-1 infection among palm civets at the Quandong markets was high, palm civets at the neighbouring farms or from the wild were mostly negative for the virus or virus-specific antibodies (Tu et al. Citation2004; Kan et al. Citation2005; Poon et al. Citation2005). This suggests that infected palm civets were a likely immediate source for human infection (intermediate host) but they were probably infected at the market from other animals, and were unlikely to be the natural wildlife reservoir host for the virus. In contrast, a large proportion of healthy camels in Saudi Arabia were positive for MERS-CoV (Drosten et al. Citation2014). The virus most likely originated in bats and was transmitted at some point in the past to camels, but by the time MERS-CoV was detected in people, the virus circulated endemically among camels in the Arabian Peninsula without causing any overt clinical illness. The camels were the source of infection for people (intermediate hosts), but they also became the reservoir host for the virus.
At present, neither an intermediate nor reservoir host for SARS-CoV-2 has been conclusively determined. Bats are considered to be the most likely reservoir host based on the fact that they harbour a large number of diverse coronaviruses (Lin et al. Citation2017). However, none of the viruses identified from bats thus far is similar enough to SARS-CoV-2 to be considered a direct source of the zoonotic transmission to people (Lytras et al. Citation2021). In addition, the 2019 outbreak was epidemiologically linked to wet animal markets in Wuhan, which is geographically distant from Yunnan, where bat viruses similar to SARS-CoV-2 were identified. Finally, bats were hibernating at the time of SARS-CoV-2’s emergence and no bats were sold at the Wuhan market. Pangolins (Manis javanica) have been suggested as an alternative intermediate host (Lytras et al. Citation2021). While sequence similarity of SARS-CoV-2-like viruses obtained from pangolins to human SARS-CoV-2 is lower than that of bat viruses over the full genome length, their RBD on the S protein is very similar to that of human SARS-CoV-2 (Lytras et al. Citation2021). In fact, pangolin viruses bind to human ACE in vitro better than SARS-CoV-2 (Dicken et al. Citation2021). This led to a suggestion that pandemic SARS-CoV-2 may have arisen by recombination between bat and pangolin SARS-CoV-2-like viruses within an unidentified intermediate host (Lytras et al. Citation2022). Recombination between coronaviruses that leads to emergence of new pathogens has been described before (Focosi and Maggi Citation2022). An example may be an intertangled history of several events of the emergence of new viruses through recombination between canine coronaviruses, feline coronaviruses, and porcine TGEV (Pratelli et al. Citation2021). While most of those recombinant viruses were identified in dogs or cats, one was detected in hospitalised children in Malaysia, further highlighting the ability of coronaviruses to cross species barriers (Vlasova et al. Citation2022).
Availability of suitable receptors on the new host’s cells
Following a chance encounter with a non-natural host, the virus must be able to enter appropriate tissues and establish productive infection in those tissues. The binding between the virus and its receptor is a prerequisite for infection, so this single factor plays an important role in the species-specificity of any virus. Viruses that utilise molecules that are conserved between various animal species or viruses that can utilise more than one receptor for entry tend to have a broader host range than viruses that enter through a highly species-specific receptor. The results of the comparative genomic, evolutionary and structural analysis of ACE2 orthologs from 410 vertebrate species including selected species of fish, amphibians, birds, reptiles and mammals suggested that the SARS-CoV-2 receptor on ACE has very high (18 species), high (28 species) or medium (57 species) likelihood of being able to bind to SARS-CoV-2 S protein (Damas et al. Citation2020). The species with very high and high similarity to human ACE included old-world primates, great apes, other primates, old-world monkeys, some rodents, and cervids, as well as whales and dolphins. Several domesticated animals were within the medium likelihood group, including dog, cat, hamster, cattle, sheep, alpaca, horse and donkey. The predicted binding capacity differed between species from the same order. For example, 9/43 carnivorans scored medium, 9/43 scored low, and 25/43 scored very low, with all medium-scoring carnivorans being felids.
While such analyses can help to assess the theoretical likelihood of cross-species transmission of SARS-CoV-2, they cannot replace the data derived either from transmission studies or from investigations of field cases. To exemplify this, ACE from ferret, American mink (Neovison vison) and European mink (Mustela nivalis) all scored very low for the likelihood of binding SARS-CoV-2, and yet all three animal species are susceptible to SARS-CoV-2 infection, with outbreaks of SARS-CoV-2 among farmed American mink reported on 400 mink farms in six European countries (Boklund et al. Citation2021). In agreement with the importance of RBD for successful cross-species transfer, the viruses that circulated among mink had mutations within the RBD of the S protein that increased the efficiency of viral RBD binding to mink ACE, highlighting the propensity of coronaviruses for change not only through recombination but also through mutations (Su et al. Citation2022).
On-going transmission between new hosts
Finally, for the virus to establish itself in a new host species, enough infectious virus needs to be generated to allow infection to be propagated to another individual from that species. This is not easy for the virus to accomplish, as there are several host- and virus-related restrictions that prevent effective establishment of infection in a non-natural host (Parrish et al. Citation2008; Plowright et al. Citation2017). In addition to the availability of suitable cellular receptors for the virus entry, these include restrictions at various steps of intracellular virus replication or innate immune responses. Even if successful, the virus typically needs to adjust to its new host to allow for a sustained spread between the new hosts (Parrish et al. Citation2008; Plowright et al. Citation2017). Viruses that are highly virulent in the new host may not spread further, as the host is killed before transmission can occur. In the opposite scenario, infection may be initially established only briefly, without overt clinical disease and without further transmission of the virus, as was observed in dogs experimentally infected with SARS-CoV-2 (Shi et al. Citation2020). Such undetected, sporadic spill-over events are likely to occur on a regular basis whenever there is frequent direct or indirect contact between wildlife and people or domesticated animals, for example in rural areas or among people with occupational exposure to wildlife. If some ongoing transmission between the new hosts does occur, the virus can accumulate mutations that are necessary for adaptation to specific restriction factors, with the possibility of subsequent epidemic or pandemic spread within an immunologically naïve population. This is what has been observed with SARS-CoV-2’s spread among people and, on a smaller scale, among farmed mink. Factors such as density of the new hosts in the given area and the frequency of direct or indirect contact between them are likely to be important for the successful adaptation of a virus to the new population.
To illustrate these concepts, antibodies to SARS-CoV-1 were retrospectively detected in 17/938 (1.7%) adults tested in 2001 in Hong Kong, indicating that sporadic infections with SARS-CoV-1-like virus were occurring in Hong Kong at least 2 years before the 2003 pandemic (Zheng et al. Citation2004). Similarly, antibodies reactive with SARS-CoV-2 were retrospectively detected in 8/478 (1.7%) healthy individuals from Saudi Arabia in samples collected between October 2019 and January 2020, i.e. before the pandemic spread of the virus (Mahallawi and Ibrahim Citation2022). All eight seropositive individuals in that study had a history of travel to China within 4 weeks prior to blood collection. In another study, limited serological evidence indicated that SARS-CoV-2-like viruses may have circulated in Africa before the emergence of COVID-19 in China (Souris et al. Citation2022).
Conclusion
Thus far, the spread of SARS-CoV-2 has been largely mediated by human-to-human transmission. However, the virus can infect animals of many different species including pets and livestock. With the notable exception of mink and deer, occasional animal infections have not thus far led to sustained animal-to-animal transmission. The source of animal infections has most likely been human-derived virus, although occasional natural transmission between cats cannot be fully excluded. Considering the capacity of the virus to evolve through mutation and recombination, together with close contact between humans and animals, the on-going frequent transmission of the virus between people poses a risk of the emergence of animal-adapted variants. If such variants remain infectious to humans, this could lead to the establishment of animal reservoirs for the virus, which would complicate control efforts. As such, minimising human-to-animal transmission of SARS-CoV-2 should be considered as part of infection control efforts.
References
- Amer HM. Bovine-like coronaviruses in domestic and wild ruminants. Animal Health Research Reviews 19, 113–24, 2018. https://doi.org/10.1017/S1466252318000117
- Bae DY, Tark D, Moon SH, Oem JK, Kim WI, Park C, Na KJ, Park CK, Oh Y, Cho HS. Evidence of exposure to SARS-CoV-2 in dogs and cats from households and animal shelters in Korea. Animals 12, 2786, 2022. https://doi.org/10.3390/ani12202786
- Bahl P, Doolan C, de Silva C, Chughtai AA, Bourouiba L, MacIntyre CR. Airborne or droplet precautions for health workers treating COVID-19? The Journal of Infectious Diseases 225, 1561–8, 2022. https://doi.org/10.1093/infdis/jiaa189
- Barrs VR, Peiris M, Tam KWS, Law PYT, Brackman CJ, To EMW, Yu VYT, Chu DKW, Perera R, Sit THC. SARS-CoV-2 in quarantined domestic cats from COVID-19 households or close contacts, Hong Kong, China. Emerging Infectious Diseases 26, 3071–4, 2020. https://doi.org/10.3201/eid2612.202786
- Berryhill EH, Magdesian KG, Aleman M, Pusterla N. Clinical presentation, diagnostic findings, and outcome of adult horses with equine coronavirus infection at a veterinary teaching hospital: 33 cases (2012–2018). Veterinary Journal 248, 95–100, 2019. https://doi.org/10.1016/j.tvjl.2019.05.001
- Boklund A, Gortazar C, Pasquali P, Roberts H, Nielsen SS, Stahl K, Stegeman A, Baldinelli F, Broglia A, Van Der Stede Y, et al. Monitoring of SARS-CoV-2 infection in mustelids. European Food Safety Authority Journal 19, e06459, 2021. https://doi.org/10.2903/j.efsa.2021.6459
- Bosco-Lauth AM, Hartwig AE, Porter SM, Gordy PW, Nehring M, Byas AD, VandeWoude S, Ragan IK, Maison RM, Bowen RA. Experimental infection of domestic dogs and cats with SARS-CoV-2: pathogenesis, transmission, and response to reexposure in cats. Proceedings of the National Academy of Sciences of the USA 117, 26382–8, 2020. https://doi.org/10.1073/pnas.2013102117
- Brussow H, Brussow L. Clinical evidence that the pandemic from 1889 to 1891 commonly called the Russian flu might have been an earlier coronavirus pandemic. Microbial Biotechnology 14, 1860–70, 2021. https://doi.org/10.1111/1751-7915.13889
- Bushmaker T, Yinda CK, Morris DH, Holbrook MG, Gamble A, Adney D, Bushmaker C, van Doremalen N, Fischer RJ, Plowright RK, et al. Comparative aerosol and surface stability of SARS-CoV-2 variants of concern. bioRxiv, 2022. https://doi.org/10.1101/2022.11.21.517352
- Calvet GA, Pereira SA, Ogrzewalska M, Pauvolid-Corrêa A, Resende PC, Tassinari WS, Costa AP, Keidel LO, da Rocha ASB, da Silva MFB, et al. Investigation of SARS-CoV-2 infection in dogs and cats of humans diagnosed with COVID-19 in Rio de Janeiro, Brazil. PLoS ONE 16, e0250853, 2021. https://doi.org/10.1371/journal.pone.0250853
- *CDC. Prevalence of IgG antibody to SARS-associated coronavirus in animal traders – Guangdong Province, China, 2003. Morbidity and Mortality Weekly Report 52, 986–7, 2003
- *CDC. Coronaviruses. https://www.cdc.gov/coronavirus/types.html (accessed 29 November 2022). Centers for Disease Control and Prevention, Atlanta, GA, USA, 2020
- Cebra CK, Mattson DE, Baker RJ, Sonn RJ, Dearing PL. Potential pathogens in feces from unweaned llamas and alpacas with diarrhea. Journal of the American Veterinary Medical Association 223, 1806–8, 2003. https://doi.org/10.2460/javma.2003.223.1806
- Chan JF, Yuan S, Kok K, To KK, Chu H, Yang J, Xing F, Liu J, Yip CC, Poon RW, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The Lancet 395, 514–23, 2020a. https://doi.org/10.1016/S0140-6736(20)30154-9
- Chan JF, Zhang AJ, Yuan S, Poon VK, Chan CC, Lee AC, Chan WM, Fan Z, Tsoi HW, Wen L, et al. Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID-19) in a golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clinical Infectious Diseases 71, 2428–46, 2020b. https://doi.org/10.1093/cid/ciaa325
- Cheng YR, Li X, Zhao X, Lin H. Cell entry of animal coronaviruses. Viruses 13, 1977, 2021. https://doi.org/10.3390/v13101977
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nature Microbiology 5, 536–44, 2020. https://doi.org/10.1038/s41564-020-0695-z
- Crossley BM, Mock RE, Callison SA, Hietala SK. Identification and characterization of a novel alpaca respiratory coronavirus most closely related to the human coronavirus 229E. Viruses 4, 3689–700, 2012. https://doi.org/10.3390/v4123689
- Damas J, Hughes GM, Keough KC, Painter CA, Persky NS, Corbo M, Hiller M, Koepfli KP, Pfenning AR, Zhao H, et al. Broad host range of SARS-CoV-2 predicted by comparative and structural analysis of ACE2 in vertebrates. Proceedings of the National Academy of Sciences of the USA 117, 22311–22, 2020. https://doi.org/10.1073/pnas.2010146117
- de Oliveira-Filho EF, de Carvalho OV, Carneiro IO, Fernandes FD, Vaz SN, Pedroso C, Gonzalez-Auza L, Urbieta VC, Kühne A, Mayoral R, et al. Frequent infection of cats with SARS-CoV-2 irrespective of pre-existing enzootic coronavirus immunity, Brazil 2020. Frontiers in Immunology 13, 857322, 2022. https://doi.org/10.3389/fimmu.2022.857322
- de Wit JJS, Cook JKA. Spotlight on avian coronaviruses. Avian Pathology 49, 313–6, 2020. https://doi.org/10.1080/03079457.2020.1761010
- Decaro N, Buonavoglia C. Canine coronavirus: not only an enteric pathogen. Veterinary Clinics of North America: Small Animal Practice 41, 1121–32, 2011. https://doi.org/10.1016/j.cvsm.2011.07.005
- Deng J, Jin Y, Liu Y, Sun J, Hao L, Bai J, Huang T, Lin D, Jin Y, Tian K. Serological survey of SARS-CoV-2 for experimental, domestic, companion and wild animals excludes intermediate hosts of 35 different species of animals. Transboundary and Emerging Diseases 67, 1745–9, 2020. https://doi.org/10.1111/tbed.13577
- Dicken SJ, Murray MJ, Thorne LG, Reuschl AK, Forrest C, Ganeshalingham M, Muir L, Kalemera MD, Palor M, McCoy LE, et al. Characterisation of B.1.1.7 and pangolin coronavirus spike provides insights on the evolutionary trajectory of SARS-CoV-2. bioRxiv, 2021. https://doi.org/10.1101/2021.03.22.436468
- Dileepan M, Di D, Huang Q, Ahmed S, Heinrich D, Ly H, Liang Y. Seroprevalence of SARS-CoV-2 (COVID-19) exposure in pet cats and dogs in Minnesota, USA. Virulence 12, 1597–609, 2021. https://doi.org/10.1080/21505594.2021.1936433
- Drosten C, Kellam P, Memish ZA. Evidence for camel-to-human transmission of MERS coronavirus. New England Journal of Medicine 371, 1359–60, 2014. https://doi.org/10.1056/NEJMc1409847
- Epstein JH, Anthony SJ. Viral discovery as a tool for pandemic preparedness. Revue Scientifique et Technique 36, 499–512, 2017. https://doi.org/10.20506/rst.36.2.2669
- Erles K, Brownlie J. Canine respiratory coronavirus: an emerging pathogen in the canine infectious respiratory disease complex. Veterinary Clinics of North America: Small Animal Practice 38, 815–25, 2008. https://doi.org/10.1016/j.cvsm.2008.02.008
- Fang P, Fang L, Zhang H, Xia S, Xiao S. Functions of coronavirus accessory proteins: overview of the state of the art. Viruses 13, 1139, 2021. https://doi.org/10.3390/v13061139
- Fenwick C, Croxatto A, Coste AT, Pojer F, André C, Pellaton C, Farina A, Campos J, Hacker D, Lau K, et al. Changes in SARS-CoV-2 spike versus nucleoprotein antibody responses impact the estimates of infections in population-based seroprevalence studies. Journal of Virology 95, e01828–20, 2021. https://doi.org/10.1128/JVI.01828-20
- Focosi D, Maggi F. Recombination in coronaviruses, with a focus on SARS-CoV-2. Viruses 14, 1239, 2022. https://doi.org/10.3390/v14061239
- Freuling CM, Breithaupt A, Muller T, Sehl J, Balkema-Buschmann A, Rissmann M, Klein A, Wylezich C, Hoper D, Wernike K, et al. Susceptibility of raccoon dogs for experimental SARS-CoV-2 infection. Emerging Infectious Diseases 26, 2982–5, 2020. https://doi.org/10.3201/eid2612.203733
- Fritz M, Rosolen B, Krafft E, Becquart P, Elguero E, Vratskikh O, Denolly S, Boson B, Vanhomwegen J, Gouilh MA, et al. High prevalence of SARS-CoV-2 antibodies in pets from COVID-19 + households. One Health 11, 100192, 2021. https://doi.org/10.1016/j.onehlt.2020.100192
- Fritz M, de Riols de Fonclare D, Garcia D, Beurlet S, Becquart P, Rosolen SG, Briend-Marchal A, Leroy EM. First evidence of natural SARS-CoV-2 infection in domestic rabbits. Veterinary Sciences 9, 49, 2022. https://doi.org/10.3390/vetsci9020049
- Gaudreault NN, Trujillo JD, Carossino M, Meekins DA, Morozov I, Madden DW, Indran SV, Bold D, Balaraman V, Kwon T, et al. SARS-CoV-2 infection, disease and transmission in domestic cats. Emerging Microbes & Infections 9, 2322–32, 2020. https://doi.org/10.1080/22221751.2020.1833687
- Gilbert PB, Donis RO, Koup RA, Fong Y, Plotkin SA, Follmann D. A COVID-19 milestone attained – a correlate of protection for vaccines. New England Journal of Medicine 387, 2203–6, 2022. https://doi.org/10.1056/NEJMp2211314
- Gorbalenya AE, Enjuanes L, Ziebuhr J, Snijder EJ. Nidovirales: evolving the largest RNA virus genome. Virus Research 117, 17–37, 2006. https://doi.org/10.1016/j.virusres.2006.01.017
- Grome HN, Meyer B, Read E, Buchanan M, Cushing A, Sawatzki K, Levinson KJ, Thomas LS, Perry Z, Uehara A, et al. SARS-CoV-2 outbreak among Malayan tigers and humans, Tennessee, USA, 2020. Emerging Infectious Diseases 28, 833–6, 2022. https://doi.org/10.3201/eid2804.212219
- Guan Y, Zheng BJ, He YQ, Liu XL, Zhuang ZX, Cheung CL, Luo SW, Li PH, Zhang LJ, Guan YJ, et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 302, 276–8, 2003. https://doi.org/10.1126/science.1087139
- Guo Z, Wang Z, Zhang S, Li X, Li L, Li C, Cui Y, Fu R, Dong Y, Chi X, et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerging Infectious Diseases 26, 1583–91, 2020. https://doi.org/10.3201/eid2607.200885
- Gussow AB, Auslander N, Faure G, Wolf YI, Zhang F, Koonin EV. Genomic determinants of pathogenicity in SARS-CoV-2 and other human coronaviruses. Proceedings of the National Academy of Sciences of the USA 117, 15193–9, 2020. https://doi.org/10.1073/pnas.2008176117
- Haake C, Cook S, Pusterla N, Murphy B. Coronavirus infections in companion animals: virology, epidemiology, clinical and pathologic features. Viruses 12, 1023, 2020. https://doi.org/10.3390/v12091023
- Hale VL, Dennis PM, McBride DS, Nolting JM, Madden C, Huey D, Ehrlich M, Grieser J, Winston J, Lombardi D, et al. SARS-CoV-2 infection in free-ranging white-tailed deer. Nature 602, 481–6, 2022. https://doi.org/10.1038/s41586-021-04353-x
- Halfmann PJ, Hatta M, Chiba S, Maemura T, Fan S, Takeda M, Kinoshita N, Hattori SI, Sakai-Tagawa Y, Iwatsuki-Horimoto K, et al. Transmission of SARS-CoV-2 in domestic cats. New England Journal of Medicine 383, 592–4, 2020. https://doi.org/10.1056/NEJMc2013400
- Hamer SA, Pauvolid-Corrêa A, Zecca IB, Davila E, Auckland LD, Roundy CM, Tang W, Torchetti MK, Killian ML, Jenkins-Moore M, et al. SARS-CoV-2 infections and viral isolations among serially tested cats and dogs in households with infected owners in Texas, USA. Viruses 13, 938, 2021. https://doi.org/10.3390/v13050938
- Hassell JM, Begon M, Ward MJ, Fèvre EM. Urbanization and disease emergence: dynamics at the wildlife–livestock–human interface. Trends in Ecology and Evolution 32, 55–67, 2017. https://doi.org/10.1016/j.tree.2016.09.012
- Holmes EC, Goldstein SA, Rasmussen AL, Robertson DL, Crits-Christoph A, Wertheim JO, Anthony SJ, Barclay WS, Boni MF, Doherty PC, et al. The origins of SARS-CoV-2: a critical review. Cell 184, 4848–56, 2021. https://doi.org/10.1016/j.cell.2021.08.017
- Huang AT, Garcia-Carreras B, Hitchings MDT, Yang B, Katzelnick LC, Rattigan SM, Borgert BA, Moreno CA, Solomon BD, Rodriguez-Barraquer I, et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nature Communications 11, 4704, 2020a. https://doi.org/10.1038/s41467-020-18450-4
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 395, 15–21, 2020b. https://doi.org/10.1016/S0140-6736(20)30183-5
- Jackson CB, Farzan M, Chen B, Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nature Reviews: Molecular Cell Biology 23, 3–20, 2022. https://doi.org/10.1038/s41580-021-00418-x
- Kan B, Wang M, Jing H, Xu H, Jiang X, Yan M, Liang W, Zheng H, Wan K, Liu Q, et al. Molecular evolution analysis and geographic investigation of severe acute respiratory syndrome coronavirus-like virus in palm civets at an animal market and on farms. Journal of Virology 79, 11892–900, 2005. https://doi.org/10.1128/JVI.79.18.11892-11900.2005
- Kannekens-Jager MM, de Rooij MMT, de Groot Y, Biesbroeck E, de Jong MK, Pijnacker T, Smit LAM, Schuurman N, Broekhuizen-Stins MJ, Zhao S, et al. SARS-CoV-2 infection in dogs and cats is associated with contact to COVID-19-positive household members. Transboundary and Emerging Diseases 69, 4034–40, 2022. https://doi.org/10.1111/tbed.14713
- Kayode AJ, Banji-Onisile FO, Olaniran AO, Okoh AI. An overview of the pathogenesis, transmission, diagnosis, and management of endemic human coronaviruses: a reflection on the past and present episodes and possible future outbreaks. Pathogens 10, 1108, 2021. https://doi.org/10.3390/pathogens10091108
- Korner RW, Majjouti M, Alcazar MAA, Mahabir E. Of mice and men: the coronavirus MHV and mouse models as a translational approach to understand SARS-CoV-2. Viruses 12, 880, 2020. https://doi.org/10.3390/v12080880
- Kuchipudi SV, Surendran-Nair M, Ruden RM, Yon M, Nissly RH, Vandegrift KJ, Nelli RK, Li L, Jayarao BM, Maranas CD, et al. Multiple spillovers from humans and onward transmission of SARS-CoV-2 in white-tailed deer. Proceedings of the National Academy of Sciences of the USA 119, e2121644119, 2022. https://doi.org/10.1073/pnas.2121644119
- Kutter JS, de Meulder D, Bestebroer TM, Lexmond P, Mulders A, Richard M, Fouchier RAM, Herfst S. SARS-CoV and SARS-CoV-2 are transmitted through the air between ferrets over more than one meter distance. Nature Communications 12, 1653, 2021. https://doi.org/10.1038/s41467-021-21918-6
- Lan J, Chen P, Liu W, Ren W, Zhang L, Ding Q, Zhang Q, Wang X, Ge J. Structural insights into the binding of SARS-CoV-2, SARS-CoV, and hCoV-NL63 spike receptor-binding domain to horse ACE2. Structure 30, 1432–42, 2022. https://doi.org/10.1016/j.str.2022.07.005
- Lawton KOY, Arthur RM, Moeller BC, Barnum S, Pusterla N. Investigation of the role of healthy and sick equids in the COVID-19 pandemic through serological and molecular testing. Animals 12, 614, 2022. https://doi.org/10.3390/ani12050614
- Licitra BN, Duhamel GE, Whittaker GR. Canine enteric coronaviruses: emerging viral pathogens with distinct recombinant spike proteins. Viruses 6, 3363–76, 2014. https://doi.org/10.3390/v6083363
- Lin XD, Wang W, Hao Z, Wang Z, Guo W, Guan X, Wang M, Wang H, Zhou R, Li M, et al. Extensive diversity of coronaviruses in bats from China. Virology 507, 1–10, 2017. https://doi.org/10.1016/j.virol.2017.03.019
- Lytras S, Xia W, Hughes J, Jiang X, Robertson DL. The animal origin of SARS-CoV-2. Science 373, 968–70, 2021. https://doi.org/10.1126/science.abh0117
- Lytras S, Hughes J, Martin D, Swanepoel P, de Klerk A, Lourens R, Kosakovsky Pond SL, Xia W, Jiang X, Robertson DL. Exploring the natural origins of SARS-CoV-2 in the light of recombination. Genome Biology and Evolution 14, evac018, 2022. https://doi.org/10.1093/gbe/evac018
- Mahallawi W, Ibrahim N. Unexpected detection of anti-SARS-CoV-2 antibodies before the declaration of the COVID-19 pandemic. Frontiers in Medicine 9, 923715, 2022. https://doi.org/10.3389/fmed.2022.923715
- Martins M, Boggiatto PM, Buckley A, Cassmann ED, Falkenberg S, Caserta LC, Fernandes MHV, Kanipe C, Lager K, Palmer MV, et al. From deer-to-deer: SARS-CoV-2 is efficiently transmitted and presents broad tissue tropism and replication sites in white-tailed deer. PLoS Pathogens 18, e1010197, 2022. https://doi.org/10.1371/journal.ppat.1010197
- McAloose D, Laverack M, Wang L, Killian ML, Caserta LC, Yuan F, Mitchell PK, Queen K, Mauldin MR, Cronk BD, et al. From people to Panthera: natural SARS-CoV-2 infection in tigers and lions at the Bronx Zoo. mBio 11, e02220–20, 2020. https://doi.org/10.1128/mBio.02220-20
- Michelitsch A, Hoffmann D, Wernike K, Beer M. Occurrence of antibodies against SARS-CoV-2 in the domestic cat population of Germany. Vaccines 8, 772, 2020. https://doi.org/10.3390/vaccines8040772
- Michelitsch A, Schon J, Hoffmann D, Beer M, Wernike K. The second wave of SARS-CoV-2 circulation-antibody detection in the domestic cat population in Germany. Viruses 13, 1009, 2021. https://doi.org/10.3390/v13061009
- *MPI. Registers and Lists for Pests and Diseases. https://www.mpi.govt.nz/biosecurity/how-to-find-report-and-prevent-pests-and-diseases/registers-and-lists/ (accessed 29 November 2022). Biosecurity New Zealand, Ministry for Primary Industries, Wellington, NZ, 2022
- Murray J, Kiupel M, Maes RK. Ferret coronavirus-associated diseases. Veterinary Clinics of North America: Exotic Animal Practice 13, 543–60, 2010. https://doi.org/10.1016/j.cvex.2010.05.010
- Musso N, Costantino A, La Spina S, Finocchiaro A, Andronico F, Stracquadanio S, Liotta L, Visalli R, Emmanuele G. New SARS-CoV-2 infection detected in an Italian pet cat by RT-qPCR from deep pharyngeal swab. Pathogens 9, 746, 2020. https://doi.org/10.3390/pathogens9090746
- Mykytyn AZ, Lamers MM, Okba NMA, Breugem TI, Schipper D, van den Doel PB, van Run P, van Amerongen G, de Waal L, Koopmans MPG, et al. Susceptibility of rabbits to SARS-CoV-2. Emerging Microbes & Infections 10, 1–7, 2021. https://doi.org/10.1080/22221751.2020.1868951
- Oreshkova N, Molenaar RJ, Vreman S, Harders F, Oude Munnink BB, Hakze-van der Honing RW, Gerhards N, Tolsma P, Bouwstra R, Sikkema R, et al. SARS-CoV-2 infection in farmed minks, the Netherlands, April and May 2020. EuroSurveillance 25, 2001005, 2020. https://doi.org/10.2807/1560-7917.ES.2020.25.23.2001005
- Osterrieder N, Bertzbach LD, Dietert K, Abdelgawad A, Vladimirova D, Kunec D, Hoffmann D, Beer M, Gruber AD, Trimpert J. Age-dependent progression of SARS-CoV-2 infection in Syrian hamsters. Viruses 12, 779, 2020. https://doi.org/10.3390/v12070779
- Parrish CR, Holmes EC, Morens DM, Park EC, Burke DS, Calisher CH, Laughlin CA, Saif LJ, Daszak P. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiology and Molecular Biology Reviews 72, 457–70, 2008. https://doi.org/10.1128/MMBR.00004-08
- Pasternak AO, Spaan WJM, Snijder EJ. Nidovirus transcription: how to make sense … ? Journal of General Virology 87, 1403–21, 2006. https://doi.org/10.1099/vir.0.81611-0
- Pastorino B, Touret F, Gilles M, de Lamballerie X, Charrel RN. Prolonged infectivity of SARS-CoV-2 in fomites. Emerging Infectious Diseases 26, 2256–7, 2020. https://doi.org/10.3201/eid2609.201788
- Patterson EI, Elia G, Grassi A, Giordano A, Desario C, Medardo M, Smith SL, Anderson ER, Prince T, Patterson GT, et al. Evidence of exposure to SARS-CoV-2 in cats and dogs from households in Italy. Nature Communications 11, 6231, 2020. https://doi.org/10.1038/s41467-020-20097-0
- Pedersen NC. An update on feline infectious peritonitis: virology and immunopathogenesis. Veterinary Journal 201, 123–32, 2014. https://doi.org/10.1016/j.tvjl.2014.04.017
- Plowright RK, Parrish CR, McCallum H, Hudson PJ, Ko AI, Graham AL, Lloyd-Smith JO. Pathways to zoonotic spillover. Nature Reviews: Microbiology 15, 502–10, 2017. https://doi.org/10.1038/nrmicro.2017.45
- Poon LL, Chu DK, Chan KH, Wong OK, Ellis TM, Leung YH, Lau SK, Woo PC, Suen KY, Yuen KY, et al. Identification of a novel coronavirus in bats. Journal of Virology 79, 2001–9, 2005. https://doi.org/10.1128/JVI.79.4.2001-2009.2005
- Pratelli A, Buonavoglia A, Lanave G, Tempesta M, Camero M, Martella V, Decaro N. One world, one health, one virology of the mysterious labyrinth of coronaviruses: the canine coronavirus affair. Lancet Microbe 2, e646–7, 2021. https://doi.org/10.1016/S2666-5247(21)00282-2
- Pusterla N, Chaillon A, Ignacio C, Smith DM, Barnum S, Lawton KOY, Smith G, Pickering B. SARS-CoV-2 seroconversion in an adult horse with direct contact to a COVID-19 individual. Viruses 14, 1047, 2022. https://doi.org/10.3390/v14051047
- Ramassy L, Oumarou Hama H, Costedoat C, Signoli M, Verna E, La Scola B, Aboudharam G, Barbieri R, Drancourt M. Paleoserology points to coronavirus as possible causative pathogens of the ‘Russian flu’. Microbial Biotechnology 15, 1943–5, 2022. https://doi.org/10.1111/1751-7915.14058
- Rasmussen TB, Fonager J, Jorgensen CS, Lassaunière R, Hammer AS, Quaade ML, Boklund A, Lohse L, Strandbygaard B, Rasmussen M, et al. Infection, recovery and re-infection of farmed mink with SARS-CoV-2. PLoS Pathogens 17, e1010068, 2021. https://doi.org/10.1371/journal.ppat.1010068
- Roundy CM, Nunez CM, Thomas LF, Auckland LD, Tang W, Richison III JJ, Green BR, Hilton CD, Cherry MJ, Pauvolid-Corrêa A, et al. High seroprevalence of SARS-CoV-2 in white-tailed deer (Odocoileus virginianus) at one of three captive cervid facilities in Texas. Microbiology Spectrum 10, e0057622, 2022. https://doi.org/10.1128/spectrum.00576-22
- Sailleau C, Dumarest M, Vanhomwegen J, Delaplace M, Caro V, Kwasiborski A, Hourdel V, Chevaillier P, Barbarino A, Comtet L, et al. First detection and genome sequencing of SARS-CoV-2 in an infected cat in France. Transboundary and Emerging Diseases 67, 2324–8, 2020. https://doi.org/10.1111/tbed.13659
- Schlottau K, Rissmann M, Graaf A, Schon J, Sehl J, Wylezich C, Hoper D, Mettenleiter TC, Balkema-Buschmann A, Harder T, et al. SARS-CoV-2 in fruit bats, ferrets, pigs, and chickens: an experimental transmission study. Lancet Microbe 1, e218–25, 2020. https://doi.org/10.1016/S2666-5247(20)30089-6
- Segalés J, Puig M, Rodon J, Avila-Nieto C, Carrillo J, Cantero G, Terrón MT, Cruz S, Parera M, Noguera-Julián M, et al. Detection of SARS-CoV-2 in a cat owned by a COVID-19-affected patient in Spain. Proceedings of the National Academy of Sciences of the USA 117, 24790–3, 2020. https://doi.org/10.1073/pnas.2010817117
- Shi J, Wen Z, Zhong G, Yang H, Wang C, Huang B, Liu R, He X, Shuai L, Sun Z, et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science 368, 1016–20, 2020. https://doi.org/10.1126/science.abb7015
- Sit THC, Brackman CJ, Ip SM, Tam KWS, Law PYT, To EMW, Yu VYT, Sims LD, Tsang DNC, Chu DKW, et al. Infection of dogs with SARS-CoV-2. Nature 586, 776–8, 2020. https://doi.org/10.1038/s41586-020-2334-5
- Song Z, Xu Y, Bao L, Zhang L, Yu P, Qu Y, Zhu H, Zhao W, Han Y, Qin C. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses 11, 59, 2019. https://doi.org/10.3390/v11010059
- Souris M, Tshilolo L, Parzy D, Lobaloba Ingoba L, Ntoumi F, Kamgaing R, Ndour M, Mbongi D, Phoba B, Tshilolo MA, et al. Pre-pandemic cross-reactive immunity against SARS-CoV-2 among central and west African populations. Viruses 14, 2259, 2022. https://doi.org/10.3390/v14102259
- Sreedharan J, Nair SC, Muttappallymyalil J, Gopakumar A, Eapen NT, Satish KP, Manda V. Case fatality rates of COVID-19 across the globe: are the current draconian measures justified? Journal of Public Health 30, 2575–83, 2021. https://doi.org/10.1007/s10389-021-01491-4
- Stevanovic V, Vilibic-Cavlek T, Tabain I, Benvin I, Kovac S, Hruskar Z, Mauric M, Milasincic L, Antolasic L, Skrinjaric A, et al. Seroprevalence of SARS-CoV-2 infection among pet animals in Croatia and potential public health impact. Transboundary and Emerging Diseases 68, 1767–73, 2021. https://doi.org/10.1111/tbed.13924
- Stout AE, André NM, Jaimes JA, Millet JK, Whittaker GR. Coronaviruses in cats and other companion animals: where does SARS-CoV-2/COVID-19 fit? Veterinary Microbiology 247, 108777, 2020. https://doi.org/10.1016/j.vetmic.2020.108777
- Su C, He J, Han P, Bai B, Li D, Cao J, Tian M, Hu Y, Zheng A, Niu S, et al. Molecular basis of mink ACE2 binding to SARS-CoV-2 and its mink-derived variants. Journal of Virology 96, e0081422, 2022. https://doi.org/10.1128/jvi.00814-22
- Suarez DL, Pantin-Jackwood MJ, Swayne DE, Lee SA, DeBlois SM, Spackman E. Lack of susceptibility to SARS-CoV-2 and MERS-CoV in poultry. Emerging Infectious Diseases 26, 3074–6, 2020. https://doi.org/10.3201/eid2612.202989
- Tellier R. COVID-19: the case for aerosol transmission. Interface Focus 12, 20210072, 2022. https://doi.org/10.1098/rsfs.2021.0072
- Temmam S, Barbarino A, Maso D, Behillil S, Enouf V, Huon C, Jaraud A, Chevallier L, Backovic M, Perot P, et al. Absence of SARS-CoV-2 infection in cats and dogs in close contact with a cluster of COVID-19 patients in a veterinary campus. One Health 10, 100164, 2020. https://doi.org/10.1016/j.onehlt.2020.100164
- Thakor JC, Dinesh M, Manikandan R, Bindu S, Sahoo M, Sahoo D, Dhawan M, Pandey MK, Tiwari R, Emran TB, et al. Swine coronaviruses (SCoVs) and their emerging threats to swine population, inter-species transmission, exploring the susceptibility of pigs for SARS-CoV-2 and zoonotic concerns. Veterinary Quarterly 42, 125–47, 2022. https://doi.org/10.1080/01652176.2022.2079756
- Trichel AM. Overview of nonhuman primate models of SARS-CoV-2 infection. Comparative Medicine 71, 411–32, 2021. https://doi.org/10.30802/AALAS-CM-20-000119
- Tu C, Crameri G, Kong X, Chen J, Sun Y, Yu M, Xiang H, Xia X, Liu S, Ren T, et al. Antibodies to SARS coronavirus in civets. Emerging Infectious Diseases 10, 2244–8, 2004. https://doi.org/10.3201/eid1012.040520
- van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. New England Journal of Medicine 382, 1564–7, 2020. https://doi.org/10.1056/NEJMc2004973
- Vergara-Alert J, van den Brand JM, Widagdo W, Muñoz MT, Raj S, Schipper D, Solanes D, Cordón I, Bensaid A, Haagmans BL, et al. Livestock susceptibility to infection with Middle East respiratory syndrome coronavirus. Emerging Infectious Diseases 23, 232–40, 2017. https://doi.org/10.3201/eid2302.161239
- Vijgen L, Keyaerts E, Moes E, Thoelen I, Wollants E, Lemey P, Vandamme AM, Van Ranst M. Complete genomic sequence of human coronavirus OC43: molecular clock analysis suggests a relatively recent zoonotic coronavirus transmission event. Journal of Virology 79, 1595–604, 2005. https://doi.org/10.1128/JVI.79.3.1595-1604.2005
- Villanueva-Saz S, Giner J, Tobajas AP, Pérez MD, González-Ramírez AM, Macías-León J, González A, Verde M, Yzuel A, Hurtado-Guerrero R, et al. Serological evidence of SARS-CoV-2 and co-infections in stray cats in Spain. Transboundary and Emerging Diseases 69, 1056–64, 2022a. https://doi.org/10.1111/tbed.14062
- Villanueva-Saz S, Martínez M, Giner J, González A, Tobajas AP, Pérez MD, Lira-Navarrete E, González-Ramírez AM, Macías-León J, Verde M, et al. A cross-sectional serosurvey of SARS-CoV-2 and co-infections in stray cats from the second wave to the sixth wave of COVID-19 outbreaks in Spain. Veterinary Research Communications, 1–15, 2022b. https://doi.org/10.1007/s11259-022-10016-7
- Virtanen J, Aaltonen K, Kegler K, Venkat V, Niamsap T, Kareinen L, Malmgren R, Kivelä O, Atanasova N, Österlund P, et al. Experimental infection of mink with SARS-COV-2 Omicron variant and subsequent clinical disease. Emerging Infectious Diseases 28, 1286–8, 2022. https://doi.org/10.3201/eid2806.220328
- Vlasova AN, Saif LJ. Bovine coronavirus and the associated diseases. Frontiers in Veterinary Science 8, 643220, 2021. https://doi.org/10.3389/fvets.2021.643220
- Vlasova AN, Diaz A, Damtie D, Xiu L, Toh TH, Lee JS, Saif LJ, Gray GC. Novel canine coronavirus isolated from a hospitalized patient with pneumonia in east Malaysia. Clinical Infectious Diseases 74, 446–54, 2022. https://doi.org/10.1093/cid/ciab456
- Wang LF, Eaton BT. Bats, civets and the emergence of SARS. Current Topics in Microbiology and Immunology 315, 325–44, 2007. https://doi.org/10.1007/978-3-540-70962-6_13
- Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. Journal of the American Medical Association 323, 1843–4, 2020. https://doi.org/10.1001/jama.2020.3786
- *WHO. WHO MERS-CoV Global Summary and Risk Assessment. World Health Organization, Geneva, Switzerland, 2016
- *WHO. Origins of SARS-CoV-2. https://apps.who.int/iris/bitstream/handle/10665/332197/WHO-2019-nCoV-FAQ-Virus_origin-2020.1-eng.pdf (accessed 29 November 2022). World Health Organization, Geneva, Switzerland, 2020
- *WOAH. SARS-CoV-2 in Animals – Situation Report 20. https://www.woah.org/app/uploads/2023/01/sars-cov-2-situation-report-20.pdf (accessed 6 March 2023). World Organisation for Animal Health, Paris, France, 2022
- Wolters WJ, de Rooij MMT, Molenaar RJ, de Rond J, Vernooij JCM, Meijer PA, Oude Munnink BB, Sikkema RS, van der Spek AN, Spierenburg MAH, et al. Manifestation of SARS-CoV-2 infections in mink related to host-, virus- and farm-associated factors, the Netherlands 2020. Viruses 14, 1754, 2022. https://doi.org/10.3390/v14081754
- Woo PC, Lau SK, Lam CS, Lau CC, Tsang AK, Lau JH, Bai R, Teng JL, Tsang CC, Wang M, et al. Discovery of seven novel mammalian and avian coronaviruses in the genus Deltacoronavirus supports bat coronaviruses as the gene source of Alphacoronavirus and Betacoronavirus and avian coronaviruses as the gene source of Gammacoronavirus and Deltacoronavirus. Journal of Virology 86, 3995–4008, 2012. https://doi.org/10.1128/JVI.06540-11
- Woo PYC. Family: Coronaviridae. https://ictv.global/report/chapter/coronaviridae/coronaviridae (accessed 13 March 2023). International Committee on Taxonomy of Viruses, 2023
- Zappulli V, Ferro S, Bonsembiante F, Brocca G, Calore A, Cavicchioli L, Centelleghe C, Corazzola G, De Vreese S, Gelain ME, et al. Pathology of coronavirus infections: a review of lesions in animals in the One-Health perspective. Animals 10, 2377, 2020. https://doi.org/10.3390/ani10122377
- Zhang Q, Zhang H, Gao J, Huang K, Yang Y, Hui X, He X, Li C, Gong W, Zhang Y, et al. A serological survey of SARS-CoV-2 in cat in Wuhan. Emerging Microbes & Infections 9, 2013–9, 2020. https://doi.org/10.1080/22221751.2020.1817796
- Zhang G, Li B, Yoo D, Qin T, Zhang X, Jia Y, Cui S. Animal coronaviruses and SARS-CoV-2. Transboundary and Emerging Diseases 68, 1097–110, 2021. https://doi.org/10.1111/tbed.13791
- Zhao Y, Wang J, Kuang D, Xu J, Yang M, Ma C, Zhao S, Li J, Long H, Ding K, et al. Susceptibility of tree shrew to SARS-CoV-2 infection. Scientific Reports 10, 16007, 2020. https://doi.org/10.1038/s41598-020-72563-w
- Zheng BJ, Wong KH, Zhou J, Wong KL, Young BW, Lu LW, Lee SS. SARS-related virus predating SARS outbreak, Hong Kong. Emerging Infectious Diseases 10, 176–8, 2004. https://doi.org/10.3201/eid1002.030533
- Zhou P, Yang X, Wang X, Hu B, Zhang L, Zhang W, Si H, Zhu Y, Li B, Huang C, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–3, 2020. https://doi.org/10.1038/s41586-020-2012-7
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine 382, 727–33, 2020. https://doi.org/10.1056/NEJMoa2001017
- *Non-peer-reviewed
