Reports of neoplasia in deer remain rare (Hill and Staples Citation1999), despite the conviction that as deer farming became more common, a greater number of pathological processes, including tumours, would be recognised in deer (Pérez et al. Citation1998). Skin tumours are among the most common neoplasms reported in red deer (Cervus elaphus) and are usually papillomavirus-associated dermal fibropapillomas and papillomas (Erdélyi et al. Citation2009; Vaatstra et al. Citation2014; Garcês et al. Citation2020). Additional reports of cutaneous and subcutaneous tumours in red deer include malignant schwannoma and dermal malignant melanoma (Pérez et al. Citation1998; Scandrett and Wobeser Citation2004). In related deer species, subcutaneous dermoid cysts have been described in caribou (Rangifer tarandus) (Wobeser et al. Citation2009) and cutaneous fibromas in predominantly male white-tailed deer (Odocoileus virginianus) (Berry Citation1925; Friend Citation1967; Sundberg and Nielsen Citation1982).
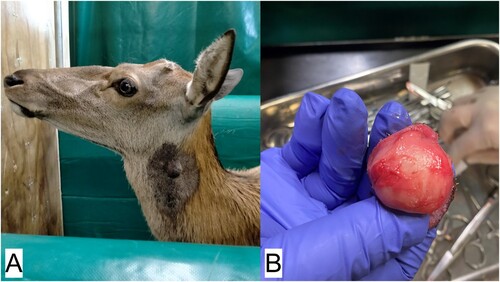
A 17-year-old, 187-kg, castrated, male red deer was examined at the Massey University (Palmerston North, NZ) deer unit on 27 January 2022, with a spherical, deep dermal mass about 3 cm in diameter, raised above the surrounding skin level. The mass was identified on the upper left neck about 7 cm below the base of the left ear, at the angle of the mandible (a). At re-examination 1 week later, the mass had almost doubled in size. The mass appeared discrete from the underlying tissue, so a decision was made to remove it that day, while the size of the mass was still manageable.
Figure 1. Images from surgery undertaken to remove a spherical, deep dermal mass, about 3 cm in diameter, from the upper neck of a 17-year-old castrated male red deer (Cervus elaphus): A) the site of the mass on the left side of the deer’s neck at the angle of the jaw; B) the spherical mass after removal.
The deer had been abandoned by its mother after dystocia and was hand reared. It had been castrated at a very young age and has resided at the deer unit ever since and been in good health. The deer had been paddocked alone most of its life, excluding short periods when it was paddocked with other deer that had undergone surgical or medical procedures and needed to be separated from the main herd.
The animal was placed in a Heenan hydraulic crush (Farmquip Ltd., Napier, NZ) and lightly restrained during the surgery. The sides of the crush were manipulated to give good access to the surgery site. The deer was sedated with 0.2 mg/kg of 5% xylazine (Phoenix Pharm Distributors Ltd., Auckland, NZ) given IM into the neck, and a local anaesthetic block using SC 2% lignocaine (Nopaine 2%; Phoenix Pharm Distributors Ltd.) was placed around the mass. The site was surgically scrubbed and prepared while the local anaesthetic block took effect. An elliptical skin incision was made, and the mass was removed via blunt dissection, with a 1-cm margin (b). The wound was closed using simple interrupted suture pattern with 4 metric PDS absorbable suture (Ethicon, Somerville, NJ, USA). Bleeding was minimal and there were no complications during the procedure, although the deer lay down once the crush was opened. The sedation was reversed using 0.2 mg/kg yohimbine IV (Reversal Injection; Phoenix Pharm Distributors Ltd.). Post-operative analgesia was administered using 0.5 mg/kg SC meloxicam (Metacam; Boehringer Ingelheim (NZ) Ltd., Auckland, NZ). No antibiotics were given. The excised mass was placed in 10% buffered formalin and submitted for histology to Massey University (Palmerston North, NZ) Pathobiology Department.
The deer recovered well from the surgery. There was slight post-surgical swelling on day 5, which rapidly improved, with no swelling evident 2 weeks after surgery.
The mass was sectioned into four and fixed in 10% neutral buffered formalin for 48 hours, processed and embedded into paraffin blocks. Routine H&E stains were performed on multiple 5-µm sections of tissue cut from the formalin-fixed, paraffin-embedded blocks. One section of the mass was additionally stained with Masson’s trichrome and phosphotungstic acid haematoxylin (PTAH).
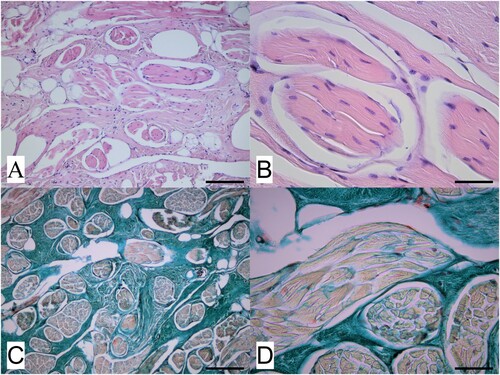
Histological examination revealed an unencapsulated but generally well demarcated deep dermal proliferation of muscle bundles below the overlying haired skin. Muscle bundles were generally of small to medium uniform size, well differentiated, and surrounded and separated by an extensive collagen stroma (A and B), confirmed by Masson’s trichrome staining (C and D). PTAH staining did not reveal the presence of obvious cross-striations within the muscle fibres comprising the bundles, typical of a smooth muscle lineage. These findings were considered consistent with a benign smooth muscle hamartoma.
Figure 2. Photomicrographs of a dermal mass removed from the neck of a red deer (Cervus elaphus) showing A) a representative area of the mass located within the deep dermis and comprising well differentiated muscle bundles surrounded and often widely separated by an extensive collagen stroma (H&E; bar = 75 µm) and B) detail of several muscle bundles within the mass and, in the upper right of the image, an area of collagen stroma (H&E; bar = 35 µm). Slides shown in C) (low magnification; bar = 75 µm) and D (high magnification; bar = 35 µm) are stained with Masson’s trichrome stain, and show the different components of the mass, with muscle bundles staining pale orange-red while the surrounding collagen stroma stains blue-green.
Hamartomas are focal malformations comprising the abnormal proliferation of mature mesenchymal or epithelial tissues normally present within the site of development (Mauldin and Peters-Kennedy Citation2016; Jacinto et al. Citation2021). They are typically benign and present as a solitary mass or plaque, and, while sometimes congenital, may also be an acquired lesion. Smooth muscle hamartomas are benign dermal proliferations of smooth muscle cells arranged in bundles with disorganised orientation (Sourreil et al. Citation1969). Lesions typically grow for several years, are asymptomatic, and do not undergo malignant transformation (Holst et al. Citation2002; Bartyzel et al. Citation2017). Hamartomas are rarely reported in production animals, although they have been described as congenital lesions in calves (Martin et al. Citation2021). They may be under-diagnosed in production animals due to the lack of histopathological examination of excised masses, and a lack of urgency to remove masses that are slow-growing and appear well circumscribed from the surrounding tissue. Their cause is unknown, but when growth is rapid, as in this case, they can give the impression of a malignant neoplastic lesion. A literature search in Scopus (www.scopus.com), on 23 March 2023, using the search terms “hamartoma AND cervus elaphus” retrieved one paper in a closely related species, a case report of a pulmonary hamartoma in an elk (Cervus elaphus canadensis) calf (Boggiatto et al. Citation2023), confirming how unusual this diagnosis is in deer.
Acknowledgements
The authors would like to acknowledge the help of Geoff Purchas in the management of this case.
References
- Bartyzel BJ, Max A, Gruszczyńska J, Sobczak-Filipiak M, Męcik-Kronenberg T, Pankowski F. Hamartoma: a rare developmental disorder. Medycyna Weterynaryjna 73, 202–7, 2017. https://doi.org/10.21521/mw.5683
- Berry E. Fibroma in a Virginia deer. Journal of Mammalogy 6, 130, 1925. https://doi.org/10.1093/jmammal/6.2.130
- Boggiatto PM, Olsen SC, Palmer MV. Pulmonary hamartoma in an elk calf. Journal of Veterinary Diagnostic Investigation 35, 193–5, 2023. https://doi.org/10.1177/10406387221141091
- Erdélyi K, Gál J, Sugár L, Ursu K, Forgách P, Szeredi L, Steineck T. Papillomavirus-associated fibropapillomas of red deer (Cervus elaphus). Acta Veterinaria Hungarica 57, 337–44, 2009. https://doi.org/10.1556/avet.57.2009.2.14
- Friend M. Skin tumors in New York deer. Bulletin of the Wildlife Disease Association 3, 102–4, 1967. https://doi.org/10.7589/0090-3558-3.3.102
- Garcês A, Pires I, Savini F, Scagliarini A, Gallina L. Cutaneous fibropapilloma in a red deer (Cervus elaphus) associated with Cervus elaphus papillomavirus in Portugal. Journal of Wildlife Diseases 56, 636–9, 2020. https://doi.org/10.7589/2019-03-070
- Hill F, Staples P. Common diseases of deer diagnosed at the AgriQuality Animal Health Laboratory, Palmerston North (1995–98). Proceedings of a Deer Course for Veterinarians. Deer Branch of the New Zealand Veterinary Association 16, 109–16, 1999
- Holst VA, Junkins-Hopkins JM, Elenitsas R. Cutaneous smooth muscle neoplasms: clinical features, histologic findings, and treatment options. Journal of the American Academy of Dermatology 46, 477–94, 2002. https://doi.org/10.1067/mjd.2002.121358
- Jacinto JG, Bolcato M, Sheahan BJ, Muscatello LV, Gentile A, Avallone G, Benazzi C. Congenital tumours and tumour-like lesions in calves: a review. Journal of Comparative Pathology 184, 84–94, 2021. https://doi.org/10.1016/j.jcpa.2021.02.003
- Martin B, Mason R, Lawrence K, Castillo-Alcala F. Congenital oral vascular hamartoma in a Jersey cross calf. New Zealand Veterinary Journal 69, 131–3, 2021. https://doi.org/10.1080/00480169.2020.1823280
- *Mauldin EA, Peters-Kennedy J. Integumentary system. In: Maxie MG (ed). Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals: Volume 1. Sixth Edition. Pp 509–736. Elsevier, St Louis, MO, USA, 2016
- Perez J, Martin De Las Mulas J, Arenas A, Luque I, Carrasco L. Malignant schwannoma in a red deer (Cervus elaphus). Veterinary Record 143, 585–7, 1998. https://doi.org/10.1136/vr.143.21.585
- Scandrett B, Wobeser G. Malignant melanoma in a captive red deer (Cervus elaphus elaphus). Journal of Wildlife Diseases 40, 808–10, 2004. https://doi.org/10.7589/0090-3558-40.4.808
- Sourreil P, Beylot C, Delfour M. Hamartoma caused by hyperplasia of the arrectores pilorum in a 1-month-old infant. Bulletin de la Société Française de Dermatologie et de Syphiligraphie 76, 602, 1969
- Sundberg JP, Nielsen SW. Prevalence of cutaneous fibromas in white-tailed deer (Odocoileus virginianus) in New York and Vermont. Journal of Wildlife Diseases 18, 359–60, 1982. https://doi.org/10.7589/0090-3558-18.3.359
- Vaatstra B, Munday J, Morriss P. Pigmented chin papillomas in red (Cervus elaphus) × wapiti (Cervus canadensis) stags associated with a novel papillomavirus. New Zealand Veterinary Journal 62, 96–9, 2014. https://doi.org/10.1080/00480169.2013.840943
- Wobeser G, Bollinger T, Neimanis A, Beckmen K. Dermoid cysts in caribou. Journal of Wildlife Diseases 45, 505–7, 2009. https://doi.org/10.7589/0090-3558-45.2.505
