Mammary neoplasia is uncommon in the mare and often mistaken for mastitis (Reppas et al. Citation1996). Incidence rates of mammary tumours in horses of 0.1–1.9% are reported, with carcinomas the most common (Brito et al. Citation2008). Due to the low incidence, and similarity of initial signs to those of mastitis, diagnosis is often delayed until after antibiotic and anti-inflammatory treatment has failed (Boyce and Goodwin Citation2017). Mares are often, therefore, presented in an advanced stage of disease with a poor prognosis (Ferreira Júnior et al. Citation2019). Due to the infrequency of mammary neoplasia in horses it has been difficult to develop an evidence based approach to treatment. Studies in domestic animals report a low success rate with conservative management of these neoplasms. Combined with their highly aggressive and metastatic nature, surgical excision has been perceived as the treatment of choice in the initial management of these neoplasms (Seahorn et al. Citation1992).
Mammary tubulopapillary carcinomas have been reported in multiple species, including horses. They are more malignant than other forms of mammary carcinoma; lymphovascular and lymph node metastasis occurs commonly (Goldschmidt et al. Citation2016).
This case report describes the surgical treatment of a mare with advanced bilateral mammary tubulopapillary carcinoma and describes the use of a barbed wound closure device to reduce surgical dead space to aid in complete wound closure.
An 18-year-old, Thoroughbred, multiparous, barren mare was presented to the referring veterinarian with an enlarged left mammary gland during a routine reproduction examination. Over the next 3 months the mare received three courses of anti-inflammatory drugs and antibiotic therapy based on culture and sensitivity, but clinical signs persisted. The left mammary gland was scanned, revealing a mass in the interstitial tissue. The mare was then referred for further investigation, 3.5 months after initial presentation.
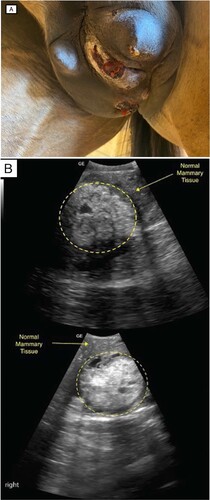
On presentation, the mare underwent a general physical examination, the results of which were within normal limits except that the left mammary gland was enlarged and firm, with three ulcerative lesions (A). No pain response was elicited upon palpation. The teat was also firm and enlarged. Palpation of the right gland revealed some firm masses throughout, with a normal teat. Serosanguinous secretions were easily expressed from each teat.
Figure 1. (A) Photograph of lesions on the mammary gland of an 18-year-old Thoroughbred mare, showing visible enlargement of the left gland with multiple exudative ulcerated lesions and discharge from the left teat. (B) Ultrasound images of the left (top) and right (bottom) mammary glands showing areas of mixed echogenicity with microlobulated margins and an abrupt interface between the mass and normal mammary tissue (circle).
A jugular blood sample was submitted for routine haematology and biochemistry (including serum amyloid A) and all parameters were within normal limits.
Ultrasound examination of both glands was performed using a 2–5 MHz curved array transducer. The left gland and teat revealed extensive areas of mixed echogenicity with microlobulated margins and an abrupt interface between the mass and normal mammary tissue in some areas (B) with more diffuse and generalised margins elsewhere. The right gland contained multiple focal areas of mixed echogenicity with microlobulated margins, and an abrupt interface between the mass and normal mammary tissue (B). The larger focal areas were located closer to the intermammary groove, with smaller focal patches seen laterally. The right teat appeared normal.
Due to the extent of the pathology in both glands, and the owner’s desire to maximise the mare’s breeding potential, the treatment elected was bilateral mastectomy. The authors acknowledge that further investigation and potential staging of any metastatic disease would have been appropriate in this case. However, these were not performed due to financial constraints of the owner and their desire to proceed to surgery irrespective of the prognosis.
Prior to surgery, the mare was premedicated with 0.1 mg/kg morphine (Hameln Pharma, Hameln, Germany) and 0.04 mg/kg acepromazine (Acezine 10; Ethical Agents Ltd., Auckland, NZ) given IM, and 6.6 mg/kg gentamicin (Gentamax 100; Ceva Animal Health Ltd., Gelenorie, Australia), 4.4 mg/kg ceftiofur (Calefur 4 g; Dechra New Zealand, Paraparaumu, NZ) and 1.1 mg/kg flunixin meglumine (Colix; Randlab, Revesby, Australia) given IV. The mare was then anaesthetised routinely with 1.1 mg/kg xylazine (Xylazine 10%; PhoenixPharm, Auckland, NZ), 3.3 mg/kg ketamine (Randlab) and 0.05 mg/kg diazepam (Pamlin; CEVA) given IV, and placed in dorsal recumbency. The site was routinely prepared for aseptic surgery. An elliptical skin incision was made immediately lateral to each teat, preserving as much skin as possible to aid with closure. Using blunt dissection, the entire udder was debulked. An electrocautery device (LigaSure; Covidien/Medtronic, Minneapolis, MN, USA) was used to seal blood vessels, reducing blood loss. Major blood vessels (the external pudendal artery and veins, and the superficial epigastric veins) were electrosurgically sealed and then ligated with 0 polyglactin 910 (Vicryl; Ethicon, Somerville, NJ, USA). The surgical site was lavaged with sterile saline. To reduce the extensive dead space created, two barbed suture wound closure devices (V-Loc 0-USP, 3.5 M; Covidien, Dublin, Ireland) were used in a multilayer walking pattern. This enabled apposition of the incisional margins with minimal tension and reduced dead space. It also reduced surgical time as knots were not required between layers. A SC suture, in a simple continuous pattern, was placed along the incisional margin using 2-0 polyglactin 910. Finally, the skin was closed using 1 polyprolene (Prolene; Ethicon) in a cruciate pattern. A sterile stent tie-over dressing was placed to protect the surgical site during recovery.
The mare recovered uneventfully. Following removal, cut sections of both glands were sent for histological analysis.
The mare remained hospitalised for 3 days on 6.6 mg/kg gentamicin (Gentamax 100; CEVA) once daily, 4.4 mg/kg ceftiofur (Calefur 4 g; Dechra) twice daily, and 1.1 mg/kg flunixin meglumine (Colix; Randlab) twice daily, all given IV. The stent was removed the day after surgery, with the surgical site remaining clean and dry during the remainder of hospitalisation. On the day of discharge, the mare was given 6.6 mg/kg long-acting ceftiofur (Excede; Zoetis, Auckland, NZ) IM which was repeated 4 days later (for a total of 10-days of antimicrobial coverage) and 4.4 mg/kg phenylbutazole (PhenylBute, Dechra) given orally twice daily for 4 days. Skin sutures were removed 14 days after surgery, with good appositional healing and no evidence of wound dehiscence. Two months after removal of the skin sutures, the referring veterinarian reported a 3-cm separation of the surgical site at the most distal point with no discharge or signs of inflammation or infection.
The mare was served twice over the remainder of the breeding season but did not conceive. If she had, it was planned to supplement the foal with colostrum and immediately foster it onto another mare. This remains the plan for subsequent breeding seasons.
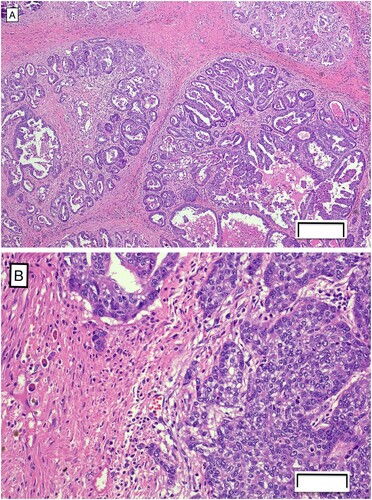
Histologic examination revealed both samples contained neoplasm extending from the deep dermis to all other tissue margins. The neoplasm consisted of epithelial cells forming tubules and papillary projections, as well as cystic and solid areas supported by scant fibrovascular stroma (A). The cystic areas and papillary projections were lined by one to multiple layers of cells. Several small clusters of cells invaded beyond the basement membrane and were surrounded by abundant fibrous tissue, which is an important feature of malignancy (B). The cells had scant to moderate amounts of eosinophilic cytoplasm and round oval nuclei often having single prominent nucleoli with mild anisocytosis and anisokaryosis and a mitotic count of 37 figures per 10 high powered fields. As there was minimal peritumoral tissue to evaluate on the samples submitted, the full extent of the margins could not be assessed. No evidence of lymphatic or vascular invasion was observed but metastatic disease could not be ruled out. A diagnosis of mammary tubulopapillary carcinoma was made.
Figure 2. Photomicrographs of sections of mammary tissue from a Thoroughbred mare, showing (A) epithelial cells forming tubules and papillary projections within the neoplasia of the mammary tissue (H&E; bar = 200 µm) and (B) clusters of cells invading the basement membrane (H&E; bar = 50 µm).
Mammary neoplasia is relatively rare in horses and often carries a poor prognosis (Shark Citation2009). This type of tumour is usually encountered in mares > 12 years old with tumours appearing deep and multifocal with ulcerating skin nodules (Prendergast et al. Citation1999; Knottenbelt Citation2003), as seen in this case. Tubulopapillary carcinoma is aggressive and locally invasive with a high degree of metastasis to other tissues (Avci and Toplu Citation2012). It is a variant of the tubular form, having papillae that extend into the tubular luminae. These papillae are supported by a fine fibrovascular connective stroma. When the neoplastic cells infiltrate the surrounding tissue, they evoke a stromal response, including extensive myofibroblast proliferation (Goldschmidt et al. Citation2011). This neoplasm has been previously diagnosed in many species, including horses.
Due to the low incidence of mammary tumours in horses and the non-specific nature of the initial clinical signs, neoplasia is frequently misdiagnosed as mastitis (Munson Citation1987). This is complicated further as mastitis can be a secondary complication of mammary tumours (Hughes et al. Citation2015). Delayed diagnosis contributes to the guarded prognosis attributed to these cases as they are often in an advanced state by the time appropriate interventions are initiated (Smiet et al. Citation2012). Diagnosis of mammary neoplasia requires a multimodal approach. Ultrasound examination of the mammary gland can be useful to evaluate the extent of neoplasia. This should be accompanied by rectal examination to assess iliac lymphadenopathy (Knottenbelt Citation2003). Cytology of fine needle aspirates or biopsies can be useful in distinguishing between mastitis and mammary neoplasia. However, it is not as reliable a diagnostic procedure as histopathology of intact tissue samples because the findings may be unrepresentative and misleading (Brito et al. Citation2008). A wedge or excisional biopsy for histological analysis is required for a definitive diagnosis (Brendemuehl Citation2008).
The treatment of choice for mammary neoplasia is complete surgical resection of the affected tissue and all accessible regional lymph nodes (Smiet et al. Citation2012). This surgery can create dead space following removal of the mammary glands, which may lead to post-operative infections and wound dehiscence (Ferreira Júnior et al. Citation2019). Techniques that reduce dead space, aid in complete surgical closure, and reduce the need for wound drainage are advantageous in reducing post-operative complications and recovery time (Celeste Citation2016).
The locally aggressive and metastatic nature of tubulopapillary carcinomas often means that by the time a definitive diagnosis is made, and surgery is performed, metastasis to regional lymph nodes and other tissues may have occurred. Following surgical excision, recurrence or metastatic disease must be considered during follow-up examinations (Shark Citation2009). In this case the extent of metastatic disease was not investigated to the extent it could have been. Thoracic radiographs, checking for lung metastasis or fine needle aspirates of regional lymph nodes were not performed. Despite the success of the surgical procedure and immediate post-operative recovery period, the long-term prognosis of this case must remain guarded as the likelihood of metastatic disease or recurrence is high.
In the authors’ opinion, the use of the barbed suture wound closure device was useful in reducing post-operative complications such as dehiscence or excessive discharge from the surgical site. Self-anchoring, barbed, monofilament sutures were developed to eliminate knots. Knots are the weakest point in a suture line and reduce its strength by 40% (Von Frauhoffer and Chu Citation2018). By using this material, the tensile strength can be increased, and a stronger repair can be achieved while reducing surgical time (Warner and Gutowski Citation2009). Barbed sutures also prevent micromotion associated with traditional suture material, further reducing post-operative complications (Mitchell and Bengtson Citation2015).
In conclusion, the mare in this case recovered well from the surgical procedure and did not experience any of the post-operative complications commonly reported for bilateral mastectomy. A previously unreported approach to surgical site closure of a bilateral mastectomy is described, using a barbed wound closure device to reduce dead space and improve skin apposition. However, due to the lack of assessment of concurrent metastatic disease and the high likelihood of recurrence associated with this type of neoplasm, the long-term prognosis of this case remains guarded.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Avci H, Toplu N. Tetrathyridiosis and tubilopapillary carcinoma occurring simultaneously in the mammary gland of a cat. Reproduction in Domestic Animals 47, 36–8, 2012. http://doi.org/10.1111/j.1439-0531.2011.01903.x
- Boyce SD, Goodwin SL. Mammary gland neoplasia in a Canadian mare: challenges of diagnosis and treatment in a rural setting. Canadian Veterinary Journal 58, 628–30, 2017
- Brendemuehl J. Mammary gland enlargement in the mare. Equine Veterinary Education 20, 8–9, 2008. http://doi.org/10.2746/095777308X265687
- Brito MDF, Seppa GS, Teixeira LG, Rocha, TG, França TDN, Hess TM, Peixoto PV. Mammary adenocarcinoma in a mare. Ciência Rural 38, 556–60, 2008. http://doi.org/10.1590/S0103-84782008000200045
- *Celeste C. Selection of suture materials, suture patterns, and drains for wound closure. In: Theoret C, Schumacher J (eds). Equine Wound Management. Pp 173–99. John Wiley & Sons, Ames, IA, USA, 2016. http://doi.org/10.1002/9781118999219.ch9
- Ferreira Júnior JA, Pavarini SP, dos Santos MC, Alvarenga K, Ocampos Pedroso PM, Almeida e Macêdo JTS. Tubulopapillary carcinoma of the mammary gland in a mare. Acta Scientiae Veterinariae 47 (Suppl), 2019. http://doi.org/10.22456/1679-9216.96389
- Goldschmidt M, Peña L, Rasotto R, Zappulli V. Classification and grading of canine mammary tumours. Veterinary Pathology 48, 117–31, 2011. http://doi.org/10.1177/0300985810393258
- *Goldschmidt MH, Peña L, Zappulli V. Tumors of the mammary gland. In: Meuten DJ (ed). Tumors in Domestic Animals. Fifth Edtn. Pp 723–65. Wiley-Blackwell, Chichester, UK, 2016. http://doi.org/10.1002/9781119181200.ch17
- Hughes K, Scase TJ, Foote AK. Estrogen receptor and signal transducer and activator of transcription 3 expression in equine mammary tumours. Veterinary Pathology 52, 631–4, 2015. http://doi.org/10.1177/0300985814559400
- *Knottenbelt DC. The mammary gland. In: Knottenbelt DC, Le Blanc M, Lopate Pascoe RR (eds). Equine Stud Farm Medicine and Surgery. Pp 343–52. Saunders, London, UK, 2003
- Mitchell RTM, Bengtson BP. Clinical applications of barbed suture in aesthetic breast surgery. Clinics in Plastic Surgery 42, 595–604, 2015. http://doi.org/10.1016/j.cps.2015.06.003
- Munson L. Carcinoma of the mammary gland in a mare. Journal of the American Veterinary Medical Association 191, 71–2, 1987
- Prendergast M, Bassett H, Larkin HA. Mammary carcinoma in three mares. Veterinary Record 144, 731–2, 1999. http://doi.org/10.1136/vr.144.26.731
- Reppas GP, McClintock SA, Canfield PJ, Watson GF. Papillary ductal adenocarcinoma in the mammary glands of two horses. Veterinary Record 123, 518–9, 1996. http://doi.org/10.1136/vr.138.21.518
- Seahorn TL, Hall G, Brumbaugh GW, Honnas CM, Lovering SL, Snyder JR. Mammary adenocarcinoma in four mares. Journal of the American Medical Association 200, 1675–7, 1992
- Shark AM. Mare mammary neoplasia: difficulties in diagnosis and treatment. Equine Veterinary Education 21, 475–7, 2009. http://doi.org/10.2746/095777309X466070
- Smiet E, Grinwis G, Van Den Top J, Sloet van Oldruitenborgh-Oosterbaan M. Equine mammary gland disease with a focus on botryomycosis: a review and case study. Equine Veterinary Education 24, 357–66, 2012. http://doi.org/10.1111/j.2042-3292.2011.00352.x
- *Von Fraunhofer JA, Chu CC. Mechanical properties. In: Chu CC, Von Fraunhofer JA, Greisler HP (eds). Wound Closure Biomaterials and Devices. Pp 122. CRC Press, New York, NY, USA, 2018
- Warner JP, Gutowski KA. Abdominoplasty with progressive tension closure using a barbed suture technique. Aesthetic Surgical Journal 29, 221–5, 2009. http://doi.org/10.1016/j.asj.2009.01.009
- *Non-peer-reviewed
