ABSTRACT
Case histories
Medical records of four dogs diagnosed with protothecosis in New Zealand were reviewed. The dogs were aged between 4 and 9 years and three of the four dogs were female. Breeds were one Labrador, one Miniature Schnauzer and two crossbreeds. The reasons for initial veterinary evaluation were a cough and opaque appearance of the right eye (Case 1), diarrhoea (Cases 2 and 3), and cutaneous disease (Case 4).
Clinical findings
The ocular signs were characterised by panuveitis, retinal detachment and secondary glaucoma. Gastrointestinal signs included chronic haemorrhagic diarrhoea due to colitis. Three cases had disseminated infection and developed both bilateral, blinding, ocular disease and chronic gastrointestinal disease. Cutaneous signs consisted of draining fistulae over the olecranon, multifocal cutaneous nodules, and ulceration and tracts of the foot pads. Disseminated protothecosis was confirmed by histopathology of biopsied ocular tissues in Cases 1 and 2 and by gastrointestinal biopsies in Case 3. Prototheca spp. were also identified in cytological specimens from Cases 1 and 4 and recovered by culture in Cases 2 and 4. Cutaneous protothecosis was diagnosed in Case 4 initially by cytology and histopathology of skin lesions, and Prototheca zopfii was confirmed by PCR of cultured organisms.
Treatment and outcome
Prior to diagnosis of protothecosis, a variety of treatments were prescribed to treat the gastrointestinal and ocular signs. After diagnosis, only Cases 2 and 4 received medication aimed at treating the protothecal infection, which was itraconazole in both cases. Following the progression of clinical signs and concerns about quality of life, all four dogs were euthanised.
Diagnosis
Disseminated protothecosis in three dogs, cutaneous protothecosis in one dog.
Clinical relevance
Canine protothecosis is rarely reported, despite the ubiquity of the causal algae, and the disease usually carries an extremely grave prognosis when infection is generalised. In New Zealand, protothecosis should be considered as a differential diagnosis in dogs with panuveitis, chorioretinitis or retinal detachment, colitis, or nodular, ulcerative or fistulating cutaneous lesions.
Introduction
Prototheca spp. are algae that cause rare but serious disease in humans and animals (Kano Citation2020). The organism can be found in a wide range of wet habitats including slime flux of trees, sewage, and water and feed sources on farms (Libisch et al. Citation2022). Between 1988 and 2005 there were 17 confirmed cases of canine protothecosis in Australia and none in New Zealand (Stenner et al. Citation2007). Since then, there has been one case report of neurological protothecosis in a 1-year-old male Border Terrier dog in New Zealand (Walker et al. Citation2022).
In dogs, the disease is most often disseminated, with gastrointestinal, ocular and central nervous system signs predominating (Stenner et al. Citation2007). Cutaneous protothecal infections have been reported in both dogs and cats (Huth et al. Citation2015; Papadogiannakis et al. Citation2016; Gmyterco et al. Citation2023). Protothecal mastitis has been reported with increasing frequency in cows in New Zealand, in multiple North Island locations and in Canterbury (Hodges et al. Citation1985; Hulme-Moir Citation2020). Humans develop cutaneous signs, olecranon bursitis and disseminated forms of protothecosis (Lass-Flörl and Mayr Citation2007).
The most common species of Prototheca reported to cause disease in dogs are P. wickerhamii and P. zopfii (Stenner et al. Citation2007). Recently, the taxonomy of Prototheca spp. has been revised, and P. zopfii genotype 1 and genotype 2 are now considered separate species and named P. ciferrii and P. bovis, respectively (Jagielski et al. Citation2019). Clinical signs of protothecosis can be insidious, and treatment is usually unrewarding, with the systemic disease in dogs being nearly always fatal (Stenner et al. Citation2007). This report summarises the diagnosis and progression of protothecosis in four dogs in New Zealand between 2015 and 2018.
Case histories
The diagnosis of protothecosis in a dog with bilateral retinal detachment and colitis prompted the collation and review of clinical information of three additional cases of canine protothecosis from other veterinary clinics in New Zealand, diagnosed between 2015 and 2018. The information is summarised in . The reason for initial veterinary evaluation varied and was due to a cough and opaque appearance of the right eye in Case 1, diarrhoea in Cases 2 and 3, and cutaneous disease in Case 4. Breeds varied and the dogs’ ages ranged from 4 to 9 years.
Table 1. Signalment, diagnostic findings, details of case management and outcome in four dogs diagnosed with protothecosis in New Zealand.
Clinical findings
Cases 1–3 had both gastrointestinal and ocular signs throughout the evolution of their disease, however none had disease detected in both systems at the first examination. Chronic/intermittent diarrhoea with haematochezia was the predominant gastrointestinal sign present. Specialist ophthalmic examinations were performed on two of the three dogs with ocular abnormalities, and unilateral retinal detachments, panuveitis and secondary glaucoma were identified. In Case 1 the retinal detachment was complete, opaque and there was suspicion for subretinal exudate; in Case 2 the retinal detachments were multifocal and haemorrhagic. Progression to bilateral ocular disease occurred over a period of weeks to months in the three disseminated cases. Case 1 developed head tilt and dysphoria 19 weeks after the first presenting signs.
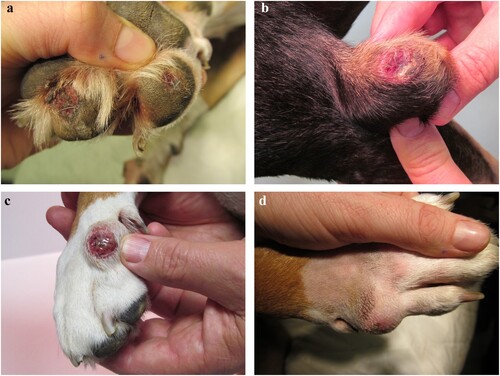
Case 4 was evaluated multiple times for cutaneous disease over a period of 7 months. The first cutaneous lesions observed included multiple cutaneous nodules and thickened skin over the olecranons with pyoderma. The dog was pruritic, and pruritus improved after administration of 0.7 mg/kg prednisone (Apotex NZ Ltd., Auckland, NZ) twice daily orally, on a tapering schedule. Later, multifocal tracts were identified on the digital pads of the right forelimb, and after 3 months the digital pads became ulcerated ((a)). Concurrently, the dog developed bilateral, non-painful elbow swellings with palpable nodules, skin ulceration of the left elbow ((b)), and increased heat in the right elbow. Firm nodular swellings were present within the skin overlying the dorsal aspect of the second metacarpal bones in both forelimbs ((c, d)), with ulceration of the right forelimb swelling ((c)). No gastrointestinal or ocular signs were reported at any time.
Figure 1. Photographs of a dog (Case 4) with (a) multiple foot pad ulcerations, (b) an ulcerated swelling of the left elbow and (c, d) firm nodular swellings within the skin of the metacarpi with ulceration of the swelling of the right metacarpus (c).
Laboratory and pathology findings
Serum biochemistry and complete blood count findings were non-specific when measured (Cases 1–3).
Histological examination of tissues was performed in all cases and identified endosporulating organisms consistent with Prototheca spp. (). Histology of intraocular contents (Case 1) and enucleated globe (Case 2) was consistent with protothecal chorioretinitis, and gastrointestinal biopsies (Case 3) identified caecal protothecosis with regional lymph node spread. A post-mortem examination of Case 1 was completed and histologically there was evidence of chronic granulomatous panophthalmitis, colitis and nephritis with endosporulating organisms morphologically consistent with Prototheca spp. Organisms were also identified in the mesenteric lymph nodes and kidneys. Multiple sections of the brain and spinal cord were examined but no explanation for the head tilt or dysphoria was identified nor were there algal organisms in the lungs that would implicate protothecosis as the cause of the initial transient cough.
Figure 2. Photomicrograph of section from the (a) colon of Case 1 and (b) skin of Case 4 showing endosporulating algal organism (arrows) with internal septation in both locations and empty theca (arrow heads) in the section of skin (H&E; bar = 30 μm). These are magnified in the insets.
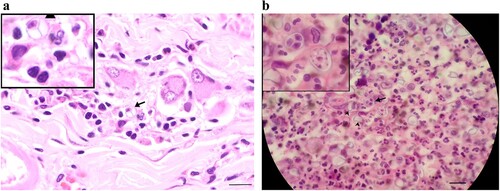
In Case 4, fine needle aspirates were collected from the swellings on the left elbow and right metacarpus. On cytological examination, ovoid bodies, variable in size and up to 20 µm in diameter, were detected within both samples under microscopy. The ovoid bodies were blue with homogeneous centres. Some had pale rims and dark round, oval or dumbbell-shaped central areas, and others had pale centres containing multiple small round bodies. The ovoid bodies stained positively with periodic acid-Schiff and the internal round structures within the cell wall were consistent with Prototheca organisms. Histologic examination of skin biopsies identified pyogranulomatous, necrotising, diffuse, severe dermatitis and panniculitis and endosporulating organisms consistent with Prototheca spp. ((b)).
Culture of urine, kidney, faeces, and cutaneous biopsies from all four dogs in this series were performed by laboratories according to standard culture techniques, and these successfully recovered Prototheca spp. from Cases 2 and 4. Skin biopsies from Case 4 were cultured to isolate Prototheca with a generic protocol of Sabouraud dextrose agar at 25°C, in aerobic conditions. DNA was extracted with the QIAamp DNA mini kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol for tissue samples. Extracted DNA was used as the template in an end-point PCR with universal primers targeting the 28S rRNA gene (primers NL1: 5′-GCATATCAATAAGCGGAGGAAAAG-3′ and NL4: 5′-GGTCCGTGTTTCAAGACGG-3′) (Fell Citation1995). Negative extraction controls and duplicate non-template controls were included. Resulting amplicons were sequenced (Ecogene, Landcare Research, Auckland, NZ) and compared to known strains in GenBank using the Basic Local Alignment Search Tool (Altschul et al. Citation1990), showing highest similarity (99.5%) to P. zopfii isolates (accessions: MG827372.1, MG827343.1, KX353632.1, KX353631.1, KX353630.1, MG827374.1).
Treatment and outcome
Prior to diagnosis a variety of treatments were prescribed. A full list of treatments is available in Supplementary Material. Dogs with diarrhoea were prescribed a variety of supportive treatments, anthelmintics, anti-inflammatories, and antibiotics. Treatment for panuveitis and glaucoma included topical carbonic anhydrase inhibitors, topical corticosteroids, systemic anti-inflammatories and analgesics followed by surgical enucleation or cosmetic alternative (intraocular evisceration and intraocular implant) to treat intractable pain and inflammation. Following the diagnosis of protothecosis, medication aimed at treating the protothecal infection was prescribed only in Cases 2 and 4. Case 2 was administered 10.8 mg/kg itraconazole (Itrazole; Viatrus, Auckland, NZ) twice daily, orally for a short period before euthanasia. The cutaneous case (Case 4) was administered prednisone for pruritus initially, and then systemic antibiotics and analgesics. To treat the protothecal infection 5.6 mg/kg itraconazole (Itrazole; Viatrus) was administered twice daily, orally. After 2 months the itraconazole was withdrawn at the owner’s request and the dog was maintained on prednisone and analgesics. After a period of 9 months, systemic antibiotics were again administered to manage suspected secondary infection, in addition to the other treatments.
The disseminated cases (Cases 1–3) had chronic colitis and ocular disease that became bilateral. Euthanasia was elected in all cases, with post-mortem examination occurring in Case 1. For the cutaneous case (Case 4), despite persistence of lesions on the feet and elbows and intermittent drainage of the left elbow lesion, the dog’s quality of life was determined to be good for an extended period. Foot pad ulceration progressed to involve all feet, and new cutaneous nodules developed. The dog was euthanised after 30 months due to open, necrotic cutaneous lesions and concerns regarding quality of life. Post-mortem examination was not performed.
Discussion
This case series highlights the occurrence of protothecosis in four dogs in New Zealand, all of which were euthanised due to the disease. Three dogs exhibited disseminated protothecosis, characterised by chronic gastrointestinal disease and severe ocular disease, diagnosed by histopathology. The fourth dog exhibited chronic cutaneous protothecosis, initially diagnosed through cytology, and later confirmed by histology, culture, and PCR analysis of cultured skin biopsies. These cases emphasise the need for veterinarians in New Zealand to consider protothecosis as a differential diagnosis in dogs presenting with chronic/haemorrhagic diarrhoea, chorioretinitis, retinal detachment, and nodular, ulcerative, or fistulating skin disease. While not used in the disseminated cases in this series, non-invasive cytological examinations can serve as a readily available diagnostic tool when protothecosis is clinically suspected.
Stenner et al. (Citation2007) reported that large intestinal signs were the most common reason for presentation in dogs with disseminated protothecosis, followed by ocular abnormalities, although a wide range of body systems can be affected. Disease may present simultaneously in multiple organs at the first examination or be limited to one body system, often with progression to involve multiple body systems over time (Stenner et al. Citation2007; Shank et al. Citation2015). Gastrointestinal colonisation after ingestion is the suspected mode of infection; however, gastrointestinal disease is not always reported at the onset of infection (Stenner et al. Citation2007; Shank et al. Citation2015). Subclinical or unobserved gastrointestinal disease may be one explanation for an apparent lack of gastrointestinal signs observed initially in some dogs.
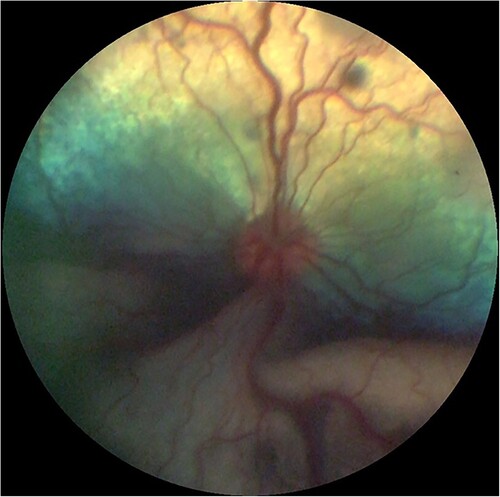
Ocular protothecosis occurs through haematogenous spread to the vascular choroid and is often bilateral, although eyes may be sequentially affected (Stenner et al. Citation2007). Vision loss and ocular discomfort are due to chorioretinitis and resulting exudative retinal detachment and glaucoma secondary to inflammation (Shank et al. Citation2015). A fundic photograph depicting chorioretinitis and exudative retinal detachment can be seen in .
Figure 3. Fundic photograph of a dog with ocular protothecosis showing multifocal hypo-reflective areas of active chorioretinitis and an opaque, ventral, exudative retinal detachment. Image courtesy of Marnie Ford, Animal Eye Care, Melbourne, Australia.
Case 4 in this case series exhibited protothecosis limited to the skin, characterised by foot pad ulceration and multifocal cutaneous nodules on the limbs, consistent with previous reports (Papadogiannakis et al. Citation2016; Carfora et al. Citation2017). Other documented locations of protothecal infection include the sacrum, zygomatic arch, scrotum and nares (Macartney et al. Citation1988; Ginel et al. Citation1997; Hsu et al. Citation2013). In addition, Case 4 had bilateral nodules, swellings and fistulae at the olecranon from which P. zopfii was recovered. Protothecal olecranon bursitis is recognised as an entity in humans (Lass-Flörl and Mayr Citation2007); however, to our knowledge olecranon bursitis has not previously been demonstrated in dogs. Some canine cutaneous cases appear to be confined to the skin (Macartney et al. Citation1988; Papadogiannakis et al. Citation2016), while others have evidence of dissemination (Carfora et al. Citation2017; Silveira et al. Citation2018). Case 4 did not have systemic signs of protothecosis, but post-mortem examination was not undertaken to exclude dissemination. P. wickerhamii has been recovered from samples of most reported cutaneous protothecosis cases (Hsu et al. Citation2013; Papadogiannakis et al. Citation2016; Gmyterco et al. Citation2023); however, P. zopfii was identified in Case 4 by PCR, and has been reported in two other cases of cutaneous and systemic protothecosis (Carfora et al. Citation2017; Silveira et al. Citation2018).
Protothecal skin infections are postulated to occur opportunistically through breaks in the skin barrier in humans (Lass-Flörl and Mayr Citation2007). The distribution of foot pad, forelimb and face lesions seems to support a cutaneous entry of infection in dogs with subsequent haematogenous dissemination in some cases. A gastrointestinal route with spread to the skin is, however, also possible.
The discovery of Prototheca spp. on histopathology and cytology of specimens led to the diagnosis of protothecosis in all four dogs of this series. Prototheca spp. can be identified by cytology, although less experienced cytologists may misinterpret the algae as yeast forms (Masuda et al. Citation2021). Cytological features of Prototheca include variably sized organisms that are ovoid, round or reniform with a defined colourless cell wall/capsule and basophilic central regions (Ribeiro et al. Citation2009; Whipple et al. Citation2020). Internal septation and endosporulation can be seen on cytological and histologic examinations (). The species of Prototheca can be determined by mass spectrometry of cultured specimens and sequencing of PCR products amplified from DNA extracted from cultures or formalin-fixed paraffin-embedded tissues (Kano Citation2020; Falcaro et al. Citation2021; Masuda et al. Citation2021). Matrix-assisted laser desorption ionisation-time-of-flight mass spectrometry (MALDI-TOF) is an inexpensive mass spectrometry test now available at most diagnostic laboratories in New Zealand. These additional tests may be helpful in surveillance of the organism and in establishing potential environmental sources of Prototheca spp. (Libisch et al. Citation2022) or to confirm the diagnosis and species of Prototheca.
Two dogs in this series were diagnosed with protothecosis through ocular histopathology. Diagnosis can also be achieved through cytological examination or culture of sub-retinal or vitreal aspirates (Schultze et al. Citation1998; Rizzi et al. Citation2006; Beribè et al. Citation2014). Examination of ocular fluid aspirates before surgery can provide helpful diagnostic information but may require additional anaesthesia. In cases where dogs are already blind and have painful eyes, proceeding with enucleation is justifiable both to alleviate pain and to obtain diagnostic samples in a single procedure.
Beyond ocular fluids, cytology of other more readily obtainable samples such as rectal scrapings (Stenner et al. Citation2007) and urine sediment (Pressler et al. Citation2005; Stenner et al. Citation2007; Ribeiro et al. Citation2009) can reveal Prototheca spp. but were not performed ante-mortem in the dogs in this series. Prototheca organisms were seen on histology sections of the kidney in Case 1 as well as on cytology of post-mortem rectal scrapings, suggesting that Prototheca organisms might have been identified on urine sediment or rectal scrapings had they been performed in life. It is important to note that a negative urine sediment does not rule out protothecosis, as Prototheca organisms can be identified on renal histopathology despite a negative urine sediment (Lane et al. Citation2012).
In this series, culture for Prototheca of urine, kidney, faeces, and cutaneous biopsies were performed following an established diagnosis of protothecosis, with mixed success. Negative culture results in some cases may be due to delayed submission and freezing of the samples (Case 1), intermittent gastrointestinal or renal shedding (Pressler et al. Citation2005; Ribeiro et al. Citation2009), or lack of renal involvement. Most Prototheca spp. can be cultured within 3 days on standard dextrose culture media such as Sabouraud dextrose agar, although some strains require 7 days’ incubation before growth can be observed (Libisch et al. Citation2022). Overgrowth with fungi and bacteria can result in a failure to isolate Prototheca spp., particularly if the samples are densely contaminated. To assist growth of Prototheca spp., Prototheca isolation medium can be used, or chloramphenicol can be added to the agar to inhibit bacterial growth (Pore Citation1973).
Protothecosis is regarded as difficult to treat, with a grave prognosis and median survival time of 4 months in dogs with disseminated disease (Stenner et al. Citation2007), which is consistent with our experience in this case series. Late diagnosis with advanced dissemination due to low index of suspicion of protothecosis may have contributed to the poor outcomes of these and other reported cases. Amphotericin B and azole anti-fungal medications are the drugs most often prescribed in an attempt to treat the infection (Hollingsworth Citation2000; Stenner et al. Citation2007). Evidence for treatment outcomes is lacking due to the rarity of published reports. Only one dog with disseminated protothecosis has been reported in published literature to have survived, and was treated with amphotericin B and ongoing itraconazole (Stenner et al. Citation2007). Anecdotally, oral posaconazole and amphotericin B infusions have been successful in achieving clinical remission and occasional cure. Sustained-release posaconazole may be superior to itraconazole and can be used in combination with terbinafine (R Malik, pers. comm).Footnote1 The single case of cutaneous protothecosis in this series was administered systemic itraconazole but the drug was withdrawn after 2 months, and the patient lived a further 20 months. The limited reports of cutaneous protothecosis describe variable responses to anti-fungal medications; however, a recent report describes a long-term remission with ongoing pulse therapy of itraconazole (Gmyterco et al. Citation2023). Surgical excision of a protothecal scrotal granuloma was reported in conjunction with oral ketoconazole administration (Ginel et al. Citation1997). Intralesional injection of amphotericin B has been used to treat localised fungal infections, and could have application for cutaneous protothecal infections (Malik et al. Citation2009).
The occurrence of protothecal infections in dogs in New Zealand is of interest globally, as the previous lack of canine cases from New Zealand and Tasmania supported the speculation that cooler climates may inhibit infection (Libisch et al. Citation2022). Furthermore, the onset of clinical signs for the disseminated cases in this series occurred in June, July and October, which are typically colder months in New Zealand. The wet habitat of Prototheca spp. and occurrence of protothecal mastitis in dairy cows in New Zealand raises the possibility that exposure to waterways in farming areas may be a risk factor. Two of the dogs in this series were from northern Waikato and South Auckland, regions where protothecal mastitis has occurred; however, the same correlation cannot be drawn for the dogs from Ōamaru and Lower Hutt. There was no pertinent history available regarding the dogs’ access to waterways or farms, but it should be noted that the three disseminated cases did reside on lifestyle blocks, or with lifestyle and reserve surrounds, rather than urban environments. Case 4 resided in a provincial but urban environment, although the dog’s prior history is unknown.
Prototheca spp. infections are not thought to be transmissible between people and there are no reports of spread of protothecal infections between people and dogs (Libisch et al. Citation2022). Despite a lack of evidence for zoonosis so far, the potential for zoonotic infection should remain a consideration, particularly if owners are immunocompromised or dogs have draining lesions, as for Case 4.
Conclusion
The four cases in this series provide evidence for the occurrence of protothecosis as a cause of ocular, gastrointestinal and cutaneous disease in dogs in New Zealand. Prototheca infection should be considered as a differential diagnosis in New Zealand for dogs with haemorrhagic or chronic colitis, or panuveitis, chorioretinitis or retinal detachment, especially when both ocular and gastrointestinal disease coexist. Prototheca spp. infection should also be considered in nodular, ulcerative or fistulating cutaneous disease. Diagnosis can be made by cytology of rectal scrapes, urine sediment, ocular or lesion aspirates, and by culture and histopathology. The diagnosis can be confirmed and speciated with mass spectrometry and PCR.
Acknowledgements
The authors thank veterinarians Felicity Morris, Estelle Louarduzzi, Ivan Aleksic, Peter Collinson, Amy O’Sullivan, David Kettles, Angela Schumacher and all veterinarians involved in the clinical care of dogs. The authors also thank the Ministry for Primary Industries, Massey University, Gribbles Veterinary and New Zealand Veterinary Pathology Ltd. for their diagnostic work, and Marnie Ford (Animal Eye Care, Melbourne, Australia) for the image reproduced in this article.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes
1 R. Malik, University of Sydney, Sydney, Australia
References
- Altschup SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology 215, 403–10, 1990. https://doi.org/10.1016/S0022-2836(05)80360-2
- Beribè F, Miglio A, Cassarani MP, Magi G, Passamonti F, Laus F, Cerquetella M, Spaterna A. What is your diagnosis? Systemic lymphadenopathy and blindness in a dog from Italy. Veterinary Clinical Pathology 43, 605–6, 2014. https://doi.org/10.1111/vcp.12189
- Carfora V, Noris G, Caprioli A, Iurescia M, Stravino F, Franco A. Evidence of a Prototheca zopfii genotype 2 disseminated infection in a dog with cutaneous lesions. Mycopathologia 182, 603–8, 2017. https://doi.org/10.1007/s11046-016-0108-2
- Falcaro C, Furlanello T, Binanti D, Fondati A, Bonfanti U, Krockenberger M, Malik R, Danesi P. Molecular characterization of Prototheca in 11 symptomatic dogs. Journal of Veterinary Diagnostic Investigation 33, 156–61, 2021. https://doi.org/10.1177/1040638720976423
- Fell JW. rDNA targeted oligonucleotide primers for the identification of pathogenic yeasts in a polymerase chain reaction. Journal of Industrial Microbiology 14, 475–7, 1995. https://doi.org/10.1007/BF01573961
- Ginel PJ, Pérez J, Molleda JM, Lucena R, Mozos E. Cutaneous protothecosis in a dog. Veterinary Record 140, 651–3, 1997. https://doi.org/10.1136/vr.140.25.651
- Gmyterco VC, Jagielski T, Baldasso G, Bacher LH, Ribeiro MG, de Farias MR. Cutaneous protothecosis in a dog successfully treated with oral itraconazole in pulse dosing. Acta Veterinaria Scandinavica 65, 7, 2023. https://doi.org/10.1186/s13028-022-00662-x
- Hodges RT, Holland JTS, Neilson FJA, Wallace NM. Prototheca zopfii mastitis in a herd of dairy cows. New Zealand Veterinary Journal 33, 108–11, 1985. https://doi.org/10.1080/00480169.1985.35187
- Hollingsworth SR. Canine protothecosis. Veterinary Clinics of North America: Small Animal Practice 30, 1091–101, 2000. https://doi.org/10.1186/s13028-022-00662-x
- Hsu C, Liu K, Shen W, Yen IF, Chien Y, Liu C. Canine cutaneous protothecosis caused by Prototheca wickerhamii in a dog. Taiwan Veterinary Journal 39, 50–8, 2013
- *Hulme-Moir L. Udder disease: Prototheca mastitis – the emergence continues. HoofPrint 38(4), 20–3, 2020
- Huth N, Wenkel RF, Roschanski N, Rösler U, Plagge L, Schöniger S. Prototheca zopfii genotype 2-induced nasal dermatitis in a cat. Journal of Comparative Pathology 152, 287–90, 2015. https://doi.org/10.1016/j.jcpa.2015.02.001
- Jagielski T, Bakuła Z, Gawor J, Maciszewski K, Kusber W-H, Dyląg M, Nowakowska J, Gromadka R, Karnkowska A. The genus Prototheca (Trebouxiophyceae, Chlorophyta) revisited: implications from molecular taxonomic studies. Algal Research 43, 101639, 2019. https://doi.org/10.1016/j.algal.2019.101639
- Kano R. Emergence of fungal-like organisms: Prototheca. Mycopathologia 185, 947–54, 2020. https://doi.org/10.1007/s11046-020-00455-8
- Lane LV, Meinkoth JH, Brunker J, Li SKS, Snider TA, Thomas J, Bradway D, Love BC. Disseminated protothecosis diagnosed by evaluation of CSF in a dog. Veterinary Clinical Pathology 41, 147–52, 2012. https://doi.org/10.1111/j.1939-165X.2011.00395.x
- Lass-Flörl C, Mayr A. Human protothecosis. Clinical Microbiology Reviews 20, 230–42, 2007. https://doi.org/10.1128/CMR.00032-06
- Libisch B, Picot C, Ceballos-Garzon A, Moravkova M, Klimesová M, Telkes G, Chuang ST, Le Pape P. Prototheca infections and ecology from a One Health perspective. Microorganisms 10, 938, 2022. https://doi.org/10.3390/microorganisms10050938
- Macartney L, Rycroft AN, Hammil J. Cutaneous protothecosis in the dog: first confirmed case in Britain. Veterinary Record 123, 494–6, 1988. https://doi.org/10.1136/vr.123.19.494
- Malik R, Krockenberger MB, O'Brien CR. Intra-lesional amphotericin B—worth a try, maybe for lots of things, but we need more data! Journal of Feline Medicine and Surgery 11, 621–3, 2009. https://doi.org/10.1016/j.jfms.2009.02.007
- Masuda M, Jagielski T, Danesi P, Falcaro C, Bertola M, Krockenberger M, Malik R, Kano R. Protothecosis in dogs and cats – new research directions. Mycopathologia 186, 143–52, 2021. https://doi.org/10.1007/s11046-020-00508-y
- Papadogiannakis EI, Velonakis EN, Spanakos GK, Koutinas AF. Cutaneous disease as sole clinical manifestation of protothecosis in a Boxer dog. Case Reports in Veterinary Medicine, e2878751, 2016. https://doi.org/10.1155/2016/2878751
- Pore RS. Selective medium for the isolation of Prototheca. Applied Microbiology 26, 648–9, 1973. https://doi.org/10.1128/am.26.4.648-649.1973
- Pressler BM, Gookin JL, Sykes JE, Wolf AM, Vaden SL. Urinary tract manifestations of protothecosis in dogs. Journal of Veterinary Internal Medicine 19, 115–9, 2005. https://doi.org/10.1111/j.1939-1676.2005.tb02669.x
- Ribeiro MG, Rodrigues de Farias M, Roesler U, Roth K, Rodigheri SM, Ostrowsky MA, Salerno T, Siqueira AK, Fernandes MC. Phenotypic and genotypic characterization of Prototheca zopfii in a dog with enteric signs. Research in Veterinary Science 87, 479–81, 2009. https://doi.org/10.1016/j.rvsc.2009.04.015
- Rizzi TE, Cowell RL, Meinkoth JH, Gilmour MA. More than meets the eye: subretinal aspirate from an acutely blind dog. Veterinary Clinical Pathology 35, 111–13, 2006. https://doi.org/10.1111/j.1939-165X.2006.tb00099.x
- Schultze AE, Ring RD, Morgan RV, Patton CS. Clinical, cytologic and histopathologic manifestations of protothecosis in two dogs. Veterinary Ophthalmology 1, 239–43, 1998. https://doi.org/10.1046/j.1463-5224.1998.00034.x
- Shank AMM, Dubielzig RD, Teixeira LBC. Canine ocular protothecosis: a review of 14 cases. Veterinary Ophthalmology 18, 437–42, 2015. https://doi.org/10.1111/vop.12239
- Silveira CS, Cesar D, Keating MK, DeLeon-Carnes M, Armién AG, Luhers M, Riet-Correa F, Giannitti F. A case of Prototheca zopfii genotype 1 infection in a dog (Canis lupus familiaris). Mycopathologia 183, 853–8, 2018. https://doi.org/10.1007/s11046-018-0274-5
- Stenner VJ, MacKay B, King T, Barrs VRD, Irwin P, Abraham L, Swift N, Langer N, Bernays M, Hampson E, et al. Protothecosis in 17 Australian dogs and a review of the canine literature. Medical Mycology 45, 249–66, 2007. https://doi.org/10.1080/13693780601187158
- Walker A, MacEwan I, Fluen T, Hardcastle M. Disseminated protothecosis with central nervous system involvement in a dog in New Zealand. New Zealand Veterinary Journal 70, 238–43, 2022. https://doi.org/10.1080/00480169.2022.2056539
- Whipple KM, Wellehan JF, Jeon AB, Sabatino BR, Frasca Jr S, Popov VL, Ossiboff R, Leissinger MK. Cytologic, histologic, microbiologic, and electron microscopic characterization of a canine Prototheca wickerhamii infection. Veterinary Clinical Pathology 49, 326–32, 2020. https://doi.org/10.1111/vcp.12864
- *Non-peer-reviewed
