?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Case history
Necropsies on Toggenburg goats culled from a small farm in the Manawatū district of New Zealand, performed at Massey University (Palmerston North, NZ) over a period of 29 years (1991–2019), revealed soft tissue mineralisation, particularly of cardiovascular tissues. The farm spans 10 acres and runs between 15 and 30 Toggenburg goats. The goats are predominantly on pasture comprising a variety of types.
Pathological findings
Necropsies were performed on all adult goats (n = 45) that died or were euthanised. Histopathology was performed on 42 goats (93%), of which 33 (73%) included sufficient tissues diagnostically relevant to soft tissue mineralisation. The most significant gross findings were in various arteries, with the aorta most commonly affected, followed by the heart and lungs. The aortic intima showed prominent, multifocal to coalescing, raised, wrinkled, white plaques. Microscopically there were multiphasic lesions of mineralisation, chondroid, and osseous metaplasia in the elastic arteries, aorta, heart and lungs. A lumbar vertebra from one goat had prominent, basophilic, fibrillar, tangled matrix lining Haversian canals and lamellae.
Laboratory findings
Blood samples were collected from 15 adult goats in the affected herd and from 10 adult Toggenburg goats from an unaffected herd. Samples were collected by jugular venipuncture at 2-month intervals for 12 months (April 2018–March 2019). Concentrations of calcium, phosphorus, 25-hydroxyvitamin D2 and D3 (25OHD2, 25OHD3) in serum were analysed. The concentration of total 25OHD in serum was 34.2 (95% CI = 18.9–49.4) nmol/L (p < 0.001) higher in goats from the affected herd than in goats from the unaffected herd. Serum 25OHD2 concentration was 46.2 (95% CI = 39.2–53.2) nmol/L higher (p < 0.001) in goats from the affected herd compared to the unaffected herd. Serum Ca concentrations in affected goats were 0.101 (95% CI = 0.005–0.196) mmol/L higher (p = 0.039) than unaffected goats, but remained within the reference range. There was no evidence of a difference in serum 25OHD3 and P concentration between the herds.
Vegetation survey
All paddocks on the property were surveyed every 2 months along evenly spaced line transects, and then further traversed perpendicularly to form a grid. No known calcinogenic species were identified. Known plant sources of vitamin D identified on the farm included mushrooms (species not defined), Dactylis glomerata, lichen, pine pollen, and algae.
Diagnosis
Soft tissue mineralisation and enzootic calcinosis.
Clinical relevance
Veterinarians are alerted to the possibility of either enzootic calcinosis in goats and the potential occurrence of calcinogenic plants in New Zealand; or chronic vitamin D toxicosis of non-plant origin.
Introduction
Soft tissue mineralisation secondary to metastatic mineralisation can occur in a number of different species. Potential causes of metastatic mineralisation include: granulomatous disease resulting in activated macrophages (which can produce 1,25-dihydroxyvitamin D) (Lopez et al. Citation2021), neoplasia producing calcinogenic compounds, chronic kidney disease, and vitamin D toxicosis (Miller et al. Citation2022). Additionally, an inherited disorder of arterial calcification should be considered as a cause of widespread vascular mineralisation.
Enzootic calcinosis is a syndrome of extensive mineralisation of soft tissues in grazing animals caused by the ingestion of plants that contain increased concentrations of vitamin D, in particular 1,25-dihydroxyvitamin D (active form) which may be in the form of glycosides (Jäpelt and Jakobsen Citation2013; Machado et al. Citation2020a). Plants known to contain these vitamin D compounds include members of the Solanaceae and Poaceae (Riet-Correa et al. Citation2023). Three species known to have calcinogenic properties have been identified in New Zealand: Trisetum flavescens (three plants are catalogued), Solanum torvum (a single specimen identified several years ago in Auckland) and Nierembergia rivularis syn. repens (identified in Wairarapa, Whanganui and Christchurch) (Anonymous Citation2013). Furthermore, outbreaks of enzootic calcinosis with unknown aetiology have occurred worldwide (Machado et al. Citation2020a).
Animals affected by enzootic calcinosis develop anorexia, emaciation, and stiffness (Machado et al. Citation2020a). They may become recumbent and have difficulty rising. Those with severe cardiovascular mineralisation may develop dyspnoea, cardiac arrhythmia and pulmonary oedema. On post-mortem examination affected animals have mineralisation of the elastic and muscular arteries, the heart (particularly the left side) and lungs, along with nephrocalcinosis. Some animals have tendon mineralisation and osteopetrosis. Mineralisation of medium and large-sized arteries is considered to be the hallmark of enzootic calcinosis (Machado et al. Citation2020a).
Necropsies on Toggenburg goats culled from a small farm in the Manawatū district of New Zealand have been performed at Massey University (Palmerston North, NZ) over a period of 29 years (1991–2019). These have revealed soft tissue mineralisation, particularly of cardiovascular tissues consistent with enzootic calcinosis. This report describes an investigation into enzootic calcinosis in this herd, including macroscopic and microscopic findings, assessments of serum Ca, phosphorus (P) and 25-hydroxyvitamin D concentrations within the herd, and the presence of sources of vitamin D such as calcinogenic plants on the farm. While enzootic calcinosis occurs around the world, this is the first report of an enzootic calcinosis-type syndrome in New Zealand.
Case history and clinical findings
All goats (n = 45, age 4–16 years) selected for culling from a small dairy goat farm in the Manawatū district during the period 1991–2019 were subjected to euthanasia and necropsy at the Massey University School of Veterinary Science (Palmerston North, NZ). Animals were necropsied after they died or were culled due to a variety of clinical signs, most commonly ill thrift, scouring or advanced age. Only one animal presented with lameness, and none with severe cachexia.
The farm runs between 15 and 30 Toggenburg goats, of which bucks or wethers comprise 20% of the herd, the remainder being lactating females and their offspring. Full details on farm characteristics, vegetation information, supplementary feeding and treatments, fertiliser application and pest control are included in Supplementary Information 1.
Pathological findings
A retrospective study of gross and microscopic necropsy reports (n = 45) was performed, and all glass slides retrieved (n = 42) and examined under light microscopy by author SEB. Tissues had been fixed in 10% neutral buffered formalin for ≥ 24 hours, processed, embedded, and sectioned at 4 µm. All sections had been stained with H&E, and selected sections with Von Kossa stain for calcium, Masson’s trichrome stain for collagen and smooth muscle, Alcian blue pH 2.5 and periodic acid-Schiff stain for mucopolysaccharides, and Verhoeff-Van Gieson stain for elastin.
In the absence of a grossly visible soft tissue mineralisation, sufficient tissues had been sampled from 33 goats to allow for microscopic assessment of soft tissue mineralisation (i.e. lung, heart, aorta, kidney, gastrointestinal tract, thyroid, parathyroid gland). A description of the spectrum of histopathological findings was compiled for each organ. Cases were classified as either affected or not-affected by mineralisation, and affected or not-affected by osseous and chondroid metaplasia, and animals with mineralisation and/or osseous and chondroid metaplasia were considered to have enzootic calcinosis.
In total, 35 animals had either a macroscopic diagnosis of soft tissue mineralisation made at necropsy (n = 25) and/or had sufficient tissues sampled to allow a microscopic diagnosis of soft tissue mineralisation (n = 33). Overall, 32/35 (91%) goats were affected by mineralisation. Macroscopic lesions were present in 25/32 (78%) affected goats, while the remaining seven goats had microscopic lesions only. Peaks in numbers of affected goats necropsied occurred in 2003 and 2012. The mean lifespan of affected goats (7 male, 25 female) was 9.9 (min 4, max 16) years, while for non-affected goats (1 male, 2 female) it was 7.7 (min 7, max 8) years.
The most common gross findings were in various arteries, with the aorta most commonly affected (a), followed by the heart (b), and lungs (c). The aortic intima showed prominent, multifocal to coalescing, raised, wrinkled, white plaques (a). In severe cases the aortic wall was diffusely thickened and rigid. Variably, the carotid, brachial, coronary and mesenteric arteries were rigid and brittle. In mild cases, plaques were restricted to the distal abdominal aorta, in some cases to foci less than 2 cm in diameter. Microscopically there were multiphasic lesions of mineralisation, chondroid, and osseous metaplasia in the elastic arteries and aorta (a and b). Microscopic evidence of mineral was also present in abomasal muscular arteries, atrioventricular valves and atria of the heart, coronary arteries, and hilar and arcuate arteries and arterioles of the kidney.
Figure 1. Photographs of tissues from a 13-year-old female Toggenburg goat with soft tissue mineralisation showing a) thoracic aorta with raised, white intimal plaques; b) heart with white mineralised plaques on the aorta, left atrioventricular valves and aortic outflow tract; and c) lung with raised, firm, mineralised nodules.
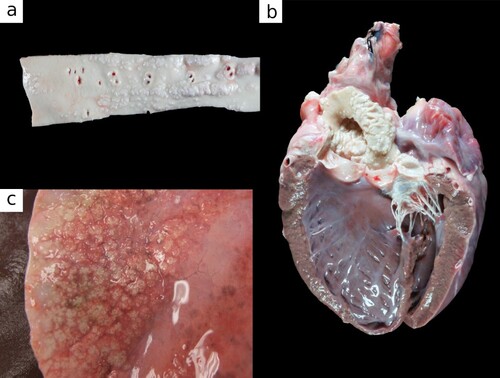
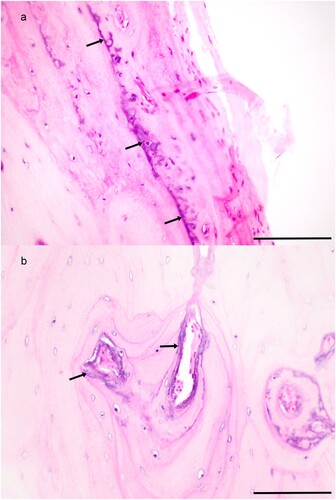
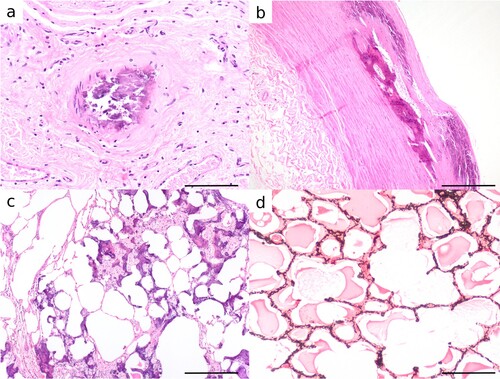
Figure 2. Photomicrographs of tissues from Toggenburg goats with soft tissue mineralisation. a) Mineralisation of small muscular artery in the abomasal submucosa in an 11-year-old female goat (H&E; bar = 100 μm). b) Osteochondroid metaplasia of aorta with osteoid in areas where vessels have infiltrated mineralised foci in a 6-year-old male goat (H&E; bar = 400 μm). c) Extensive basophilic amorphous mineral and early osseous metaplasia expanding alveolar septa of the lung in an 11-year-old female goat (H&E; bar = 400 μm). d) The presence of mineral in alveolar septa is highlighted by Von Kossa stain showing a honeycomb arrangement in a 9-year-old male goat (bar = 200 μm).
In the heart, there were multifocal, 1–3-mm firm, gritty nodules on the aortic valve semilunar cusps, or the left atrioventricular valve leaflets and chordae tendineae (b). The right atrioventricular valve was only sporadically involved, and the pulmonary valve was spared.
In the lung, mineralisation of the dorsocaudal lungs was evident macroscopically in 8/32 affected goats and comprised numerous (10–50) gritty, pink-white, 1–10-mm nodules, which coalesced to form irregularly stellate concretions of mineralised parenchyma up to 4 × 5 × 1.5 cm (c). Microscopically, in the lung, the alveolar septa, peribronchial interstitium, and tunica media of large vessels were multifocally expanded by amorphous basophilic granular mineral, which stained positively with Von Kossa for Ca. Multifocally alveolar septa were expanded by osteoid (osseous metaplasia) (c and d). Chondroid metaplasia was infrequent.
The kidneys were macroscopically unremarkable but microscopically, mineral expanded the renal interstitium, lined tubular basement membranes and replaced tubular epithelial cells. In the medulla and at the corticomedullary junction tubular lumina also contained mineral.
There was no microscopic evidence of hyperplasia in the internal parathyroid gland or of C-cells in the thyroid gland. The parathyroid glands were small or normal-sized.
There was no macroscopic mineralisation of musculoskeletal tissues, or osteosclerosis. Bone was microscopically examined in four goats. A lumbar vertebra from one goat had prominent, basophilic, fibrillar, tangled matrix lining Haversian canals and lamellae consistent with chronic vitamin D toxicosis (a and b). In this goat, rare osteoclasts were present. However, there was no evidence of osteocyte necrosis, or trabecular sclerosis.
Laboratory findings
Blood samples were collected from 25 adult goats. The sampled animals were from two different herds. The first group were from the affected herd described above with soft tissue mineralisation, and comprised 15 adult Toggenburg does (n = 13) and wethers (n = 2). Lactating does within the affected herd (n = 3) received goat pellets (Reliance Goat Performance, Reliance Feeds, Rolleston, NZ) providing approximately 2 μg/goat/day (80 IU) of vitamin D (unspecified as to vitamin D2 or D3). The second group was a herd of 10 Toggenburg goats with no history of soft tissue mineralisation (unaffected herd), comprising does (n = 7) and bucks (n = 3), from a small herd in the same region, reared in full sunshine but with access to shade and fed ad libitum pasture and a salt lick with no added vitamin supplementation. During the period of sampling there were three lactating does in the unaffected herd. This herd was chosen for comparison due to being the same breed, having a similar sex distribution, and living in the same area, in a similar farm environment to the herd with soft tissue mineralisation. The mean age of goats in the unaffected herd was 4 (min 1, max 11) years, and in the affected herd with soft tissue mineralisation was 4.8 (min 1, max 9) years.
Blood was collected from all 25 goats by jugular venipuncture into 10-mL plain vacutainer tubes (BD, Plymouth, UK) at 2-monthly intervals for 12 months (April 2018–March 2019). Serum was separated by centrifugation at 3500 g for 15 minutes within 5 hours of collection and transferred to 1.5-mL tubes for storage at −20°C until further analysis. All procedures involving animals were approved by the Massey University Animal Ethics Committee (protocol 17/24).
Serum 25-hydroxyvitamin D2 (25OHD2) and 25-hydroxyvitamin D3 (25OHD3) concentrations were measured using isotope-dilution liquid chromatography-tandem mass spectrometry (LC-MS/MS) at Canterbury Health Laboratories (Christchurch, NZ). Assays conducted by this laboratory have a lower quantifiable limit for 25OHD2 and 25OHD3 concentration of 4 nmol/L, and limit of detection of 1 nmol/L. Serum phosphorus and total Ca concentrations were measured using a Roche Hitachi 911 chemistry analyser (Roche Diagnostics, Indianapolis, IN, USA) at IDEXX/New Zealand Veterinary Pathology (Palmerston North, NZ).
Statistical analyses were performed using Rstudio (R Foundation for Statistical Computing, Vienna, Austria) with the ggplot2 (Wickham Citation2016) package for plots and the lme4 package (Bates et al. Citation2015) for the model. Serum concentrations of total Ca, P, 25OHD2, 25OHD3 and total 25OHD were expressed as absolute values and plotted per sample month.
A linear mixed effects model was developed for each serum analyte (Ca, P, total 25OHD, 25OHD2 and 25OHD3) to analyse the relationship between each analyte and class (affected vs. control goats). For each analyte, the level analyteij for goat i during month j was modelled allowing average levels to differ by month (May, July, September, November, January, March) and classi (affected vs. control), and accounting for goat variation via a random effect goati:
All models were repeated in a reduced form that excluded the fixed effect of class (affected vs. control) and compared with their original by likelihood ratio testing with ANOVA to obtain p-values, and thus determine whether there was a significant difference resulting from class (affected vs. control).
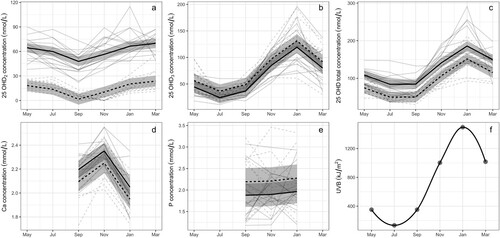
The model showed that the total serum 25OHD concentration from goats in the affected herd with soft tissue mineralisation was 34.2 (95% CI = 18.9–49.4) nmol/L greater (p < 0.001) than in goats from the unaffected herd, when controlling for the month of sampling. Similarly, serum 25OHD2 concentration was also higher (p < 0.001) in the goats from the affected herd compared with goats from the unaffected herd by 46.2 (95% CI = 39.2–53.2) nmol/L. However, no evidence for a difference in serum 25OHD3 concentrations was found (95% CI = −1.77 to 25.8 nmol/L; p = 0.084) (). Although the means of total serum calcium concentrations in goats from the herds with and without soft tissue mineralisation remained within the laboratory reference interval (2.0–2.52 mmol/L), serum Ca concentrations were 0.101 (95% CI = 0.005–0.196) mmol/L higher (p = 0.039) in goats from the affected herd compared with goats from the unaffected herd. There was no evidence for a difference in serum P concentration between goats in the two herds (95% CI for difference between herds = −0.056 to 0.682 mmol/L; p = 0.092).
Figure 4. Graph of absolute values for concentrations of (a) 25-hydroxyvitamin D2 (25OHD2; nmol/L), (b) 25-hydroxyvitamin D3 (25OHD3; nmol/L), (c) 25-hydroxyvitamin total D2 and D3 (25OHD total; nmol/L), (d) calcium (Ca; mmol/L), and e) phosphorus (P; mmol/L) in serum between April 2018 and March 2019 for goats from a herd affected by soft tissue mineralisation (n = 15; grey solid line), and an unaffected herd (n = 10; grey dotted line). Overlaid are the results of the linear mixed effects model for affected goats (black solid line) and unaffected goats (black dashed line), with the grey shading indicating the 95% CI. (f) Graph of monthly ultraviolet B (UVB) radiation.
Vegetation survey
Pasture, shrubs and trees on the property were surveyed every 2 months during the year of blood sampling along evenly spaced line transects through all paddocks at even widths, which were further traversed perpendicularly to form a grid array. The property was assessed for the following known calcinogenic plants: T. flavescens, S. glaucophyllum, Cestrum diurnum, N. veitchii, N. rivularis; any plant sharing these genera; and other plants known to contain vitamin D (mushrooms, lichen, algae, cocksfoot (Dactylis glomerata), capsicum (Capsicum anuum)). Grass specimens were sent to the Allan Herbarium (Manaaki Whenua – Landcare Research, Lincoln, NZ) and the Dame Ella Campbell Herbarium (Massey University, Palmerston North, NZ) for identification. Exclosure cages were placed in three paddocks for 12 months to permit seed-head maturation for grass species identification. A vegetation survey of all identified grasses, weeds, shrubs and trees and their location was compiled (see Supplementary Table 1).
No known calcinogenic species were identified. Known plant sources of vitamin D identified on the farm included mushrooms (Laccaria sp., Cortinarius sp. and Leucoagaricus sp.) in March–May; cocksfoot (D. glomerata) and lichen (probable Cladina sp.) year round; and pine pollen (Pinus radiata) and algae (species unidentified) in July.
Discussion
The main finding in this study was mineralisation of the aorta, heart valves and blood vessels throughout the body with macroscopic and microscopic lesions consistent with published reports of enzootic calcinosis, albeit at the mild end of the spectrum. This New Zealand goat herd had only a single case of lameness accompanied by musculoskeletal mineralisation, and no cachexia, whereas these are the predominant clinical signs observed in severe enzootic calcinosis outbreaks overseas (Krook et al. Citation1975; Braun et al. Citation2000; Gufler et al. Citation2005). However, other studies do report more subtle clinical signs, such as decreased body weight, stiffness and decreased reproductive performance with vascular mineralisation (Schild et al. Citation2021), which are more comparable to the findings in this case series.
The low severity of soft tissue mineralisation in this herd, the small herd size and small genetic pool of Toggenburg goats in New Zealand make a hereditary disorder of cardiovascular calcification a possible cause (Rutsch et al. Citation2021). However, with soft tissue mineralisation identified in 91% of goats analysed by histopathology between 1991 and 2019, this is a higher prevalence than would be expected for a genetic disease, even with autosomal dominant inheritance (Dittmer & Thompson Citation2015).
In the absence of an obvious genetic cause the most likely cause of the soft tissue mineralisation is enzootic calcinosis due to ingestion of a toxic plant containing a form of vitamin D. The milder disease seen in these goats compared with much of the disease described in the literature could hypothetically result from either a smaller magnitude spike of hypercalcaemia, a shorter duration of hypercalcaemia, or both. Low-magnitude hypercalcaemia could result from ingesting small quantities of plant due to sparse growth on the farm, low-level hay contamination, or low calcinogenic potency of the offending plant, whereas a short duration of hypercalcaemia could be more consistent with contamination of purchased hay, a short plant-growth season, or toxicity of a particular life-stage (e.g. flowering). Experimental intoxication of cattle with S. malacoxylon (syn. S. glaucophyllum), administered as either multiple small doses or a single high dose, resulted in similar lesions (Done et al. Citation1976). Additionally, N. veitchii is considered to be 32–78 times less toxic than S. malacoxylon (syn S. glaucophyllum), and sheep and cattle fed N. rivularis have lesions similar to those described in the goats in this case series (García y Santos et al. Citation2012; Schild et al. Citation2021).
Compared with goats from the unaffected herd, those in the affected herd had significantly increased concentrations of total 25OHD, 25OHD2, and total Ca; however, the Ca concentration remained within the reference range. Three aspects of the results warrant consideration. The first is whether the finding that serum Ca and P concentrations were within the reference range in affected animals excludes the possibility of vitamin D intoxication. The second is whether the total serum 25OHD concentration in the present study constitutes toxic concentrations. Finally, how does the finding of high serum 25OHD2 concentration in the present study affect which plants are identified as candidates for the cause of vitamin D intoxication in this herd, given that previous studies have attributed enzootic calcinosis to plants containing one of, or a combination of, vitamin D3, 25OHD3 or 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) glycosides but not vitamin D2 (Machado et al. Citation2020a)?
In this study, mean serum Ca and P concentrations in the affected goats remained within the laboratory reference ranges, which raises concern over the physiological significance of the increased total 25OHD concentration in serum. However, given the infrequency of blood sampling, changes in serum Ca and P concentrations may not have been detected if they were transient. The half-life of vitamin D is up to 2 months (Jones Citation2008; Cusano, et al. Citation2018), that of 25OHD is 15–20 days (Jones Citation2008) and 4–15 hours for 1,25(OH)2D (Gattineni et al. Citation2011; Cusano et al. Citation2018). If ingestion of the calcinogenic plant was sporadic, the resulting short period of hypercalcaemia could easily have been missed due to the blood sampling interval. Additionally, if the calcinogenic principle was 1,25(OH)2D, this would not be detected by serum 25OHD assays regardless of timing of sampling. Unfortunately, serum 1,25(OH)2D analysis is not available in New Zealand, and given the short half-life it is unlikely to have been of diagnostic assistance in this case series.
While hypercalcaemia and hyperphosphataemia are the hallmarks of vitamin D toxicosis on serum biochemistry, in published cases of naturally occurring enzootic calcinosis serum Ca and P concentrations are only reported sporadically, and of those that do, many animals with severe mineralisation have normal serum Ca and P concentrations at presentation, or a normal mean herd Ca concentration in serum is reported (Gill et al. Citation1976; Gufler et al. Citation1999, Citation2005). As such, some authors suggest that the absence of hypercalcaemia and/or hyperphosphataemia does not rule out a diagnosis of enzootic calcinosis (Machado et al. Citation2020b). Additionally, rapid normalisation of increased Ca and P concentrations after cessation of grazing S. glaucophyllum occurs within 48–72 hours in cattle (Worker and Carrillo Citation1967), and 1 week in goats (Gotardo et al. Citation2014), and so hypercalcaemia and hyperphosphataemia may simply reflect recent ingestion of the toxic principle (Machado et al. Citation2020b). In fact, a recent paper has suggested that the 1,25(OH)2D3 glycoside binds to vitamin D receptors on vascular smooth muscle cells resulting in a change in cell type to one that produces an extracellular matrix that can undergo mineralisation and subsequently formation of bone and cartilage (Machado et al. Citation2022); as such, the serum Ca and P concentrations may be a clinical sign of poisoning rather than a cause of the soft tissue mineralisation.
The safe upper limit for serum 25OHD concentrations in goats is not clear. Published values vary markedly between serum assay methods (Holick et al. Citation2011), particularly between radioimmunoassay and LC-MS/MS, making comparisons difficult. The highest total 25OHD concentration reported in serum of healthy goats is 261 nmol/L (herd range 117–261 nmol/L) in a pasture-fed goat at altitude, during summer, measured by LC-MS/MS (Kohler et al. Citation2013). Concentration of total 25OHD in serum of affected goats in the current study exceeded the previously reported upper normal maximum (261 nmol/L) reported by Kohler et al. (Citation2013) on only one occasion. This suggests that the serum total 25OHD concentrations in these goats may not be toxic, but further work is required to confirm this.
The affected herd in this study had increased serum 25OHD2 concentrations compared with the unaffected herd. Significant vitamin D2 is present in good-quality hay (Jäpelt and Jakobsen Citation2013), and the affected herd had a greater hay intake compared with the unaffected goats, which may explain the greater serum 25OHD2 concentrations. However, as a similar proportion of hay is commonly included in production animal rations, the increased 25OHD2 concentration is unlikely to be the cause of the soft tissue mineralisation. The goats from the unaffected herd had similar 25OHD3 concentrations to the affected herd, suggesting a 25OHD3 plant toxin is unlikely. As such, given excessive 25OHD of any form is an unlikely cause of the soft tissue mineralisation, an undetected plant containing 1,25(OH)2D3 as the toxic principle is the most likely cause of enzootic calcinosis in these goats.
One of the limitations of this study is the small sample size of animals sampled for blood analysis of vitamin D metabolites, Ca and P, which impacted power of the statistical analysis. Additionally, sampling was not random; all animals on the farm with no soft tissue mineralisation were sampled. And, while the number of males, females, lactating and pregnant animals were similar between the herds it is possible that these and other differences between the farms may have contributed to differences in the analytes. Further limiting these results is that the affected animals are from a single herd and the unaffected animals are from a single, different herd. As such, any differences could be due to farm, rather than disease status.
In conclusion, goats from a farm in the Manawatū District were diagnosed with enzootic calcinosis. This disease affects animals insidiously and in most cases is due to ingestion of a plant containing 1,25(OH)2D3 glycosides, as is likely to be the case in these goats. Unfortunately, the toxic plant could not be determined, and additional studies are required to determine the cause.
Supplemental Material
Download PDF (271.8 KB)Acknowledgements
The authors would like to acknowledge the enthusiasm and assistance of the farmer with the affected goats, and the assistance of the farmers of the unaffected goats. Additionally, thanks go to Peter Elder for the vitamin D analyses, and to Mike Hogan, Bryce Ilton, Kirsty Anderson, Evelyn Lupton and Saritha Gil, and the Massey University pathologists and pathology residents for post-mortem and histology support. This work was supported by the School of Veterinary Science Postgraduate Fund.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- *Anonymous. Allan Herbarium (CHR) Specimen Data. https://scd.landcareresearch.co.nz (accessed 8 July 2018). Manaaki Whenua – Landcare Research, Wellington, NZ, 2013
- Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 1–48, 2015. https://doi.org/10.18637/jss.v067.i01
- Braun U, Diener M, Camenzind D, Thoma R, Flückiger M. Enzootic calcinosis in goats caused by golden oat grass. Veterinary Record 146, 161–2, 2000. https://doi.org/10.1136/vr.146.6.161
- *Cusano NE, Thys-Jacobs S, Bilezikian JP. Hypercalcemia due to vitamin D toxicity. In: Feldman D (ed). Vitamin D. 3rd Edtn. Pp 1381–402. Academic Press/Elsevier, Cambridge, MA, USA, 2018
- Dittmer KE, Thompson KG. Approach to investigating congenital skeletal abnormalities in livestock. Veterinary Pathology 52, 851–61, 2015. https://doi.org/10.1177/0300985815579999
- Done SH, Döbereiner J, Tokarnia CH. Systemic connective tissue calcification in cattle poisoned by Solanum malacoxylon: a histological study. British Veterinary Journal 132, 28–38, 1976. https://doi.org/10.1016/S0007-1935(17)34785-1
- García y Santos C, Pereira R, Etcheberry G, Goyen JM, Pérez W, Capelli A, Alonso E, Ruiz-Díaz A, Riet-Correa F. Enzootic calcinosis caused by Nierembergia rivularis in sheep. Journal of Veterinary Diagnostic Investigation 24, 423–6, 2012. https://doi.org/10.1177/1040638711435143
- Gattineni J, Twombley K, Goetz R, Mohammadi M, Baum M. Regulation of serum 1,25(OH)2 vitamin D3 levels by fibroblast growth factor 23 is mediated by FGF receptors 3 and 4. American Journal of Physiology – Renal Physiology 301, 371–7, 2011. https://doi.org/10.1152/ajprenal.00740.2010
- Gill BS, Singh M, Chopra AK. Enzootic calcinosis in sheep: clinical signs and pathology. American Journal of Veterinary Research 37, 545–52, 1976
- Gotardo AT, Hueza IM, Raspantini PCF, Maiorka PC, Górniak SL. Evaluation of the use of goats as an animal model in Solanum malacoxylon toxicity: growing goats and prenatal study. Annals of Clinical Pathology 2, 1032, 2014
- Gufler H, Bagó Z, Speckbacher G, Baumgartner W. Calcinosis in goats. Deutsche Tierärztliche Wochenschrift 106, 419–24, 1999
- Gufler H, Novak J, Reifinger M. Enzootic calcinosis in sheep: clinical, sonographical, blood-chemical, and pathohistological findings. Wiener Tierärztliche Monatsschrift 92, 58–65, 2005
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. The Journal of Clinical Endocrinology & Metabolism 96, 1911–30, 2011. https://doi.org/10.1210/jc.2011-0385
- Jäpelt RB, Jakobsen J. Vitamin D in plants: a review of occurrence, analysis and biosynthesis. Frontiers in Plant Science 4, 136, 2013. https://doi.org/10.3389/fpls.2013.00136
- Jones G. Pharmacokinetics of vitamin D toxicity. The American Journal of Clinical Nutrition 88, 582–6, 2008. https://doi.org/10.1093/ajcn/88.2.582S
- Kohler M, Leiber F, Willems H, Merbold L, Liesegang A. Influence of altitude on vitamin D and bone metabolism of lactating sheep and goats. Journal of Animal Science 91, 5259–68, 2013. https://doi.org/10.2527/jas.2013-6702
- Krook L, Wasserman RH, Shively JN, Tashjian, AH Jr, Brokken TD, Morton JF. Hypercalcemia and calcinosis in Florida horses: implication of the shrub, Cestrum diurnum, as the causative agent. Cornell Veterinarian 65, 26–56, 1975
- Lopez DV, Al-Jaberi FAH, Woetmann A, Ødum N, Bonefeld CM, Kongsbak-Wismann M, Geisler C. Macrophages control the bioavailability of vitamin D and vitamin D-regulated T cell responses. Frontiers in Immunology 12, 722806, 2021. https://doi.org/10.3389/fimmu.2021.722806
- Machado M, Castro MB, Gimeno EJ, Barros SS, Riet-Correa F. Enzootic calcinosis in ruminants: a review. Toxicon 187, 1–9, 2020a. https://doi.org/10.1016/j.toxicon.2020.08.009
- Machado M, Schild CO, Preliasco M, Balserini A, Medeiros RMT, Barros SS, Riet-Correa F. Enzootic calcinosis in sheep in Uruguay: a brief review and report of two outbreaks. Pesquisa Veterinária Brasileira 40, 2020b. https://doi.org/10.1590/1678-5150-pvb-6766
- Machado M, Castro MB, Wilson TM, Gonçalves AAB, Portiansky EL, Riet-Correa F, Barros SS. Poisoning by Nierembergia veitchii: effects on vascular smooth muscle cells in the pathogenesis of enzootic calcinosis. Veterinary Pathology 59, 814–23, 2022. https://doi.org/10.1177/03009858221098430
- *Miller MA, Lyle LT, Zachary JF. Mechanisms and morphology of cellular injury, adaptation, and death. In: Zachary JF (ed). Pathologic Basis of Veterinary Disease. 7th Edtn. Pp 16–73. Elsevier, St Louis, MO, USA, 2022
- Riet-Correa F, Machado M, Micheloud JF. Plants causing poisoning outbreaks of livestock in South America: a review. Toxicon X 17, 100150, 2023. https://doi.org/10.1016/j.toxcx.2023.100150
- Rutsch F, Buers I, Nitschke Y. Hereditary disorders of cardiovascular calcification. Arteriosclerosis, Thrombosis, and Vascular Biology 41, 35–47, 2021. https://doi.org/10.1161/ATVBAHA.120.315577
- Schild CO, Boabaid F, Machado M, Saravia A, Oliveira LGS, Díaz S, Vildoza A, Martinez A, Martínez R, Barros SS, et al. Nierembergia rivularis poisoning in cattle. Toxicon 204, 21–30, 2021. https://doi.org/10.1016/j.toxicon.2021.10.009
- *Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer, New York, NY, USA, 2016
- Worker NA, Carrillo BJ. “Enteque Seco”: calcification and wasting in grazing animals in the Argentine. Nature 215, 72, 1967. https://doi.org/10.1038/215072a0
- *Non-peer-reviewed