ABSTRACT
Case history
In 2023, the New Zealand Department of Conservation seized 63 endemic reptiles that were being held without a permit. This group included three adult female West Coast green geckos (Naultinus tuberculatus) that had been illegally removed from the wild 2 years earlier. They had been held in an outdoor enclosure with a pair of goldstripe geckos (Woodworthia chrysosiretica).
Clinical findings
On physical examination, all three geckos had at least two soft palpable masses in the coelom. Repeated ultrasonographic examination over several months confirmed the diagnosis of pre-ovulatory follicular stasis (POFS) in each gecko, and in subsequent weeks, more ovarian follicles developed in each animal.
Laboratory findings
All three geckos were negative on culture of cloacal swabs for Salmonella spp., and negative on PCR assay of a cloacal flush for Cryptosporidium spp., despite other reptiles in the seized group showing positive results for multiple Salmonella spp., and one other gecko being positive for Cryptosporidium parvum, subtype IIcA5G3.
Treatment and outcome
For all three geckos, para-midline ventral coeliotomy was performed under general anaesthesia, and folliculectomy of degenerate ovarian follicles was performed. Post-operative complications were seen in all three animals, which developed suture-line infections following disruption of normal skin shedding and entrapment of shed keratin in the surgical sites. A second surgery was undertaken to remove impacted keratin and caseous inflammatory material from the surgical wounds of all three animals and buried sutures were placed to close the coelomic wounds. The geckos were treated with 20 mg/kg ceftazidime IM every second day for 2 weeks post-operatively. Subsequent ecdysis (skin shedding) occurred without complication and the geckos were released back to the wild 10 months after admission.
Clinical relevance
The recommended treatment for POFS in reptiles is ovariectomy, which is not appropriate for wild animals. The use of folliculectomy to resolve preovulatory follicular stasis should be considered for animals where retaining reproductive ability is essential.
Introduction
New Zealand endemic reptiles are threatened by invasive predators, habitat degradation and destruction, and by poaching for the pet trade (Hitchmough et al. Citation2016). In recent years, considerable research effort has resulted in taxonomic revisions, discoveries of new species, increased data availability on some populations, and changes in conservation management practices (Nelson et al. Citation2014). The New Zealand Department of Conservation currently recognises 135 taxa of native reptiles (including some undescribed species), of which 36.3% have a conservation status of “threatened” and 49.6% are “at risk” (Hitchmough et al. Citation2021).
New Zealand lizards are characterised by long lifespans and low reproductive rates (Cree and Hare Citation2016). New Zealand geckos are viviparous in contrast to the oviparity in almost all other gecko species internationally (Cree and Hare Citation2016; Girling et al. Citation1997). Female geckos in New Zealand typically mate and begin vitellogenesis in autumn, store sperm over winter and ovulate in spring. Vitellogenesis is the process in which the yolk protein precursor, vitellogenin, is produced in the liver and absorbed by growing ovarian follicles. This generally proceeds rapidly from late summer or autumn in most species. Two enlarged vitellogenic follicles remain present over winter until ovulation in spring. All studied species are lecithotrophic, in that yolk forms the major nutrient resource for embryonic development. Gestation usually lasts at least 3 months, but gestation length, which is temperature dependent, may reach 14 months in some geckos (especially nocturnally foraging species). Some female geckos and skinks reproduce less than annually (Cree and Hare Citation2016). It should be noted that these are generalisations based on limited studies in some species, and that species-specific information on reproduction is often deficient. This brings challenges to the veterinary assessment of reproductive condition, especially in trying to differentiate pathology from normal reproductive physiology (Knotek et al. Citation2017).
Pre-ovulatory follicular stasis (POFS) is a common reproductive disease of reptiles in which disruption of the ovarian cycle results in a failure of ovulation and prolonged persistence of pre-ovulatory follicles (Knotek et al. Citation2017). Causes of the condition have been reported to include keeping female lizards without contact with males (Knotek et al. Citation2017), inappropriate nutrition, or suboptimal environmental conditions (Le Souëf et al. Citation2015). This condition has previously been described in a New Zealand species, Duvaucel’s gecko (Hoplodactylus duvaucelii), with concurrent ovarian granulomas due to salmonellosis (Le Souëf et al. Citation2015), and an example of the condition has been illustrated in a Woodworthia “Otago/Southland” gecko (Cree and Hare Citation2016). Diagnosis of POFS is based on repeated ultrasonographic imaging demonstrating a failure of progression of the ovarian cycle (Cojean et al. Citation2018). In some cases, the follicles will be reabsorbed, but in prolonged stasis, the current treatment recommendation is to perform an ovario-hysterectomy (Le Souef et al. Citation2015; Knotek et al. Citation2017).
This article reports the use of ovarian folliculectomy to resolve POFS in three wild-caught West Coast green geckos (Naultinus tuberculatus), with the aim of allowing the possibility of future reproductive success, and thus, enabling rehabilitation back to their wild habitat.
Case history
In summer 2023, the Department of Conservation seized 63 native reptiles that were being held without a permit. This group included three adult female West Coast green geckos (Lewis Pass form) that were held in an outdoor enclosure with a pair of goldstripe geckos (Woodworthia chrysosiretica). Using pattern matching (from photos of their unique markings) it was found that these individual geckos had been observed in the wild by herpetologists in October 2021. The holder subsequently admitted to having illegally taken these animals from the wild. At the request of the Department of Conservation and as a preliminary health screening while awaiting the resolution of legal proceedings, a wildlife veterinarian (author BG) and two herpetologists examined all 63 animals and carried out diagnostic testing using cloacal swabs for enteric Salmonella spp. and cloacal flushes for Cryptosporidium spp. The three West Coast green geckos were transferred to Wildbase Hospital (Massey University, Palmerston North, NZ) for quarantine ahead of an expected wild rehabilitation. West Coast green geckos are classified as a nationally vulnerable threatened species (Hitchmough et al. Citation2021).
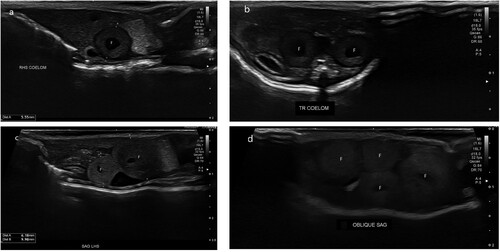
Figure 1. Ultrasonographic images of the coelomic cavity of a female West Coast green gecko (Naultinus tuberculatus) showing a) sagittal view of a single follicle (F) in the coelom on admission; b) transverse view of two follicles (F) in the coelom on admission; c) sagittal view of two follicles (F) in the coelom after 6 months of hospitalisation; d) oblique view of four follicles (F) in the coelom after 6 months of hospitalisation, suggestive of pre-ovulatory follicular stasis.
West Coast green geckos are a diurnal basking species from the South Island of New Zealand and hospital husbandry was based on climatic conditions from their home range. During hospitalisation, the geckos were held together in an indoor vivarium maintained at temperatures ranging from 15–22°C, and a relative humidity between 60 and 95%. There was a vertical thermal gradient in the enclosure, and foliage that provided basking areas close to the light source. A full spectrum light (UVB 5.0; Reptile One, Ingleburn, Australia) was provided and the day-night cycle was matched to external conditions to maintain seasonality. The geckos were offered live insects (wingless flies), fruit puree and a commercial gecko food (Grubs’n’Fruit gecko diet; Repashy, Woodville, NZ).
Clinical findings
On initial physical examination, all three female geckos had at least two soft, palpable masses in the coelom. All three were in good body condition with intact, original tails (body weight: 13.7, 14.7, and 15.6 g; snout–vent length (SVL): 78, 79, and 84 mm). The reported range for the species is an SVL up to 85 mm and weight up to 14.5 g (Anonymous Citation2021).
Due to the palpable coelomic masses, sonographic examinations were performed using an Aplio a550 ultrasound machine (CUS-AA550; Canon Medical Systems Corp., Ōtawara, Japan) with a high frequency linear array transducer (18-L7 Linear Transducer, PLT-1204BT/MA; Canon Medical Systems Corp.). Ultrasound gel (Aquasonic 100 Ultrasound Transmission Gel; Parker Laboratories Inc., Fairfield, NJ, USA) was applied to the ventrum as an ultrasound coupling medium. The image was optimised via adjustment of depth and frequency.
At each examination, the coelom was scanned in both sagittal and transverse planes. Initial ultrasonographic examination revealed each animal had two intra-coelomic spherical masses (a), but it was unclear whether these were ovarian follicles or early embryos in the oviduct. No fetal heartbeats or vasculature were detected with power Doppler interrogation. Although the three females had been held without a conspecific male, they had been housed with a male goldstripe gecko. The risk of hybridisation was thought to be low, but this possibility precluded wild release until the geckos’ reproductive status could be confirmed.
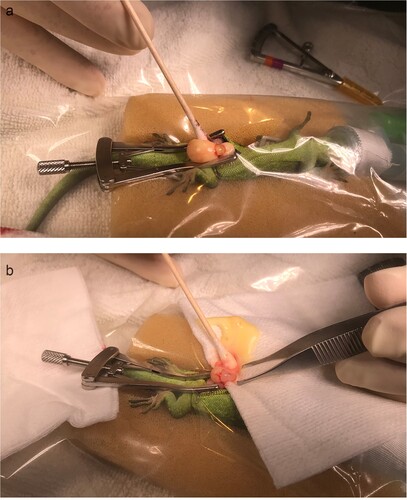
Figure 2. Intra-operative images of the exploratory coeliotomy of a female West Coast green gecko (Naultinus tuberculatus) with preovulatory follicular stasis, showing a) the ovary and two follicles exterior to the paramedian incision; b) the technique used for expressing the yolk prior to folliculectomy. The gecko is under a transparent surgical drape.
The geckos had seven ultrasonographic examinations over 6 months that showed minimal change in the sonographic appearance of these masses, suggesting a diagnosis of POFS in each gecko, and, in subsequent weeks, two additional ovarian follicles developed in each animal without regression of the original masses (b).
Laboratory findings
For the preliminary health screening tests requested by the Department of Conservation, cloacal swabs were aerobically cultured and assessed for Salmonella spp. by IDEXX Laboratories (Palmerston North, NZ) using standard methods. A sample of positive cultures was sent to the Institute of Environmental Science and Research (Auckland, NZ) for serotyping. Cloacal washes were analysed by PCR for Cryptosporidium spp. by the Hopkirk Laboratory (Palmerston North, NZ) using methods described in Garcia-R et al. (Citation2023). All three West Coast green geckos were negative on culture of cloacal swabs for Salmonella spp., and negative on PCR assay of a cloacal flush for Cryptosporidium spp., despite 16 other reptiles (including Naultinus, Woodworthia and Mokopirirakau geckos and Oligosoma grande skinks) in the seized group showing positive results for multiple Salmonella spp., and one ngahere gecko (Mokopirirakau “southern North Island”) being positive for Cryptosporidium parvum, subtype IIcA5G3.
Treatment and outcome
Six months after admission, permission was obtained to proceed to exploratory laparotomy. General anaesthesia was induced with 1 mg/kg morphine (Morphine Sulfate; Biomed, Auckland, NZ), and 9 mg/kg alfaxalone (Alfaxan; Jurox, Auckland, NZ) IM in the right biceps muscle. Loss of righting reflex and toe-pinch response occurred in approximately 5 minutes. Anaesthesia was maintained with 1% sevoflurane (Baxter Healthcare, Auckland, NZ) in oxygen via a mask. The heart rate was monitored by a Doppler probe and varied from 60–120 beats per minute during surgery. As is commonly observed (Sladky and Mans Citation2012), voluntary respiration ceased early during anaesthesia in all three geckos, and they were subsequently manually ventilated every 2–3 minutes throughout surgery. The effectiveness of ventilation was assessed by chest wall movements and capnography. The geckos were maintained at room temperature (22°C) throughout the surgery.
An operating microscope (Zeiss OPMI pico surgical microscope; Carl Zeiss, Jena, Germany) was used throughout the surgeries. The geckos were placed in dorsal recumbency and a right paramedian incision was made through the skin and then the coelomic musculature. The incision was approximately 1 cm long and located in the caudal third of the coelom. Some haemorrhage occurred on entry from the coelomic fat pad, which was controlled with pressure using a cotton tip applicator. In each gecko, the ovaries were brought outside the coelom one at a time by manipulation with a cotton tip applicator. Each ovary had two large yolky follicles in tight adherence to each other and the underlying ovary, confirming the diagnosis of POFS, as there should only be one large vitellogenic follicle present in each ovary in New Zealand geckos (a). The largest follicle on each ovary was incised and drained of yolk using pressure from cotton tip applicators (b). The remaining vitellogenic follicular tissue was dissected away from each ovary using blunt dissection with cotton tip applicators while the eviscerated follicle was held with forceps. No haemorrhage occurred during this procedure and no ligation was necessary. At the conclusion of the surgery, each ovary had only pre-vitellogenic follicles. The coelom was flushed with 0.5 mL saline and the coelomic muscle wall was closed with 5–0 polydioxanone suture with a swaged 17-mm 1/2c taperpoint needle (PDS II; Ethicon, Auckland, NZ) in single interrupted sutures. The skin was sutured to apposition also using 5–0 PDS in single interrupted sutures.
Post-operatively, 0.5 mL 0.9% normal saline (Baxter, Auckland, NZ) was given SC. The geckos regained voluntary movement 5–10 minutes after cessation of anaesthesia. The animals recovered in intensive care units at 25°C overnight and returned to their usual enclosures the next morning, once they had regained the ability to climb.
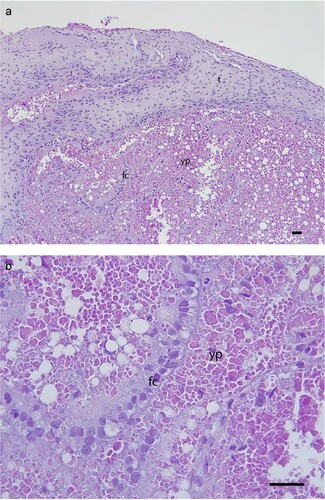
Samples of the yolk were sent to IDEXX Laboratories for aerobic bacterial culture but showed no organisms on initial Gram staining or growth on culture. The remaining follicular tissue was clamped, removed and submitted for histology, which showed no evidence of inflammation or degenerative change, although there was disruption of the follicular cells from the theca of the follicle (). It is unclear if this disruption was due to the surgical manipulation, or a change secondary to POFS.
Figure 3. Histology of the resected ovarian follicle, showing: a) relatively thick theca (t), and the single cuboidal-columnar layer of follicular cells (fc) are displaced, possibly as a result of the surgery but this may be secondary to POFS; b) the follicular cells (fc) and mature yolk protein (yp) with very few vesicles present and no evidence of inflammation or degeneration (H&E, scale bars = 50 µm).
Post-operative complications were seen in all three animals, which developed suture-line infections following disruption of normal skin shedding and entrapment of shed keratin in the surgical sites 5 weeks after the initial surgery. A second surgery was undertaken in each gecko using the same anaesthetic protocol as previously described to remove impacted keratin and caseous inflammatory material from the surgical wounds. In two geckos, the infection was confined to the skin, but the third gecko showed infection of the coelomic wall musculature that required excision of the infected material along the original incision. Simple interrupted buried sutures using 5–0 PDS were placed to close the skin in two geckos, and the skin and coelomic wall in two layers in the third gecko. The geckos were treated with 20 mg/kg ceftazidime SC (AFT Pharmaceuticals Ltd., Auckland, NZ) every second day for 2 weeks post-operatively. Subsequent ecdysis (skin shedding) occurred without complication and the three geckos were released back to the wild 10 months after admission.
Discussion
The diagnosis of POFS in this case was complicated by our limited familiarity with the normal ultrasonographic appearance of female reproductive structures in New Zealand geckos. While the imaging anatomy of leopard geckos (Eublepharis macularius) has been reported (Cojean et al. Citation2018), New Zealand geckos differ in being viviparous. Imaging studies of viviparous lizards are mostly reported for larger-bodied species with multiple offspring such as chameleons (Chamaeleo calyptratus) (Cigler et al. Citation2023) and blue-tongued lizards (Tiliqua nigrolutea) (Gartrell et al. Citation2002), although experience with smaller-bodied lizards is increasing (Gilman and Wolf Citation2007). We were expecting the ovarian follicles to have a more homogeneous inner echodensity, rather than the donut ring appearance we encountered. Matayoshi et al. (Citation2018), working with a snake, also reported that vitellogenic follicles had a hyperechoic border and hypoechoic centre. We were unable to confidently distinguish the ovarian follicles from early conceptuses in the oviduct, in which gecko embryos develop on the periphery of the large yolk ovum (Cree and Hare Citation2016). In retrospect, the spherical shape of the follicles is more consistent with ovarian follicles, as once in the oviducts the conceptuses take on an ovoid shape in related species (e.g. Wilson and Cree (Citation2003) for Naultinus gemmeus). This shape becomes increasingly more pronounced as pregnancy progresses, i.e. the embryos grow relatively more in the cranial-caudal direction. However, with progressive ultrasound examinations, the lack of developing embryonic structures, such as heart, vascular system and skeletal elements such as the spine and skull (Gartrell et al. Citation2002) enabled us to be more confident of the POFS diagnosis. The development of additional ovarian follicles in each gecko, as detected by ultrasonography prior to surgery, confirmed the diagnosis, as New Zealand geckos only produce 1–2 offspring per year, and normally recruit only one follicle per ovary into vitellogenesis (Cree Citation1994). Histological examination of the resected follicles confirmed these as degenerate follicles rather than atretic (where granulosa cells and phagocytes are actively phagocytising yolk) or corpora lutea (where the yolk is absent following ovulation of the oocyte, and the granulosa cells proliferate within the theca) (Jacobsen Citation2007).
We can only speculate as to the cause of POFS in these three geckos as information on their previous husbandry was limited. Upon admission, all three geckos were in good body condition and showed no other evidence of disease, suggesting that their husbandry and diet prior to seizure had been adequate. Cultures of the yolk removed at surgery were negative and the histology of the follicular membranes showed no evidence of inflammation or infection, unlike the Duvaucel’s gecko reported previously with salmonellosis (Le Souëf et al. Citation2015). On this basis, we suggest the most likely cause for all three animals to enter POFS was the absence of a conspecific male. Male absence has been suggested as a cause of POFS in female lizards of other species kept as solo pets (Knotek et al. Citation2017) and may be linked to pheromonal communication and associated behavioural triggers (Mason and Parker Citation2010; Schořálková et al. Citation2018). Ideally, we would have tested this hypothesis by introducing a male Naultinus tuberculatus, and monitoring subsequent reproductive activity but this was not possible. The West Coast green geckos were held in the same enclosure as goldstripe geckos prior to seizure. The wild distribution of the two species does not overlap and we have no information on whether any inter-species conflict occurred while they were held together. The stress of being held with a different species may have contributed to the failure of normal reproduction but this could not be confirmed.
We based the husbandry conditions for the geckos in hospital on average temperatures of their home range. However, the preferred body temperature of other Naultinus spp. is higher than we used. For example, the mean thermal preference in N. gemmeus reaches approximately 27°C for non-pregnant adults (females and males) during the afternoon/evening (Besson and Cree Citation2011). Higher individual values (body temperatures up to 31°C) have been reported for Naultinus spp. in the field, but these probably included pregnant females, which are likely to have a higher thermal preference (Hare and Cree Citation2016). If the temperatures we provided were sub-optimal this may have contributed to the POFS. However, at the temperatures provided, the geckos maintained good activity and bodyweight, underwent ecdysis regularly, and wound healing progressed well once the infection resolved. This uncertainty highlights the need for species-specific information on thermal preferences in New Zealand reptiles.
While regression of POFS has been seen in some animals (Knotek et al. Citation2017), there was no evidence of this in these three geckos over a 6-month period. Prior to this report, the recommended treatment for refractory POFS was ovario-hysterectomy (Le Souef et al. Citation2015; Knotek et al. Citation2017), which was not appropriate if these animals were to be returned to the wild. The wild release of non-breeding animals is contraindicated in the conservation management of threatened species, as these animals potentially displace and compete with breeding individuals. We postulated that by removing the degenerate follicles and returning the geckos to their wild habitat, the reproductive cycle could be restarted. This prompted us to attempt the novel treatment of folliculectomy for the POFS. While this return to reproductive functioning has not been confirmed, it is possible that these animals will be re-sighted, as New Zealand geckos have unique skin patterning that can be used to identify individual animals (Knox et al. Citation2013; Lettink and Monks Citation2016).
The surgical removal of the follicles was relatively simple to accomplish under magnification, and no major complications were encountered during the surgery. As the surgery was accomplished without contamination, we did not use post-operative antibiotics, and initial wound healing for the first few weeks seemed to be going well. However, all three geckos underwent ecdysis approximately 4 weeks after surgery, which resulted in dead skin becoming impacted in the suture line. Despite efforts to remove it and disinfect the site with chlorhexidine, caseous inflammatory material developed subsequently in the healing surgical wounds. Dysecydis often results in secondary bacterial or fungal infections (Hellebuyck et al. Citation2012) and can delay skin healing following surgery (Di Girolamo and Mans Citation2016). In these cases, the use of buried sutures at the second surgery allowed ecdysis to proceed normally and is recommended for future cases, particularly considering the thin skin of geckos compared to other reptiles.
These geckos were considered good cases for folliculectomy based on their general good health and the documented (based on repeated ultrasound examination) failure of progression of reproduction. During surgery, under magnification we were able to clearly distinguish and separate the degenerate follicles from the rest of the ovary. In other cases where the ovary is diseased, folliculectomy may not be feasible, for example with infected ovarian masses where the follicles and ovary have coalesced (Le Souëf et al. Citation2015).
Given the status of the West Coast green gecko as nationally vulnerable, there was a high priority to return these individuals to their wild habitat in spring to give them the best chance of survival and future reproductive success. There is no captive breeding programme for the species. It would have been more informative to hold these animals in captivity for another breeding season and continue to carry out ultrasonographic assessment of the response of the ovaries to folliculectomy. However, no males of the species were available in captivity, and male absence is a known contributing factor to POFS (Knotek et al. Citation2017), so the geckos were released to the wild once the surgical wounds had healed. In future cases, confirmation of the return of reproductive function following folliculectomy using repeated ultrasonographic examination would be useful.
Acknowledgements
We thank Tim Harker and Nick Harker for their assistance in handling and sampling the lizards. Dr Jane Girling provided advice on reptile reproductive anatomy. The Department of Conservation funded the disease screening of the reptiles and partially funded the hospitalisation and care of the West Coast green geckos. We thank the team at Wildbase Hospital, Massey University, for their diligent care of the geckos. Anthony Pita at the Hopkirk Laboratory carried out the PCR assays for Cryptosporidium spp. Dr Stuart Hunter prepared the excised follicles for histological examination.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- *Anonymous. West Coast green gecko Naultinus tuberculatus (McCann, 1955). https://www.reptiles.org.nz/herpetofauna/native/naultinus-tuberculatus (accessed 16 June 2024). New Zealand Herpetological Society, Auckland, NZ, 2021
- Besson AA, Cree A. Integrating physiology into conservation: an approach to help guide translocations of a rare reptile in a warming environment. Animal Conservation 14, 28–37, 2011. https://doi.org/10.1111/j.1469-1795.2010.00386.x
- Cigler P, Dervas E, Richter H, Hatt JM, Kummrow M. Ultrasonographic and computed tomographic characteristics of the reproductive cycle in female veiled chameleons (Chamaeleo calyptratus). Journal of Zoo and Wildlife Medicine 54, 231–43, 2023. https://doi.org/10.1638/2022-0052
- Cojean O, Vergneau-Grosset C, Masseau I. Ultrasonographic anatomy of reproductive female leopard geckos (Eublepharis macularius). Veterinary Radiology & Ultrasound 59, 333–44, 2018. https://doi.org/10.1111/vru.12599
- Cree A. Low annual reproductive output in female reptiles from New Zealand. New Zealand Journal of Zoology 21, 351–72, 1994. https://doi.org/10.1080/03014223.1994.9518005
- Cree A, Hare KM. Reproduction and life history of New Zealand lizards. In: Chapple DG (ed). New Zealand Lizards. Pp 169–206. Springer, Cham, Switzerland, 2016. https://doi.org/10.1007/978-3-319-41674-8_7
- Di Girolamo N, Mans C. Reptile soft tissue surgery. Veterinary Clinics of North America: Exotic Animal Practice 19, 97–131, 2016. https://doi.org/10.1016/j.cvex.2015.08.010
- Garcia-R JC, Pita AB, Velathanthiri N, Pas A, Hayman DTS. Mammal-related Cryptosporidium infections in endemic reptiles of New Zealand. Parasitology Research 122, 1239–44, 2023. https://doi.org/10.1007/s00436-023-07824-4
- Gartrell BD, Girling JE, Edwards A, Jones SM. Comparison of noninvasive methods for the evaluation of female reproductive condition in a large viviparous lizard, Tiliqua nigrolutea. Zoo Biology 21, 253–68, 2002. https://doi.org/10.1002/zoo.10017
- Gilman CA, Wolf BO. Use of portable ultrasonography as a nondestructive method for estimating reproductive effort in lizards. Journal of Experimental Biology 210, 1859–67, 2007. https://doi.org/10.1242/jeb.001875
- Girling JE, Cree A, Guillette LJ Jr. Oviductal structure in a viviparous New Zealand gecko, Hoplodactylus maculatus. Journal of Morphology 234, 51–68, 1997. https://doi.org/10.1002/(SICI)1097-4687(199710)234:1<51::AID-JMOR5>3.3.CO;2-0
- Hare KM, Cree A. Thermal and metabolic physiology of New Zealand lizards. In: Chapple DG (ed). New Zealand Lizards. Pp 239–67. Springer, Cham, Switzerland, 2016. https://doi.org/10.1007/978-3-319-41674-8_9
- Hellebuyck T, Pasmans F, Haesebrouck F, Martel A. Dermatological diseases in lizards. The Veterinary Journal 193, 38–45, 2012. https://doi.org/10.1016/j.tvjl.2012.02.001
- Hitchmough RA, Adams LK, Reardon JT, Monks JM. Current challenges and future directions in lizard conservation in New Zealand. Journal of the Royal Society of New Zealand 46, 29–39, 2016. https://doi.org/10.1080/03036758.2015.1108923
- *Hitchmough R, Barr B, Knox C, Lettink M, Monks JM, Patterson GB, Reardon JT, van Winkel D, Rolfe J, Pascale M. Conservation Status of New Zealand Reptiles, 2021. New Zealand Threat Classification Series 35. Department of Conservation, Wellington, NZ, 2021
- *Jacobsen ER. Overview of reptile biology, anatomy and histology. In: Jacobson ER (ed). Infectious Diseases and Pathology of Reptiles. Pp 1–131. CRC Press, Boca Raton, FL, USA, 2007. https://doi.org/10.1201/9781420004038.ch1
- Knotek Z, Cermakova E, Oliveri M. Reproductive medicine in lizards. Veterinary Clinics of North America: Exotic Animal Practice 20, 411–38, 2017. https://doi.org/10.1016/j.cvex.2016.11.006
- Knox CD, Cree A, Seddon PJ. Accurate identification of individual geckos (Naultinus gemmeus) through dorsal pattern differentiation. New Zealand Journal of Ecology 37, 60–6, 2013
- Le Souëf AT, Barry M, Brunton DH, Jakob-Hoff R, Jackson B. Ovariectomy as treatment for ovarian bacterial granulomas in a Duvaucel's gecko (Hoplodactylus duvaucelii). New Zealand Veterinary Journal 63, 340–4, 2015. https://doi.org/10.1080/00480169.2015.1063468
- Lettink M, Monks JM. Survey and monitoring methods for New Zealand lizards. Journal of the Royal Society of New Zealand 46, 16–28, 2016. https://doi.org/10.1080/03036758.2015.1108343
- Mason RT, Parker MR. Social behavior and pheromonal communication in reptiles. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology 196, 729–49, 2010. https://doi.org/10.1007/s00359-010-0551-3
- Matayoshi PM, Souza PM, Gasparotto VPO, Araujo MS, Simões CRB, Souza FF, Oba E, Machado VMV, Júnior RSF, Prestes NC. Hormonal and ultrasonographic characterization of the seasonal reproductive cycle of male and female Crotalus durissus terrificus. Animal Reproduction 15, 1236–45, 2018. https://doi.org/10.21451/1984-3143-AR2017-0019
- *Nelson NJ, Hitchmough R, Monks JM. New Zealand reptiles and their conservation. In: Stow A, Maclean N, Holwell GI (eds). Austral Ark: the State of Wildlife in Australia and New Zealand. Pp 382–404. Cambridge University Press, Cambridge, UK, 2014. https://doi.org/10.1017/CBO9781139519960.020
- Schořálková T, Kratochvíl L, Kubička L. Female sexual attractiveness and sex recognition in leopard gecko: males are indiscriminate courters. Hormones and Behavior 99, 57–61, 2018. https://doi.org/10.1016/j.yhbeh.2018.01.007
- Sladky KK, Mans C. Clinical anesthesia in reptiles. Journal of Exotic Pet Medicine 21, 17–31, 2012. https://doi.org/10.1053/j.jepm.2011.11.013
- Wilson JL, Cree A. Extended gestation with late-autumn births in a cool-climate viviparous gecko from southern New Zealand (Reptilia: Naultinus gemmeus). Austral Ecology 28, 339–48, 2003. https://doi.org/10.1046/j.1442-9993.2003.01293.x
- *Non-peer-reviewed
