Abstract
The induction assay for the cytochromes P450 (P450s) is an important tool in drug discovery and development. The inductions of dog P450 1A2 and 3A12 by omeprazole and rifampicin were functionally characterised in dog hepatocytes and were compared with induction in human HepaRG and HepaSH cells.
P450 1A2-dependent ethoxyresorufin O-deethylation was induced by R,S-omeprazole and P450 3 A-dependent midazolam 1′-hydroxylation was induced by rifampicin, and both reactions were significantly enhanced in cultured dog hepatocytes and human HepaRG and HepaSH cells.
Recombinant dog P450 1A2 exhibited activities towards R- and S-omeprazole 5-hydroxylation with low Km values of 23–28 µM, whereas dog P450 2C21 and 3A12 efficiently mediated S-omeprazole 5-hydroxylation and sulfoxidation, respectively, with high Vmax values of 12–17 min−1.
Although omeprazole 5-hydroxylation by human P450 2C19 (and sulfoxidation by P450 3A4) in human HepaSH cells were slightly (∼2-fold) induced by R,S-omeprazole, dog P450 1A2 was autoinduced by omeprazole in dog hepatocytes and showed enhanced R-omeprazole 5-hydroxylation activity (∼5-fold).
These results indicate that omeprazole can be an autoinducer of P450 1A2 in hepatocytes, and this enzyme was found to be involved in omeprazole 5-hydroxylation and sulfoxidation in dog hepatocytes and human HepaRG and HepaSH cells.
Introduction
The cytochromes P450 (P450s or CYPs) constitute a family of important drug-metabolizing enzymes that need to be taken into account in drug development, clinical study design, and regulatory reviews (Sager et al. Citation2015). In vitro P450 induction assays are important for drug discovery and development to assess the potential for drug–drug interactions in human pharmacokinetics (Huang et al. Citation2008). The guidelines of the United States Food and Drug Administration, the European Medicines Agency, and the Pharmaceuticals and Medical Devices Agency in Japan recommend evaluating the induction of human P450 1A2, 2B6, 2 C, and/or 3 A by test compounds in human hepatocytes by measuring mRNA and/or enzyme activity levels (Wong et al. Citation2021).
P450 1A2, 2B6, and 3A4 induction is routinely evaluated during drug development; however, P450 2 C induction is not always investigated by pharmaceutical companies (Yajima et al. Citation2014). The basal and induced levels of human P450 enzyme activity vary depending on the type and batch of human hepatocytes tested: this is partly determined by the commercial hepatocyte sources available and reflects variable sampling, storage, or culture conditions (Uehara et al. Citation2023). In this context, human HepaRG cells represent many lots of individual cryopreserved hepatocytes commercially available for P450 induction studies during drug development (Yajima et al. Citation2014). Recently, stable and reproducibly generated human HepaSH cells with fewer inter-individual differences in drug-metabolizing properties have been obtained from engrafted livers in immunodeficient humanized-liver mice (Uehara et al. Citation2023).
It should be noted that clear induction (from ∼5- to 60-fold) of human P450 1A2 by the typical inducer omeprazole was evident in the hepatocytes tested, in contrast to the contradictory reported induction results of P450 2C9 and 2C19 by rifampicin (Yajima et al. Citation2014). One mechanism for thalidomide activation in liver and placental cells is autoinduced metabolism regulated by the pregnane X receptor-dependent P450 3 A pathway (Yamazaki Citation2017); however, the role of human P450 1A2 induced by the important medicine omeprazole in its oxidative metabolism has not yet been reported in humans.
Because dogs are frequently used in drug metabolism studies, knowledge of dog P450 1 A, 2 C, and 3 A enzymes is essential because their homologs are abundant and play major roles in the liver and intestine in humans. In comparison studies of preclinical species, dogs showed similar hepatic metabolic activities towards typical human P450 1 A, 2 C, and 3 A substrates to those in humans (Uno et al. Citation2023a, Citation2023b, Citation2023c, Citation2023d). In the present study, the induction of dog P450 1A2 and 3A12 by omeprazole and rifampicin was functionally characterised in hepatocytes and compared to that in humans. We found that dog P450 1A2 was autoinduced by omeprazole in hepatocytes and was involved in omeprazole oxidation. Herein, we report the functions of dog P450 1A2, 2C21, and 3A12 in the regio- and stereo-selectivity of omeprazole oxidations, which are different in some details from those of human P450s.
Materials and methods
Materials
R- and S-omeprazole (purity >98%) were obtained from Toronto Research Chemicals (Toronto, ON, Canada) and Sigma-Aldrich (St. Louis, MO). Dog liver microsomes from individual beagle dogs (male, 2 years of age, weighing ∼10 kg; designated as dogs 3 and 7) and recombinant dog P450 1A2, 2C21, 2C41, 2C94, 3A12, 3A26, 3A98, and 3A99 proteins co-expressed with NADPH-P450 reductase in bacterial membranes were prepared as previously described (Uno et al. Citation2023a, Citation2023b, Citation2023c, Citation2023d). Recombinant human P450 1A2.1, 2C9.1, 2C19.1, 3A4.1, and 3A5.1 proteins co-expressed with NADPH-P450 reductase in bacterial membranes were prepared as previously described (Yamazaki et al. Citation2002, Citation2010). Human HepaRG cells (HRP116080, batch HPR11314-TA08) and pooled beagle dog hepatocytes (DBP111) were obtained from Biopredic International (Rennes, France). Human HepaSH cells (lot #1448-NCp from LHum17003) were obtained from the Central Institute for Experimental Animals (Kawasaki, Japan) (Uehara et al. Citation2023). Individual (HEP185056) and pooled (DBMP109) beagle dog hepatocytes were obtained from Discovery Life Sciences (Huntsville, AL). HepaRG and HepaSH cells and dog hepatocytes (4.8 × 105, 5.5 × 105, and 3.8 × 105cells per well, respectively) were initially seeded in collagen-coated 24-well plates and cultured with William’s E medium (Biopredic International) under conditions recommended in the manufacturers’ instructions or in reported procedures (Murayama et al. Citation2015, Citation2017; Uehara et al. Citation2023).
Enzymatic characterisation of dog and human P450 1A2, 2 C, and 3 A enzymes
Omeprazole 5-hydroxylation and sulfoxidation activities of recombinant human and dog P450 1 A, 2 C, and 3 A enzymes and liver microsomes were determined as previously described (Yamazaki et al. Citation1997; Uehara et al. Citation2016; Uno et al. Citation2023b). Briefly, typical reaction mixtures (0.20 mL) contained recombinant protein (25 pmol equivalent P450/mL) or dog liver microsomes (0.50 mg protein/mL), substrate (2.0–300 µM), and an NADPH-generating system (0.25 mM NADP+, 2.5 mM glucose 6-phosphate, and 0.25 unit of yeast glucose 6-phosphate dehydrogenase/mL) in 50 mM potassium phosphate buffer (pH 7.4). The reaction mixtures were incubated at 37 °C for 15 min and then terminated with 1.5 mL ethyl acetate. The resulting mobile phase solutions of metabolites extracted with ethyl acetate were analysed using high-performance liquid chromatography with a UV detector and a reverse-phase analytical column (C18) and eluents (Yamazaki et al. Citation1997; Uehara et al. Citation2016; Uno et al. Citation2023b). Maximum velocities (Vmax) and Michaelis constants (Km) were calculated from v versus [S] curves using the Michaelis–Menten equation fitted by nonlinear regression (mean ± standard error, n = 10 substrate concentrations, in triplicate) using Prism 9 (GraphPad Software, La Jolla, CA).
P450 induction in dog hepatocytes and human HepaRG and HepaSH cells
Human HepaRG and HepaSH cells and cryopreserved dog hepatocytes (4.8 × 105, 5.0 × 105, and 3.8 × 105 cells/well, respectively) were treated for 48 h with P450 inducers R,S-omeprazole (100 μM) or rifampicin (50 μM, Sigma-Aldrich; purity >98%) dissolved in 0.1% dimethyl sulfoxide (DMSO) (v/v). Previous single concentrations of R,S-omeprazole (50 μM) and rifampicin (10 μM) used in our induction study with 23 lots of commercially available cryopreserved human hepatocytes (Yajima et al. Citation2014) were increased in this study to 100 μM of R,S-omeprazole and 50 μM of rifampicin, according to reported findings for recommended doses for dog hepatocytes tested at 10-100 μM of these inducers (Nishibe and Hirata Citation1993; Lu and Li Citation2001). Cells treated with 0.1% DMSO (v/v) were used as the control. There were no morphological changes in dog hepatocytes and human HepaRG and HepaSH cells after treatment with these drugs. The mRNA levels of P450s (human P450 1A2, 2C9, 2C19, 3A4, and 3A5; dog P450 1A2, 2C21, 2C41, 3A12, and 3A26) were determined by quantitative polymerase chain reaction (qPCR) using total RNAs extracted from the hepatocytes using the RNeasy Mini Kit (Qiagen, Valencia, CA) as reported previously (Uno et al. Citation2023a, Citation2023b, Citation2023c, Citation2023d). Relative human P450 expression levels were determined using TaqMan probes for P450 1A2 (Hs00167927_m1), 2C9 (Hs00426397_m1), 2C19 (Hs00426380_m1), 3A4 (Hs00430021_m1), and 3A5 (Hs00241417_m1), following normalisation with glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Hs99999905_m1) levels in independent amplifications (Uehara et al. Citation2023). Results are expressed as mean values (± standard deviations) obtained from three to six wells of cultured cells. Dog P450 expression levels were determined using qPCR as described previously, including normalisation with 18S rRNA levels (Uno et al. Citation2023a, Citation2023b, Citation2023c, Citation2023d).
The P450-dependent drug oxidation activities of the hepatocytes were determined using 15 µM ethoxyresorufin (Tokyo Chemical Industry, Tokyo, Japan; purity >98%), 20 µM R- or S-omeprazole, and 5.0 µM midazolam (Sigma-Aldrich, purity >98%). The medium was collected on day 2 of culture after incubating for 2 h at 37 °C (5% CO2, v/v). The rates of metabolite formation from ethoxyresorufin and R- and S-omeprazole were determined using fluorescence and ultraviolet detection, respectively (Yamazaki et al. Citation1997; Uehara et al. Citation2016; Uno et al. Citation2023b). The rates of metabolite formation from midazolam were determined by liquid chromatography/tandem mass spectrometry as described previously (Yajima et al. Citation2014; Murayama and Yamazaki Citation2018). The results are expressed as mean values (± SD) obtained from three to six wells of cultured cells.
Results
Regio- and stereo-selectivity of omeprazole oxidations by dog P450 enzymes
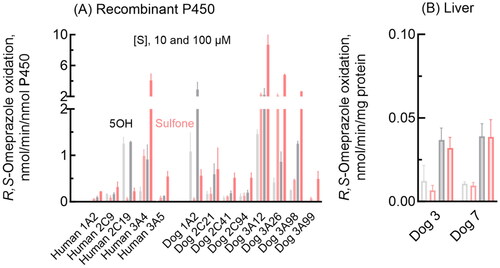
Oxidation activities towards the typical human P450 2C19 and 3A4 substrate R,S-omeprazole were determined for recombinant human and dog P450 1A2, 2 C, and 3 A enzymes and dog liver microsomes (). As expected, human P450 2C19 efficiently mediated R,S-omeprazole 5-hydroxylation at a low substrate concentration (10 µM), whereas P450 3A4 showed a high metabolic capacity for R,S-omeprazole sulfoxidation at a high substrate concentration (100 µM) (). Under the present conditions, human P450 1A2, 2C9, and 3A5 had low or negligible omeprazole oxidation activities. In contrast, dog P450 1A2 had relatively high R,S-omeprazole 5-hydroxylation activities compared to dog P450 2C21, 2C41, and 2C94 (). Dog P450 3A12 showed high R,S-omeprazole sulfoxidation and hydroxylation activities. R,S-Omeprazole sulfoxidation was also efficiently mediated by dog P450 3A26 and 3A98. In contrast, the liver microsomes from two individual dogs had similar R,S-omeprazole 5-hydroxylation activities (), despite dogs 7 and 3, respectively, being heterozygous and homozygous for the P450 1A2 null allele (Uno et al. Citation2023c), and dog 3 had undetectable P450 2C41 mRNA (Uno et al. Citation2023b).
Figure 1. Omeprazole 5-hydroxylation and sulfoxidation activities of recombinant human and dog P450 1 A, 2 C, and 3 A enzymes and dog liver microsomes. Mean activities of omeprazole 5-hydroxylation (gray bars) and sulfoxidation (pink bars) with SD values (whiskers) were determined for recombinant enzymes (A) and liver microsomes (B) using substrate concentrations of 10 µM (light color/open bars) and 100 µM (dark bars) R,S-omeprazole in triplicate reactions.
Oxidation activities towards R-omeprazole, R,S-omeprazole, and S-omeprazole were kinetically determined for three selected dog enzymes, i.e. P450 1A2, 2C21, and 3A12 (). Dog P450 1A2 similarly mediated the 5-hydroxylation of R-omeprazole (), R,S-omeprazole (), and S-omeprazole (). Moreover, for these reactions, dog P450 1A2 had the lowest Km values of 23–28 µM among the three P450s tested, but the Vmax values were low at 0.8–1.2 min−1 (). A relatively high Vmax value (3.6 min−1) for R-omeprazole sulfoxidation by dog P450 1A2 was observed under the present conditions. In contrast, dog P450 2C21 () selectively showed a high Vmax of 12 min−1 for S-omeprazole 5-hydroxylation, but with a high Km value of 220 µM (). Dog P450 3A12 () predominantly showed a high Vmax of 17 min−1 for S-omeprazole sulfoxidation activity, with a Km value of 92 µM (). In contrast, liver microsomes from two individual dogs kinetically showed roughly similar 5-hydroxylation and sulfoxidation activities for R-omeprazole and S-omeprazole, with little regio- or stereo-selectivity (). Liver microsomes from dog 7 (heterozygous for the P450 1A2 null allele) showed Km values of 67 and 110 µM, respectively, for S-omeprazole 5-hydroxylation and sulfoxidation activities (), similar to those of 68 and 92 µM for recombinant dog P450 3A12 (), respectively. Liver microsomes from dog 3 showed similar shaped substrate and velocity curves for R-omeprazole 5-hydroxylation and sulfoxidation as those of recombinant dog P450 1A2, but had a higher Km value of 110 µM for R-omeprazole 5-hydroxylation than that of 28 µM for recombinant dog P450 1A2.
Figure 2. Omeprazole 5-hydroxylation and sulfoxidation activities of recombinant dog P450 1A2, 2C21, and 3A12 enzymes. 5-Hydroxylation (open circles) and sulfoxidation (closed circles) activities of dog P450 1A2 (A-C), 2C21 (D-F), and 3A12 (G-I) towards R-omeprazole (A, D, G), R,S- omeprazole (B, E, H), and S-omeprazole (C, F, I) were determined at substrate concentrations of 2.0–300 µM. Kinetic parameters for omeprazole 5-hydroxylation and sulfoxidation activities were calculated using nonlinear regression (mean ± standard error, n = 10 substrate concentrations, in triplicate) and are shown in .
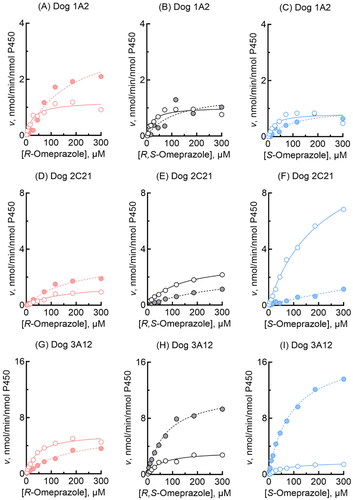
Figure 3. Omeprazole 5-hydroxylation and sulfoxidation activities of liver microsomes from two individual dogs. Activities towards R-omeprazole (A, C) and S-omeprazole (B, D) 5-hydroxylation (open circles) and sulfoxidation (closed circles) in dog liver microsomes were determined at substrate concentrations of 2.0–300 µM. Kinetic parameters for omeprazole 5-hydroxylation and sulfoxidation activities were calculated using nonlinear regression (mean ± standard error, n = 10 substrate concentrations, in triplicate) and are shown in .
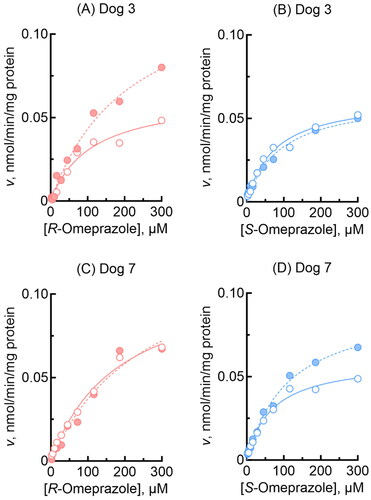
Table 1. Kinetic analysis of omeprazole oxidation by recombinant dog P450 1A2, 2C21, and 3A12 enzymes.
Table 2. Kinetic analysis of omeprazole oxidation by liver microsomes from two individual dogs.
Induced P450 1 A and 3 A enzymes in dog hepatocytes and human HepaRG and HepaSH cells involved in regio- and stereo-selective omeprazole oxidations
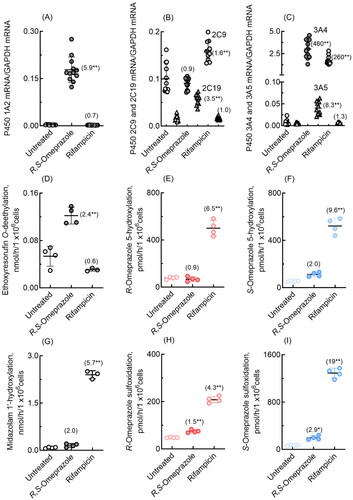
P450 induction by omeprazole, along with that by the positive control rifampicin, was investigated in cultured human HepaRG and HepaSH cells in terms of mRNA expression levels and probe drug oxidation activities. In human HepaRG cells, qPCR analysis revealed significant induction of P450 1A2, 2C19, 3A4, and 3A5 mRNAs by R,S-omeprazole (). Induction of P450 2C9 and 3A4 mRNA expression by rifampicin was also observed in human HepaRG cells. In HepaRG cells, human P450 1A2-dependent ethoxyresorufin O-deethylation activity was significantly increased by R,S-omeprazole (2.4-fold, ), and P450 3 A-dependent midazolam 1′-hydroxylation activity was significantly increased by rifampicin (5.7-fold, ). Human P450 3 A enzymes, presumably induced by rifampicin, significantly enhanced R-omeprazole 5-hydroxylation and sulfoxidation (4.3- to 6.5-fold, ) and S-omeprazole 5-hydroxylation and sulfoxidation (9.6- to 19-fold, ). Human P450 enzymes induced by R,S-omeprazole slightly but significantly enhanced R-omeprazole sulfoxidation (1.5-fold, ) and S-omeprazole sulfoxidation (2.9-fold, ).
Figure 4. mRNA expression levels of human P450 1A2, 2C9, 2C19, 3A4, and 3A5 (A–C) and drug oxidation activities (D–I) in cultured HepaRG cells treated with R,S-omeprazole or rifampicin. Numbers in parentheses indicate the fold induction relative to the untreated group. In panels B and C, mRNA levels of P450 2C19 and 3A5 are indicated by triangles. Results for drug oxidation activities are expressed as mean values (± SD) obtained from three or four wells of cultured hepatocytes on day 2 (*p < 0.05 and **p < 0.01, one-way analysis of variance with Dunnett’s post tests).
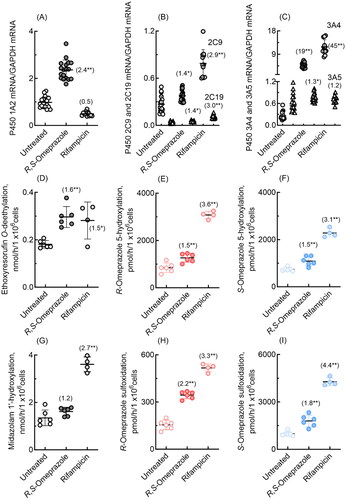
Similarly, in human HepaSH cells, qPCR analysis revealed significant induction by R,S-omeprazole of P450 1A2, 2C9, 2C19, 3A4, and 3A5 mRNAs (). In human HepaSH cells, P450 1A2-dependent ethoxyresorufin O-deethylation activity was significantly increased by R,S-omeprazole (1.6-fold, ), and P450 3 A-dependent midazolam 1′-hydroxylation activity was significantly increased by rifampicin (2.7-fold, ). R- and S-omeprazole 5-hydroxylation and sulfoxidation in freshly prepared HepaSH cells were slightly (1.5- to 2.2-fold), but significantly, induced by R,S-omeprazole. Induction of human P450 3 A enzymes by rifampicin significantly enhanced R-omeprazole 5-hydroxylation and sulfoxidation (3.3- to 3.6-fold, ) and S-omeprazole 5-hydroxylation and sulfoxidation (3.1- to 4.4-fold, ).
Figure 5. mRNA expression levels of human P450 1A2, 2C9, 2C19, 3A4, and 3A5 (A–C) and drug oxidation activities (D–I) in cultured HepaSH cells treated with R,S-omeprazole or rifampicin. Numbers in parentheses indicate the fold induction relative to the untreated group. In panels B and C, mRNA levels of P450 2C19 and 3A5 are indicated by triangles. Results for drug oxidation activities are expressed as mean values (± SD) obtained from four to six wells of cultured hepatocytes on day 2 (*p < 0.05 and **p < 0.01, one-way analysis of variance with Dunnett’s post tests).
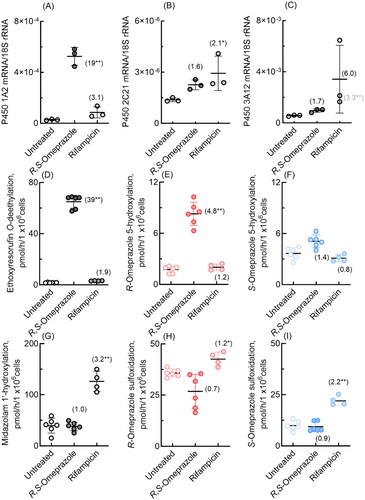
P450 induction by R,S-omeprazole and rifampicin was also investigated in cultured dog hepatocytes (). qPCR analysis similarly revealed significant induction of dog P450 1A2 mRNA by R,S-omeprazole (19-fold, ) and apparent induction of dog P450 3A12 mRNA by rifampicin (6.0-fold, ). In dog hepatocytes, P450 1A2-dependent ethoxyresorufin O-deethylation was significantly increased by R,S-omeprazole (39-fold, ), and P450 3 A-dependent midazolam 1′-hydroxylation activity was significantly increased by rifampicin (3.2-fold, ). Notably, dog P450 enzyme(s), probably P450 1A2, but not 3A12, induced by R,S-omeprazole, also showed enhanced R-omeprazole 5-hydroxylation (4.8-fold, ). The induction of dog P450 3 A enzymes by rifampin significantly enhanced R-omeprazole sulfoxidation (1.2-fold, ) and S-omeprazole sulfoxidation (2.2-fold, ). In our preliminary study with pooled dog hepatocytes obtained from a different provider, dog P450 1A2-dependent ethoxyresorufin O-deethylation activity was significantly (1.3-fold) affected by R,S-omeprazole, but R-omeprazole 5-hydroxylation was apparently enhanced (1.2-fold), probably because the hepatocytes partly originated from P450 1A2-deficient dogs. However, using other pooled dog hepatocytes, dog P450 1A2-dependent ethoxyresorufin O-deethylation and R-omeprazole 5-hydroxylation activities were significantly increased by treatment with R,S-omeprazole (11-fold and 3.0-fold, respectively), with a significant induction of dog P450 1A2 mRNA levels by R,S-omeprazole (28-fold, not shown). A role for dog P450 2 C enzymes in S-omeprazole 5-hydroxylation and/or sulfoxidation in individual dog hepatocytes cannot be ruled out under the current conditions.
Figure 6. mRNA expression levels of dog P450 1A2, 2C21, and 3A12 (A–C) and drug oxidation activities (D-I) in cultured hepatocytes derived from dog hepatocytes treated with R,S-omeprazole and rifampicin. Numbers in parentheses indicate the fold induction relative to the untreated groups. Results are expressed as mean values (± SD) obtained from three to six wells of cultured hepatocytes on day 2 (*p < 0.05 and **p < 0.01, one-way analysis of variance with Dunnett’s post tests). As shown in panel C, dog P450 3A12 was significantly induced (3.3-fold) even when the largest value, an apparent outlier, was omitted.
Discussion
Omeprazole is a well-known inducer of human P450 1A2 (Daujat et al. Citation1992; Curi-Pedrosa et al. Citation1994) and is also a typical human P450 2C19 probe substrate (Chainuvati et al. Citation2003; Turpault et al. Citation2009). Interspecific variabilities in P450 1 A mRNA inducibility by omeprazole in primary cultures of hepatocytes isolated from rabbit, rat, mouse, dog, and human livers have been assessed (Shih et al. Citation1999; Lu and Li Citation2001). Human HepaRG and HepaSH cells possess the unique ability to express various functions of mature hepatocytes, such as accurate responses to their respective prototypical P450 1A2, 2B6, and 3A4 inducers, omeprazole, phenobarbital, and rifampicin, respectively (Antherieu et al. Citation2010; Murayama et al. Citation2015; Uehara et al. Citation2023). In dogs, P450 induction has also been investigated using omeprazole, phenobarbital, and rifampicin (Nishibe et al. Citation1998; Lu and Li Citation2001; Graham et al. Citation2002, Citation2006). The reported findings recommend the use of relatively high doses (50-100 μM) of omeprazole and rifampicin in induction assays with dog hepatocytes (Nishibe and Hirata Citation1993; Lu and Li Citation2001). However, in dogs and humans, the roles of P450 1A2 induction by omeprazole in the oxidative metabolism of omeprazole, so-called autoinduction, have not yet been reported.
Human P450 2C19 efficiently mediates R-omeprazole 5-hydroxylation at low substrate concentrations, whereas P450 3A4 has high metabolic capacity for S-omeprazole sulfoxidation at high substrate concentrations (Abelo et al. Citation2000; Uehara et al. Citation2016). In contrast, of the recombinant dog P450 proteins tested, namely, 1A2, 2C21, and 3A12, dog P450 1A2 exhibited similar R- and S-omeprazole 5-hydroxylation activities with low Km values (23–28 µM), whereas dog P450 2C21 and 3A12 efficiently mediated S-omeprazole 5-hydroxylation and sulfoxidation, respectively, with high Vmax values (12–17 min−1) (), suggesting some species differences between dogs and humans in terms of regio- and stereo-selectivity of the P450 enzymes responsible for omeprazole oxidation.
In terms of the autoinduction potential of omeprazole, omeprazole 5-hydroxylation and sulfoxidation in freshly prepared HepaSH cells were slightly (∼2-fold) but significantly induced by R,S-omeprazole (). It should be noted that dog P450 enzyme(s), probably P450 1A2, but not 3A12, induced by R,S-omeprazole, also enhanced R- and S-omeprazole 5-hydroxylation (1.4- to 4.8-fold) (). Using dog hepatocytes, under the present conditions, the extents of induction of dog P450 2C94 and 3A26 by R,S-omeprazole were similar to those of dog P450 2C21 and 3A12, respectively: P450 2C41 was absent in this dog (data not shown). Dog P450 1A2 was autoinduced by R,S-omeprazole in dog hepatocytes, and thereby R-omeprazole 5-hydroxylation activity was enhanced. It may be interesting to observe the effects of P450 1A2 inhibitors on omeprazole 5-hydroxylations by whole P450 enzymes induced by omeprazole in dog hepatocytes; however, commercially available cryopreserved dog hepatocytes may not be used for further culture days after confirmation of enzyme induction. In humans, omeprazole metabolism is highly variable among patients depending on pharmacogenetic factors (Li-Wan-Po et al. Citation2010); however, the increasing rate of omeprazole metabolism in patients over time is not well recognised. Therapeutic monitoring of the drug voriconazole has facilitated the use of large doses in patients while controlling toxicity in suspected cases of autoinduction of voriconazole metabolism (Ferguson, Randles, de Freitas Citation2017).
The present study provides the first evidence of the autoinduction of omeprazole metabolism. The results indicated the autoinduction by omeprazole of P450 1A2 (which is involved in omeprazole 5-hydroxylation and/or sulfoxidation) in dog hepatocytes and human HepaRG and HepaSH cells.
Acknowledgments
We thank Yuichiro Higuchi, Nao Yoneda, Saho Morikuni, Yuki Fujiki, Yutaro Noda, Asuka Oguchi, Koichiro Adachi, and Makiko Shimizu for their support of this work. The authors also thank David Smallbones for copy-editing the draft of this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abelo A, Andersson TB, Antonsson M, Naudot AK, Skanberg I, Weidolf L. 2000. Stereoselective metabolism of omeprazole by human cytochrome P450 enzymes. Drug Metab Dispos. 28(8):966–972.
- Antherieu S, Chesne C, Li R, Camus S, Lahoz A, Picazo L, Turpeinen M, Tolonen A, Uusitalo J, Guguen-Guillouzo C, et al. 2010. Stable expression, activity, and inducibility of cytochromes P450 in differentiated HepaRG cells. Drug Metab Dispos. 38(3):516–525. doi:10.1124/dmd.109.030197.
- Chainuvati S, Nafziger AN, Leeder JS, Gaedigk A, Kearns GL, Sellers E, Zhang Y, Kashuba AD, Rowland E, Bertino JS. Jr. 2003. Combined phenotypic assessment of cytochrome p450 1A2, 2C9, 2C19, 2D6, and 3A, N-acetyltransferase-2, and xanthine oxidase activities with the "Cooperstown 5 + 1 cocktail". Clin Pharmacol Ther. 74(5):437–447. doi:10.1016/S0009-9236(03)00229-7.
- Curi-Pedrosa R, Daujat M, Pichard L, Ourlin JC, Clair P, Gervot L, Lesca P, Domergue J, Joyeux H, Fourtanier G, et al. 1994. Omeprazole and lansoprazole are mixed inducers of CYP1A and CYP3A in human hepatocytes in primary culture. J Pharmacol Exp Ther. 269(1):384–392.
- Daujat M, Peryt B, Lesca P, Fourtanier G, Domergue J, Maurel P. 1992. Omeprazole, an inducer of human CYP1A1 and 1A2, is not a ligand for the Ah receptor. Biochem Biophys Res Commun. 188(2):820–825. doi:10.1016/0006-291x(92)91130-i.
- Ferguson MJ, Randles ML, de Freitas DG. 2017. A suspected case of autoinduction of voriconazole metabolism in a patient with cerebral aspergillosis. Drug Healthc Patient Saf. 9:89–91. doi:10.2147/DHPS.S140213.
- Graham RA, Downey A, Mudra D, Krueger L, Carroll K, Chengelis C, Madan A, Parkinson A. 2002. In vivo and in vitro induction of cytochrome P450 enzymes in beagle dogs. Drug Metab Dispos. 30(11):1206–1213. doi:10.1124/dmd.30.11.1206.
- Graham RA, Tyler LO, Krol WL, Silver IS, Webster LO, Clark P, Chen L, Banks T, LeCluyse EL. 2006. Temporal kinetics and concentration-response relationships for induction of CYP1A, CYP2B, and CYP3A in primary cultures of beagle dog hepatocytes. J Biochem Mol Toxicol. 20(2):69–78. doi:10.1002/jbt.20118.
- Huang SM, Strong JM, Zhang L, Reynolds KS, Nallani S, Temple R, Abraham S, Habet SA, Baweja RK, Burckart GJ, et al. 2008. New era in drug interaction evaluation: US Food and Drug Administration update on CYP enzymes, transporters, and the guidance process. J Clin Pharmacol. 48(6):662–670. doi:10.1177/0091270007312153.
- Li-Wan-Po A, Girard T, Farndon P, Cooley C, Lithgow J. 2010. Pharmacogenetics of CYP2C19: functional and clinical implications of a new variant CYP2C19*17. Br J Clin Pharmacol. 69(3):222–230. doi:10.1111/j.1365-2125.2009.03578.x.
- Lu C, Li AP. 2001. Species comparison in P450 induction: effects of dexamethasone, omeprazole, and rifampin on P450 isoforms 1A and 3A in primary cultured hepatocytes from man, Sprague-Dawley rat, minipig, and beagle dog. Chem Biol Interact. 134(3):271–281. doi:10.1016/s0009-2797(01)00162-4.
- Murayama N, Kazuki Y, Satoh D, Arata K, Harada T, Shibata N, Guengerich FP, Yamazaki H. 2017. Induction of human cytochrome P450 3A enzymes in cultured placental cells by thalidomide and relevance to bioactivation and toxicity. J Toxicol Sci. 42(3):343–348. eng. doi:10.2131/jts.42.343.
- Murayama N, Usui T, Slawny N, Chesne C, Yamazaki H. 2015. Human HepaRG cells can be cultured in hanging-drop plates for cytochrome P450 induction and function assays. Drug Metab Lett. 9(1):3–7. eng. doi:10.2174/1872312809666150119104806.
- Murayama N, Yamazaki H. 2018. Cytochrome P450-dependent drug oxidation activities in commercially available hepatocytes derived from human induced pluripotent stem cells cultured for 3 weeks [3]. J Toxicol Sci. 43(4):241–245. doi:10.2131/jts.43.241.
- Nishibe Y, Hirata M. 1993. Effect of phenobarbital and other model inducers on cytochrome P450 isoenzymes in primary culture of dog hepatocytes. Xenobiotica. 23(6):681–692. doi:10.3109/00498259309059405.
- Nishibe Y, Wakabayashi M, Harauchi T, Ohno K. 1998. Characterization of cytochrome P450 (CYP3A12) induction by rifampicin in dog liver. Xenobiotica. 28(6):549–557. doi:10.1080/004982598239308.
- Sager JE, Yu J, Ragueneau-Majlessi I, Isoherranen N. 2015. Physiologically based pharmacokinetic (PBPK) modeling and simulation approaches: a systematic review of published models, applications, and model verification. Drug Metab Dispos. 43(11):1823–1837. doi:10.1124/dmd.115.065920.
- Shih H, Pickwell GV, Guenette DK, Bilir B, Quattrochi LC. 1999. Species differences in hepatocyte induction of CYP1A1 and CYP1A2 by omeprazole. Hum Exp Toxicol. 18(2):95–105. doi:10.1177/096032719901800206.
- Turpault S, Brian W, Van HR, Santoni A, Poitiers F, Donazzolo Y, Boulenc X. 2009. Pharmacokinetic assessment of a five-probe cocktail for CYPs 1A2, 2C9, 2C19, 2D6 and 3A. Br J Clin Pharmacol. 68(6):928–935. doi:10.1111/j.1365-2125.2009.03548.x.
- Uehara S, Higuchi Y, Yoneda N, Ito R, Takahashi T, Murayama N, Yamazaki H, Murai K, Hikita H, Takehara T, et al. 2023. HepaSH cells: experimental human hepatocytes with lesser inter-individual variation and more sustainable availability than primary human hepatocytes. Biochem Biophys Res Commun. 663:132–141. doi:10.1016/j.bbrc.2023.04.054.
- Uehara S, Kawano M, Murayama N, Uno Y, Utoh M, Inoue T, Sasaki E, Yamazaki H. 2016. Oxidation of R- and S-omeprazole stereoselectively mediated by liver microsomal cytochrome P450 2C19 enzymes from cynomolgus monkeys and common marmosets. Biochem Pharmacol. 120:56–62. ENG. doi:10.1016/j.bcp.2016.09.010.
- Uno Y, Jikuya S, Noda Y, Murayama N, Yamazaki H. 2023a. A comprehensive investigation of dog cytochrome P450 3A (CYP3A) reveals a functional role of newly identified CYP3A98 in small intestine. Drug Metab Dispos. 51(1):38–45. doi:10.1124/dmd.121.000749.
- Uno Y, Morikuni S, Shiraishi M, Asano A, Murayama N, Yamazaki H. 2023b. Novel cytochrome P450 2C94 functionally metabolizes diclofenac and omeprazole in dogs. Drug Metab Dispos. 51(5):637–644. doi:10.1124/dmd.122.001236.
- Uno Y, Noda Y, Morikuni S, Murayama N, Yamazaki H. 2023c. Liver microsomal cytochrome P450 3A-dependent drug oxidation activities in individual dogs. Xenobiotica. 53(3):140–148. doi:10.1080/00498254.2023.2211673.
- Uno Y, Noda Y, Murayama N, Tsukiyama-Kohara K, Yamazaki H. 2023d. Novel cytochrome P450 1 (CYP1) genes in tree shrews are expressed and encode functional drug-metabolizing enzymes. Comp Biochem Physiol C Toxicol Pharmacol. 265:109534. doi:10.1016/j.cbpc.2022.109534.
- Wong SG, Ramsden D, Dallas S, Fung C, Einolf HJ, Palamanda J, Chen L, Goosen TC, Siu YA, Zhang G, et al. 2021. Considerations from the innovation and quality induction working group in response to drug-drug interaction guidance from regulatory agencies: guidelines on model fitting and recommendations on time course for in vitro cytochrome P450 induction studies including impact on drug interaction risk assessment. Drug Metab Dispos. 49(1):94–110. doi:10.1124/dmd.120.000055.
- Yajima K, Uno Y, Murayama N, Uehara S, Shimizu M, Nakamura C, Iwasaki K, Utoh M, Yamazaki H. 2014. Evaluation of 23 lots of commercially available cryopreserved hepatocytes for induction assays of human cytochromes P450. Drug Metab Dispos. 42(5):867–871. doi:10.1124/dmd.113.056804.
- Yamazaki H, Inoue K, Shaw PM, Checovich WJ, Guengerich FP, Shimada T. 1997. Different contributions of cytochrome P450 2C19 and 3A4 in the oxidation of omeprazole by human liver microsomes: effects of contents of these two forms in individual human samples. J Pharmacol Exp Ther. 283(2):434–442.
- Yamazaki H, Nakamoto M, Shimizu M, Murayama N, Niwa T. 2010. Potential impact of cytochrome P450 3A5 in human liver on drug interactions with triazoles. Br J Clin Pharmacol. 69(6):593–597. doi:10.1111/j.1365-2125.2010.03656.x.
- Yamazaki H, Nakamura M, Komatsu T, Ohyama K, Hatanaka N, Asahi S, Shimada N, Guengerich FP, Shimada T, Nakajima M, et al. 2002. Roles of NADPH-P450 reductase and apo- and holo-cytochrome b5 on xenobiotic oxidations catalyzed by 12 recombinant human cytochrome P450s expressed in membranes of Escherichia coli. Protein Expr Purif. 24(3):329–337. doi:10.1006/prep.2001.1578.
- Yamazaki H. 2017. Differences in toxicological and pharmacological responses mediated by polymorphic cytochromes P450 and related drug-metabolizing enzymes. Chem Res Toxicol. 30(1):53–60. doi:10.1021/acs.chemrestox.6b00286.
