 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
P-glycoprotein (P-gp), a multidrug efflux pump encoded by the ABCB1 (formerly MDR1) gene, plays a crucial role in limiting drug absorption and eliminating toxic compounds in both humans and dogs. However, species-specific differences in P-gp substrates necessitate the development of canine-specific evaluation systems. Canine intestinal organoids derived monolayers offer a promising platform for studying drug transport, yet P-gp-mediated transport in these models remains unexplored.
We generated canine colonoid-derived 2D monolayers to investigate ABCB1 gene expression and P-gp function. We employed widely recognised P-gp substrates, Rhodamine 123 and Doxorubicin, in conjunction with the P-gp inhibitor PSC833 at Days 5 and 10 of culture.
A significant increase in gene expression of P-gp encoded by the ABCB1 was noted on Day 10 compared to Day 5 of culture. Despite this disparity in gene expression, the transport activity of P-gp, as assessed by the efflux of Rhodamine 123 and Doxorubicin with PSC833 inhibition, did not exhibit significant differences between these two time points. However, the inhibition of P-gp function by PSC833 confirms the presence of functional P-gp in our model.
Canine intestinal organoid-derived monolayers provide a valuable tool for investigating P-gp-mediated drug transport. These findings highlight the potential for predicting drug bioavailability and adverse reactions in veterinary medicine, aligning with principles of ethical and sustainable research.
Introduction
P-Glycoprotein (P-gp) is an ATP-binding efflux pump encoded by the ABCB1 (formally MDR1) gene, expressed on the apical surfaces of epithelial cells including intestinal epithelium, where it plays a key role in limiting the intestinal absorption of oral drugs and eliminating toxic xenobiotics in both humans and dogs (Barta et al. Citation2008; Martinez et al. Citation2008). Various drugs commonly used in veterinary practices have been reported to be substrates for canine P-gp, such as anticancer drug (doxorubicin and vincristine), antidiarrheal drug (loperamide) and parasiticide (ivermectin) (Katrina L Mealey Citation2008; Katrina L Mealey et al. Citation2023). Importantly, P-gp is also expressed at the blood-brain barrier, where its role in protecting the brain from potential toxins is crucial, particularly given the life-threatening interactions that can arise from compromised P-gp function at this site (Steingold et al. Citation1998). P-gp dysfunction, whether intrinsic (due to MDR1 gene polymorphism (Mealey et al. Citation2001)) or acquired (through drug interaction (Zolk and Fromm Citation2011)), can lead to severe adverse drug effects for patients (Martinez et al. Citation2008). Although canine P-gp structure is similar to humans with 93% sequence homology of MDR1 gene (Steingold et al. Citation1998), species-differences of P-gp substates have been reported (Zolnerciks et al. Citation2011). Therefore, an in vitro evaluation system for dogs is required to assess P-gp-mediated drug distribution and excretory function, instead of extrapolation of P-gp-based drug interaction in humans to dogs.
Canine intestinal organoid can be established from intestinal specific adult stem cells and develop into differentiated canine intestinal epithelial cells (Chandra et al. Citation2019). To allow easy evaluation drug absorption and secretion at the apical surfaces and lumen (Zolnerciks et al. Citation2011), canine colonoid derived 2D cell monolayer that represent physiological intestinal epithelial interface, were developed (Ambrosini et al. Citation2020; Gabriel et al. Citation2022). 2D cell monolayer provides a physiologic intestinal epithelial interface with an expression of tight junctions, and allows measurement of transepithelial electrical resistance (TEER) for functional analysis of the cell junctions (Ambrosini et al. Citation2020), and passive transport (Sahoo et al. Citation2023). Colonoid derived monolayers enable the standardised measurement of transepithelial permeability and epithelial-luminal interaction due to easier accessibility of the apical surface, which can be used for pharmaceutical and biomedical research. However, drug transport via P-gp remains unexplored in these canine monolayers.
By utilising 2D monolayers derived from 3D organoids, researchers can capitalise on the advantages of both systems—retaining the complex cellular interactions and structural complexity of 3D environments, while benefiting from the experimental ease and precision afforded by 2D formats. The specific advantages of using 2D monolayers for P-gp transport assays are manifold. These include controlled drug exposure and simplified sampling, which are essential for precise measurement; streamlined imaging and analysis, facilitating easier data acquisition; high-throughput capabilities, allowing for the efficient screening of multiple compounds; enhanced reproducibility and standardisation, ensuring consistent results across studies; and the ability to focus on specific cellular interfaces, crucial for detailed investigations of directional transport processes.
The in vitro apparent permeability (Papp) metric can mathematically estimate in vivo permeability through P-gp, as indicated in previous studies (Tavelin et al. Citation2002). There is a critical need for canine-specific permeability tools for assessing the unique absorption challenges associated with the canine intestinal tract. Utilising canine intestinal organoids for these studies could offer more precise predictions of canine intestinal P-gp permeability, compared to using conventional in vitro models such as Caco-2 or MDCK cells. Development of more physiologic in vitro model also has the benefit of being ethically and financially more sustainable than live animal experiments. Additionally, the potential for reusing stored organoids aligns well with the 3 R principles of "Replacement, Reduction, and Refinement."
In this study, we generated a physiological epithelial interface derived from canine colonoids to evaluate P-gp efflux and its inhibitory modulations. The P-gp expression and function were evaluated using P-gp substrates (the fluorescent P-gp substrate Rhodamine 123 and Doxorubicin) at Day 5 and 10 of culture to investigate the relationship with the P-gp gene expression and its function. We further assessed the P-gp efflux using the P-gp inhibitor PSC833. We envision that canine intestinal organoid-derived monolayers may enable us to predict the drug bioavailability and adverse drug reaction prior to administration to patients, which may lead to applications in personalised medicine. Utilising canine models in the early stages of drug testing is beneficial due to their physiological resemblance to humans (Coelho et al. Citation2018). We envision that monolayers derived from canine intestinal organoids could be instrumental in predicting drug bioavailability and adverse reactions before these drugs are administered to patients. This approach has the potential to enhance personalised medicine by providing earlier and more accurate assessments of drug safety and effectiveness.
Materials and methods
Generation and culture of canine colonoids
Intestinal biopsies were obtained via colonoscopy from three clinically healthy donor dogs undergoing dental procedures at Washington State University Veterinary Teaching Hospital (WSU-VTH) Community Service. These dogs were carefully selected based on physical examinations, blood work, and a lack of chronic diseases (including heart, kidney, liver, and intestinal conditions), and the age ranging for inclusion was limited to 1 to 12 years. Dogs meeting these criteria and deemed suitable for elective anaesthesia at WSU-VTH Community Service were classified as clinically healthy. All animal procedure were approved by Washington State University Institutional Animal Care and Use Committee (IACUC approval: ASAF#6993). The signalment of these healthy dogs is listed in Supplemental Table 1. Colonic stem cells were collected and cultured as previously reported (Chandra et al. Citation2019). Briefly, colonic biopsies were washed using ice-cold Dulbecco’s phosphate-buffered saline (PBS, Gibco) containing 1x Penicillin/Streptomycin (Gibco) for five times, cut into small pieces, and incubated in 30 mM EDTA solution (Invitrogen) for 60 min at 4 °C to release crypts containing intestinal stem cells. Collected intestinal crypts were suspended in Matrigel (Corning) and seeded to a 48-well plate (Thermo Scientific) in 30 µL per well. After the Matrigel dome was solidified in 37 °C incubator, 500 µl of organoid culture medium was added to each well. For composition of organoid culture medium, we followed the previous study; DMEM/F12 (Gibco) supplemented with 2 mM GultaMAX (Gibco), 10 mM HEPES (Gibco), 1x Penicillin/Streptomycin (Gibco), 10% (vol/vol) conditioned medium of Noggin (Heijmans et al. Citation2013), 20% (vol/vol) conditioned medium of R-spondin, 100 ng/ml recombinant murine Wnt-3a (PeproTech), 50 ng/ml murine Epidermal Growth Factor (EGF) (PeproTech), 10 nM Gastrin (Sigma-Aldrich), 500 nM A-83-01 (Sigma-Aldrich), 10 µM SB202190 (Sigma-Aldrich), 1 mM N-Acetyl-L-Cysteine (MP Biomedicals), 10 mM Nicotinamide (Sigma-Aldrich), 1x B27 supplement (Gibco), 1x N2 MAX media supplement (R&D Systems), 100 µg/ml Pimocin (Invitrogen). 10 µM Y-27632 (Stem Cell Technologies) and 2.5 µM CHIR 99021 (Stem Cell Technologies) were added to the organoid culture medium for the first two days after the crypts were isolated. The culture medium was changed every other day, and colonoids were passaged once a week by breaking down the colonoids using TrypLE Express (Gibco), spinning down them (200 g, 5 min, 4 °C), resuspending them in Matrigel, and seeding them in a 48-well plate.
Culture of canine colonoid-derived monolayers
Colonoid-derived monolayers were prepared as previously reported (Ambrosini et al. Citation2020). Briefly, 3D colonoids cultured in Matrigel were collected using Cell Recovery Solution (Corning), centrifuging at 200× g, 4 °C for 5 min, and incubating with 1 mL TrypLE Express (Gibco) containing 10 µM of Y-27632 for 10 min at 37 °C with mixing. The single cells dissociated from colonoids were filtered through a 70 µm cell strainer (Fisher Scientific), centrifuged at 200 g, 4 °C for 5 min, resuspended in organoid culture medium and seeded at 105 cells/well in 24 well Transwell inserts (Corning) which were pre-coated with a mixture of Matrigel (100 μg/mL; Corning) and collagen I (30 μg/mL; Gibco). The culture medium was changed every other day. The morphology of canine colonoid-derived monolayer was observed using a phase-contrast microscopy (DMi1, Leica).
Assessment of intestinal barrier integrity in colonoid-derived monolayers
The intestinal barrier integrity of the colonoid-derived monolayer was assessed by measuring the transepithelial electrical resistance (TEER) value. Electrical resistance (Ωt) was quantified using Ag/AgCl electrodes connected to a Volt-Ohm metre (Millicell ERS-2, Millipore), and this value was then transformed into a TEER measurement using the following equation: TEER = (Ωt - Ωblank) x A. Here, Ωblank represents the resistance of the blank well in ohms, and A denotes the surface area of the culture insert in cm2.
Total RNA extraction and quantitative reverse transcription polymerase chain reaction analysis
After 5- or 10-days culture, total RNA was extracted from colonoid-derived monolayer utilising the RNeasy mini kit (Qiagen) and subjected to reverse transcription using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Dog colon total RNA derived from single health beagle dog (DR-311, Zyagen) were served as the template for reverse transcription. Quantitative reverse transcription polymerase chain reaction (qPCR) was conducted using PowerUp SYBR Green Master Mix (Applied Biosystems) and the CFX96 Touch Real-time PCR Detection System (Bio-Rad) to evaluate the gene expression levels of ATP-binding cassette sub-family B member 1 (ABCB1). For cDNA normalisation, internal control genes were employed, namely, Succinate dehydrogenase complex subunit A (SDHA), Hydroxymethyl-bilane synthase (HMBS), and Hypoxanthine phosphoribosyl-transferase 1 (HPRT1), as previously reported in colon tissue (Peters et al. Citation2007). Details of the primers used in this study can be found in Supplemental Table 2.
Immunofluorescence imaging
For immunofluorescence (IF) microscopy, a confluent cell monolayer cultured on a Transwell insert was fixed using 4% (w/v) paraformaldehyde (Thermo Fisher Scientific) by immersing the insert in the fixative for 15 min at room temperature. The cells were then permeabilized with 0.3% (v/v) Triton X-100 (Thermo Fisher Scientific) and blocked using 2% (w/v) bovine serum albumin (BSA; Cytiva). After blocking, the cells were washed with PBS. The monolayer was incubated overnight at 4 °C with primary antibodies specific for P-gp (MA1-26528, 1:25, Thermo Fisher Scientific) diluted in 2% BSA in PBS. This was followed by incubation with Goat Anti-Mouse IgG H&L (DyLight 488, 1:500, Abcam) diluted in 2% BSA in PBS, protected from light, for another hour at room temperature. Nuclei were stained using DAPI (1:1000, Fisher Scientific). Imaging was performed on a SP8-X white light point scanning confocal microscope (Leica) and the images were processed using LAS X software (Leica).
Transport assay in canine colonoid-derived monolayers
All transport experiments were conducted on Days 5 and 10 after organoid-derived epithelial cell seeding on the Transwell inserts. All solutions for the transport experiments were prepared in Hank’s Balances Slat Solutions (HBSS; Corning) with calcium and magnesium. Before the experiment, both the apical (AP) and basolateral (BL) chambers were washed twice with fresh HBSS at room temperature. For the transport assay, HBSS containing 20 μM Rhodamine 123 (Rh123; Fisher Scientific) (Forster et al. Citation2012) or 25 µM Doxorubicin (Dox; Cayman Chemical) (Crowe Citation2021) was introduced to the AP or BL compartment (200 µl to AP compartment and 500 µl to BL compartment), then the plate was incubated at 37 °C with 5% CO2. The sample (50 μL) from the AP or BL side was taken every 20 min up to 60 min for Rh123 or every 60 min up to 180 min for Dox. For testing the inhibitory effect of a chemical on the Rh123 transport, 4 µM of PSC 833 (Fisher Scientific) (Feller et al. Citation1995; Atadja et al. Citation1998; Aouali et al. Citation2011; Crowe Citation2021) were added in both AP and BL chambers at the onset of transport assay. As a control, we utilised 0.01% DMSO in both AP and BL chambers. The concentration of Rh123 and Dox were measured (Rh123: excitation at 500 nm, emission at 550 nm, Dox: excitation at 470 nm, emission at 600 nm) using a microplate reader (SpectraMax; Molecular Devices) after transferring the aliquot of the sample in a fluorescence measuring plate (96 Well Black plate: Greiner Bio-One). A standard curve was prepared to estimate the concentration of Rh123 or Dox based on the fluorescence intensity in the range of 0-50 μM for Rh123 or 0-25 µM for Dox. The apparent permeability (Papp) was then calculated using the following equation (Tavelin et al. Citation2002):
Where dQ/dt (µg/sec) is the flux rate in the basolateral (AP to BL) or apical (BL to AP) direction, C0 (µg/mL) is the initial concentration of the Rh123 or Dox, and A (cm2) is the area that the cell monolayer covers (0.33 cm2).
Statistical analysis
Statistical analysis was conducted using R Studio v1.4.1717 (RStudio) and figures were generated using GraphPad Prism 10.0.3 (Dotmatics). To confirm the normality of each dataset, the Shapiro-Wilk’s test was performed. Student t-test was conducted for the comparison of ABCB1 gene expression between Day5 and Day10. The Wilcoxon test was utilised to compare the transport activity between Day5 and Day10 and between control and inhibited group. All results were presented as mean ± standard error of the mean. A significance level of p < 0.05 was considered statistically significant.
Results
P-gp was expressed in canine colonoid-derived monolayers
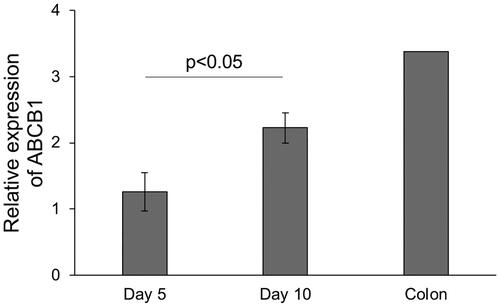
In this study, we utilised three lines of canine colonoids derived from the colonic tissue of three clinical healthy dogs. From these colonoids cultured in Matrigel, colonoid-derived monolayers were successfully formed on Transwell culture inserts () (Ambrosini et al. Citation2020). The TEER value exceeded 2000 Ω cm2 on the first two days of culture and remained constant until Day 6, after which it increased further and remained constant at over 3,500 Ω cm2 (). To investigate the expression level of P-gp in canine colonoid-derived monolayer, we compared two conditions with different culture periods, Day 5 and Day 10. The gene expression level of ABCB1 was found to be significantly increased on Day 10 compared to Day 5 (). Moreover, the expression of the ABCB1 gene on Day 10 was more similar to that in the colon tissue control ().
Figure 1. Development of canine colonoid-derived monolayers.
(A) A schematic representation of the canine colonoid-derived monolayer generation process. Colonoids, derived from colonic tissue biopsies, were dissociated into individual cells and then seeded onto nanoporous membranes. The resulting matured colonoid-derived monolayer expresses P-gp on the apical side as previously reported (Ambrosini et al. Citation2020). This schematic was created with BioRender.com.
(B) Representative phase-contrast microscopy images displaying colonoid-derived monolayer at two time points: Day 5 and Day 10. Scale bar = 100 µm.
(C) The intestinal epithelial barrier integrity was assessed through the measurement of TEER values. The TEER value progressively increased with the duration of culture days increased, and stabilising at the level exceeding over 2000 Ω cm2 after Day 2. This assessment encompassed three biological replicates, each with three technical replicates. The error bars represent the standard error of the mean (SEM).
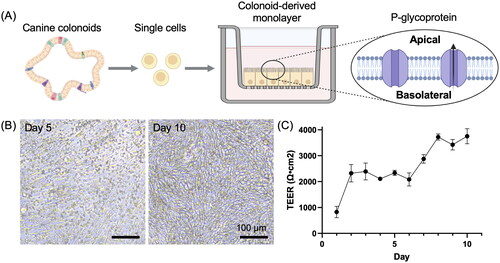
Figure 2. Gene expression level of ABCB1 in canine colonoid-derived monolayers.
Gene expression level of ABCB1 was assessed using quantitative reverse transcription polymerase chain reaction (qPCR) and compared between Day 5 and Day 10, with commercially sourced canine colonic tissue as a control. This assessment encompassed three biological replicates, each with three technical replicates. The error bars represent the standard error of the mean (SEM).
Luminal localisation of the P-gp on canine colonoid-derived monolayers
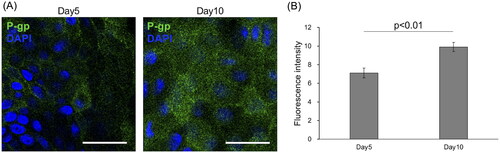
P-gp was diffusely expressed on the surface of the canine colonoid-derived monolayers (). This pattern aligns with previous findings on canine colonoid-derived monolayers (Ambrosini et al. Citation2020) and the known apical polarisation of P-gp transporters in canine intestinal tissue (Pietra et al. Citation2021). Notably, IF analysis demonstrated a significant increase (p < 0.01) in the polarised expression of P-gp on Day 10 compared to Day 5 (), consistent with an earlier report (Ambrosini et al. Citation2020).
Figure 3. P-glycoprotein expression in canine colonoid-derived epithelial monolayers.
(A) Top-down visualisation of P-glycoprotein (P-gp) expression (green) on polarised canine colonoid-derived monolayers at Day 5 and Day 10, achieved through immunofluorescence staining. Nuclei are stained blue. Scale bars represent 25 μm.
(B) Quantification of P-gp expression on Days 5 and 10. Fluorescence intensity was quantified using ImageJ. The error bars represent the standard error of the mean (SEM).
The duration of culture did not impact P-gp transport activity in canine colonoid-derived monolayers
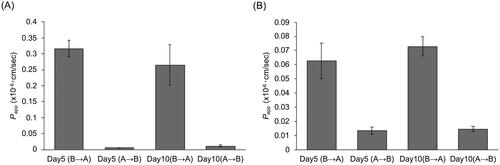
Next, we assessed transport activity of P-gp in canine colonoid-derived monolayer using Rh123 and Dox, which are well-known P-gp substrate (Forster et al. Citation2012; Crowe Citation2021). Transport activity was assessed by measuring the fluorescence intensity of the substrate in each well for P-gp substrate efflux in the AP to BL or BL to AP direction. Papp was then calculated from the total amount of these effluxed substrates and compared as an indicator of Transport activity in each direction. For Rh123, the efflux from the BL to AP direction was notably higher, with a 67.9-fold increase on Day 5 and a 24.1-fold increase on Day 10 compared to the efflux from AP to BL. This suggests that P-gp function in the monolayers predominantly facilitates transport from the BL to the AP side, consistent with observations in transport assays using Caco-2, a human intestinal epithelial cell line (Crowe Citation2021). When comparing the culture periods, there were no significant differences in transport activity, with values of 0.32 × 10−6 cm/sec at Day 5 and 0.26 × 10−6 cm/sec at Day 10 (). As for Dox, the BL to AP efflux was 4.6 times greater on Day 5 and 4.9 times greater on Day 10 than AP to BL, and there was no significant difference in Papp (BL to AP) between Day5 and Day10 (). A similar trend was observed for both Rh123 and Dox, but Papp (BL to AP) of Dox was 5.1-fold lower on Day 5 and 3.6-fold lower on Day 10 compared to that of Rh123, as previously described in a transport assays using Caco-2 (Crowe Citation2021).
Figure 4. Evaluation of P-gp-mediated transport in canine colonoid-derived monolayers.
The apparent permeability (Papp) of Rhodamine123 (A) and Doxorubicin (B), both well-established P-gp substrates, was assessed on Day 5 and Day 10. The Papp was assessed in Basolateral to Apical (B→A) and Apical to Basolateral (A→B) direction. This assessment encompassed three biological replicates, each with three technical replicates. The error bars represent the standard error of the mean (SEM).
P-gp-mediated transport was inhibited by PSC833 in canine colonoid-derived monolayers
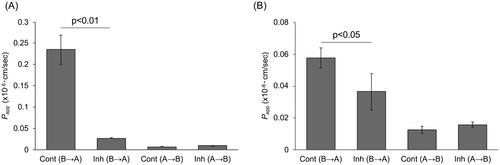
To determine whether the efflux observed with Rh123 and Dox was due to P-gp, we conducted transport assay using PSC833, a P-gp-specific inhibitor (Feller et al. Citation1995; Atadja et al. Citation1998; Aouali et al. Citation2011; Crowe Citation2021). Despite the higher gene expression level of ABCB1 on Day 10 compared to Day 5, there was no discernible difference in transport activity on either of these days. As a result, we opted to utilise the Day 5 monolayers for subsequent experiments. The addition of PSC833 significantly reduced the Rh123 efflux by 88.6% to 0.27 × 10−7 cm/sec compared to 0.23 × 10−6 cm/sec for the control Papp (p < 0.01) (). As for Dox efflux, Papp was significantly reduced by 36.6% to 0.36 × 10−7 cm/sec when PSC was added, compared to 0.58 × 10−7 cm/sec in the control (p < 0.05) ().
Figure 5. Inhibition of P-gp-mediated transport activity in canine colonoid-derived monolayers using PSC833.
On Day 5, a P-gp transport assay was performed using Rhodamine123 (A) and Doxorubicin (B) in the presence of PSC833, a specific inhibitor of P-gp. The Papp was assessed in Basolateral to Apical (B→A) and Apical to Basolateral (A→B) direction. Control group (Cont) was treated with 0.01% DMSO and Inhibitor group (Inh) was treated with 4 µM PSC833 during transport assay. This assessment encompassed three biological replicates, each with three technical replicates. The error bars represent the standard error of the mean (SEM).
Discussion
This study effectively establishes canine colonoid-derived monolayers as a dependable model for evaluating P-gp function. While previous research has explored passive drug transport in canine colonoid-derived monolayers (Sahoo et al. Citation2023), this work represents the first assessment of P-gp-specific drug transport in this in vitro system.
In this study, the expression of P-gp (ABCB1 gene) was demonstrated in these monolayers, with significantly increased gene expression observed on Day 10 compared to Day 5 of culture. However, despite this difference in gene expression, the transport activity of P-gp, as measured by the efflux of Rh123 and Dox, did not show significant variations between the two time points. This finding suggests that the Day 5 could be a suitable time point for P-gp function assessment in this given experimental design using canine colonoid-derived monolayers.
A consistent saturation of P-gp function was observed () regardless of the expression levels of P-gp genes and proteins (). Previous studies have documented the saturation of P-gp function at various culture time points in monolayer systems (Sevin et al. Citation2013). Additionally, research into the relationship between P-gp expression and function under different pathological conditions has shown inconsistent correlations (Bankstahl et al. Citation2008; Kuteykin-Teplyakov et al. Citation2009; Syvänen et al. Citation2011; Syvänen et al. Citation2012). Notably, an increase in apparent P-gp function without a change in P-gp expression was reported in epileptic models (De Lange et al. Citation2018). Conversely, increased P-gp expression accompanied by similar saturation of function was observed in acute myeloid leukaemia cells from patients (List et al. Citation1996; Broxterman et al. Citation1997). These findings highlight the need for a critical approach to interpreting changes in P-gp expression as a biomarker of its functionality. Developing in vitro models that accurately replicate P-gp functionality is crucial for characterising potential substrates of efflux proteins and assessing the risk of drug-drug interactions in the intestinal epithelium.
Both Rh123 and Dox predominantly exhibit basolateral to apical (BL to AP) efflux, aligning with the known directional transport of P-gp. Our results demonstrate a notable decrease in the Papp of both Rh123 and Dox following co-administration with PSC833 (), indicating a robust inhibition of P-gp by PSC833. This finding confirms the functional and physiological comparability of the P-gp in our model with those reported in previous studies (Van Der Sandt et al. Citation2000; Crowe Citation2021). Notably, the transport of Dox was approximately four times lower than that of Rh123, a discrepancy that aligns with prior research (Crowe Citation2021).
The functional assays employed in this study, utilising Rh123 and Dox as substrates, provide insights into P-gp drug transport processes in canine colonoid-derived monolayers. However, it is important to note that these assays may not be P-gp-specific. Indeed, previous research, suggests that substances classified as substrates or "inhibitors (competitive substrates)" may be transported through different routes other than P-gp (Katrina L Mealey et al. Citation2023; Lehne et al. Citation2000). For instance, Rh123 and doxorubicin are also a substrate for Breast Cancer Resistance Protein (BCRP) (Ni et al. Citation2010), and therefore, the observed changes might be attributed to other transport mechanisms. This phenomenon underscores the complexity of drug transport mechanisms in the intestinal epithelium.
In our experiments, we first utilised the combination of PSC833 and Rh123, which have been shown a potential to be P-gp-specific, particularly within the 60-minute experimental timeframe (Feller et al. Citation1995). Furthermore, studies have shown that PSC833, as used in our study, inhibits P-gp without affecting BRCP (Feng et al. Citation2019). These findings hold significance as they confirm the functionality of P-gp in canine colonoid-derived monolayers and validate the feasibility of conducting P-gp functional assessments in this model. However, it is important to approach these results with caution and consider the need for comprehensive assessments with multiple substrates and competitive substrates (K L Mealey et al. Citation2017).
While utilising canine intestinal tissues for assessing P-gp expression and conducting transport assays is a viable approach, employing colonoid-derived monolayers offers several advantages. These benefits include enhanced complexity and relevance, as these models closely mimic the in vivo environment, providing physiologically pertinent cell-cell and cell-matrix interactions essential for accurate studies of P-gp functionality. Additionally, colonoid-derived monolayers allow for a controlled experimental environment where variables such as growth factors and nutrients can be precisely manipulated, facilitating detailed mechanistic insights. They also offer reproducibility and scalability, which are crucial for longitudinal studies that require consistent experimental conditions. Particularly in canine models, where ethical and practical considerations may limit the use of traditional rodent models, colonoids provide a more sustainable and ethically responsible alternative. Furthermore, when reformulated into 2D monolayers, colonoids deliver enhanced functional insights by allowing focused studies on cell polarity and transporter functionality at specific cellular interfaces.
While understanding the differences between 3D and 2D models and aligning them more closely with in vivo conditions is important, our study primarily aims to establish and validate an efficient and reproducible 2D monolayer model for P-gp transport assays using canine colonoids. Future studies might explore modifications to the culture conditions, such as the differentiation medium (Nagao et al. Citation2024; Kozuka et al. Citation2017), to further recapitulate the in vivo environment. In addition to the previously discussed benefits of 2D monolayers, this model offers a simplified and effective system for studying microbial impacts on P-gp expression and function. The straightforward layout of 2D monolayers allows for direct, uniform exposure to bacterial agents, overcoming the challenges of bacterial control and measurement faced in 3D organoid models. This direct contact enables precise manipulation of experimental conditions and thorough analysis of epithelial cell responses. By providing a clearer understanding of microbial influences on drug transport mechanisms, our use of 2D monolayers not only enhances the relevance of our findings to in vivo conditions but also sets the stage for future studies exploring how the microbiome modulates drug resistance.
This study establishes the utility of canine organoid-derived monolayers for evaluating P-gp permeability using well-established compounds for such investigations. Future research endeavours can enhance our understanding by exploring a wider array of substrates and competitive substrates, unravelling the intricacies of drug transport mechanisms within this model. These considerations are pivotal in advancing our comprehension of P-gp-mediated drug transport within this model and its implications for veterinary medicine.
Animal welfare and ethics
All procedures on client-owned dogs in this study were approved by the Washington State University Institutional Animal Care and Use Committee (IACUC protocol: ASAF #6993). All clients who owned the dogs participated in this study were informed and signed the informed consent forms approved by the IACUC.
Authors’ contributions
I.N., Y.T., M.K., and Y.M.A. designed the experiments and I.N. performed the experiments. I.N., M.N. and Y.M.A. wrote and edited the manuscript, tables and figures.
Supplemental Material
Download Zip (14.4 KB)Acknowledgement
Authors would like to thank WSU Small Animal Internal Medicine service (Dr. Jillian Haines, Dr. Sarah Guess, Shelley Ensign LVT, Sybil Fiedler VTA), WSU Community Practices service (Dr. Jessica Bell, Dr. Cassidy Cordon, Dr. Matt Mason, Dr. Jenna Waltzek, Mrs. Melody Gerber, Ms. Maggie deSouza, Ms. Becky Brodie), and WSU VTH Clinical Studies Coordinator Valorie Wiss for their support in case recruitment and sample collection from citizen scientists (patient donors). We also extend our heartfelt gratitude to Mr. Neal Burke and Dr. Katrina Mealey for their invaluable scientific expertise and guidance during the design and experimental phases of this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All relevant data are within the paper and its Supporting Information files.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Ambrosini YM, Park Y, Jergens AE, Shin W, Min S, Atherly T, Borcherding DC, Jang J, Allenspach K, Mochel JP, et al. 2020. Recapitulation of the accessible interface of biopsy-derived canine intestinal organoids to study epithelial-luminal interactions. PLoS One. 15(4):e0231423. doi: 10.1371/journal.pone.0231423.
- Aouali N, El Btaouri H, Dumontet C, Eddabra L, Malagarie-Cazenave S, Madoulet C, Morjani H. 2011. Accumulation of lactosylceramide and overexpression of a PSC833-resistant P-glycoprotein in multidrug-resistant human sarcoma cells. Oncol Rep. 25(4):1161–1167. doi: 10.3892/or.2011.1180.
- Atadja P, Watanabe T, Xu H, Cohen D. 1998. PSC-833, a frontier in modulation of P-glycoprotein mediated multidrug resistance. Cancer Metastasis Rev. 17(2):163–168. doi: 10.1023/a:1006046201497.
- Bankstahl JP, Hoffmann K, Bethmann K, Löscher W. 2008. Glutamate is critically involved in seizure-induced overexpression of P-glycoprotein in the brain. Neuropharmacology. 54(6):1006–1016. doi: 10.1016/j.neuropharm.2008.02.008.
- Barta CA, Sachs-Barrable K, Feng F, Wasan KM. 2008. Effects of monoglycerides on P-glycoprotein: modulation of the activity and expression in Caco-2 cell monolayers. Mol Pharm. 5(5):863–875. doi: 10.1021/mp800050q.
- Broxterman HJ, Lankelma J, Pinedo HM, Eekman CA, Währer DC, Ossenkoppele GJ, Schuurhuis GJ. 1997. Theoretical and practical considerations for the measurement of P-glycoprotein function in acute myeloid leukemia. Leukemia. 11(7):1110–1118. doi: 10.1038/sj.leu.2400685.
- Chandra L, Borcherding DC, Kingsbury D, Atherly T, Ambrosini YM, Bourgois-Mochel A, Yuan W, Kimber M, Qi Y, Wang Q, et al. 2019. Derivation of adult canine intestinal organoids for translational research in gastroenterology. BMC Biol. 17(1):33. doi: 10.1186/s12915-019-0652-6.
- Coelho LP, Kultima JR, Costea PI, Fournier C, Pan Y, Czarnecki-Maulden G, Hayward MR, Forslund SK, Schmidt TSB, Descombes P, et al. 2018. Similarity of the dog and human gut microbiomes in gene content and response to diet. Microbiome. 6(1):72. doi: 10.1186/s40168-018-0450-3.
- Crowe A. 2021. P-glycoprotein-mediated efflux using a rapidly maturing Caco2 clone (CLEFF4) in only 5 days without requiring modified growth medium. SLAS Discov. 26(1):151–160. doi: 10.1177/2472555220942758.
- De Lange ECM, Vd Berg DJ, Bellanti F, Voskuyl RA, Syvänen S. 2018. P-glycoprotein protein expression versus functionality at the blood-brain barrier using immunohistochemistry, microdialysis and mathematical modeling. Eur J Pharm Sci. 124(November):61–70. doi: 10.1016/j.ejps.2018.08.022.
- Feller N, Kuiper CM, Lankelma J, Ruhdal JK, Scheper RJ, Pinedo HM, Broxterman HJ. 1995. Functional detection of MDR1/P170 and MRP/P190-mediated multidrug resistance in tumour cells by flow cytometry. Br J Cancer. 72(3):543–549. doi: 10.1038/BJC.1995.371.
- Feng B, West M, Patel NC, Wager T, Hou X, Johnson J, Tremaine L, Liras J. 2019. Validation of human MDR1-MDCK and BCRP-MDCK cell lines to improve the prediction of brain penetration. J Pharm Sci. 108(7):2476–2483. Elsevier Ltd: doi: 10.1016/j.xphs.2019.02.005.
- Forster S, Thumser AE, Hood SR, Plant N. 2012. Characterization of rhodamine-123 as a tracer dye for use in in vitro drug transport assays. PloS One. 7(3):e33253. doi: 10.1371/journal.pone.0033253.
- Gabriel V, Zdyrski C, Sahoo DK, Dao K, Bourgois-Mochel A, Atherly T, Martinez MN, Volpe DA, Kopper J, Allenspach K, et al. 2022. Canine intestinal organoids in a dual-chamber permeable support system. J Vis Exp. 2(181):1–24. doi: 10.3791/63612.
- Heijmans J, van Lidth de Jeude JF, Koo B-K, Rosekrans SL, Wielenga MCB, van de Wetering M, Ferrante M, Lee AS, Onderwater JJM, Paton JC, et al. 2013. ER stress causes rapid loss of intestinal epithelial stemness through activation of the unfolded protein response. Cell Rep. 3(4):1128–1139. doi: 10.1016/j.celrep.2013.02.031.
- Kozuka K, He Y, Koo-McCoy S, Kumaraswamy P, Nie B, Shaw K, Chan P, Leadbetter M, He L, Lewis JG, et al. 2017. Development and characterization of a human and mouse intestinal epithelial cell monolayer platform. Stem Cell Rep. 9(6):1976–1990. doi: 10.1016/j.stemcr.2017.10.013.
- Kuteykin-Teplyakov K, Brandt C, Hoffmann K, Löscher W. 2009. Complex time-dependent alterations in the brain expression of different drug efflux transporter genes after status epilepticus. Epilepsia. 50(4):887–897. United States: doi: 10.1111/j.1528-1167.2008.01916.x.
- Lehne G, Mørkrid L, den Boer M, Rugstad HE. 2000. Diverse effects of P-glycoprotein inhibitory agents on human leukemia cells expressing the Multidrug Resistance Protein (MRP). Int J Clin Pharmacol Ther. 38(4):187–195. doi: 10.5414/cpp38187.
- List AF, Spier CS, Grogan TM, Johnson C, Roe DJ, Greer JP, Wolff SN, Broxterman HJ, Scheffer GL, Scheper RJ, et al. 1996. Overexpression of the major vault transporter protein lung-resistance protein predicts treatment outcome in acute myeloid leukemia. Blood. 87(6):2464–2469. doi: 10.1182/blood.V87.6.2464.bloodjournal8762464.
- Martinez M, Modric S, Sharkey M, Troutman L, Walker L, Mealey K. 2008. The pharmacogenomics of P-glycoprotein and its role in veterinary medicine. J Vet Pharmacol Ther. 31(4):285–300. doi: 10.1111/j.1365-2885.2008.00964.x.
- Mealey KL. 2008. Canine ABCB1 and macrocyclic lactones: heartworm prevention and pharmacogenetics. Vet Parasitol. 158(3):215–222. doi: 10.1016/j.vetpar.2008.09.009.
- Mealey KL, Bentjen SA, Gay JM, Cantor GH. 2001. Ivermectin sensitivity in collies is associated with a deletion mutation of the Mdr1 gene. Pharmacogenetics. 11(8):727–733. doi: 10.1097/00008571-200111000-00012.
- Mealey KL, Dassanayake S, Burke NS. 2017. Establishment of a cell line for assessing drugs as canine P-glycoprotein substrates: proof of principle. J Vet Pharmacol Ther. 40(5):545–551. doi: 10.1111/jvp.12390.
- Mealey KL, Owens JG, Freeman E. 2023. Canine and feline P-glycoprotein deficiency: what we know and where we need to go. J Vet Pharmacol Ther. 46 (1):1–16. doi: 10.1111/jvp.13102.
- Nagao I, Nakazawa M, Goyama T, Court MH, Ambrosini YM. 2024. Assessment of cytochrome P450 induction in canine intestinal organoid models. Xenobiotica. :1–9. doi: 10.1080/00498254.2024.2326973.
- Ni Z, Bikadi Z, Rosenberg MF, Mao Q. 2010. Structure and function of the human breast cancer resistance protein (BCRP/ABCG2). Curr Drug Metab. 11(7):603–617. Netherlands: doi: 10.2174/138920010792927325.
- Peters IR, Peeters D, Helps CR, Day MJ. 2007. Development and application of multiple internal reference (housekeeper) gene assays for accurate normalisation of canine gene expression studies. Veterinary Immunol Immunopathol. 117(1–2):55–66. doi: 10.1016/j.vetimm.2007.01.011.
- Pietra M, Galiazzo G, Bresciani F, Morini M, Licarini S, Turba ME, Amaducci G, Bettini G, Fracassi F, Ostanello F. 2021. Evaluation of prognostic factors, including duodenal P-glycoprotein expression, in canine chronic enteropathy. Animals. 11(8):2315. doi: 10.3390/ani11082315.
- Sahoo DK, Martinez MN, Dao K, Gabriel V, Zdyrski C, Jergens AE, Atherly T, Iennarella-Servantez CA, Burns LE, Schrunk D, et al. 2023. Canine intestinal organoids as a novel in vitro model of intestinal drug permeability: a proof-of-concept study. Cells. 12(9):1269. doi: 10.3390/CELLS12091269/S1.
- Sevin E, Dehouck L, Fabulas-da Costa A, Cecchelli R, Dehouck MP, Lundquist S, Culot M. 2013. Accelerated Caco-2 cell permeability model for drug discovery. J Pharmacol Toxicol Methods. 68(3):334–339. doi: 10.1016/j.vascn.2013.07.004.
- Steingold SF, Sharp NJ, McGahan MC, Hughes CS, Dunn SE, Page RL. 1998. Characterization of canine MDR1 MRNA: its abundance in drug resistant cell lines and in vivo. Anticancer Res. 18(1A):393–400.
- Syvänen S, Luurtsema G, Molthoff CFM, Windhorst AD, Huisman MC, Lammertsma AA, Voskuyl RA, de Lange EC. 2011. (R)-[11C]Verapamil PET studies to assess changes in P-glycoprotein expression and functionality in rat blood-brain barrier after exposure to kainate-induced status epilepticus. BMC Med Imaging. 11(1):1–16. doi: 10.1186/1471-2342-11-1.
- Syvänen S, Schenke M, van den Berg D-J, Voskuyl RA, de Lange EC. 2012. Alteration in P-glycoprotein functionality affects intrabrain distribution of quinidine more than brain entry-a study in rats subjected to status epilepticus by kainate. Aaps J. 14(1):87–96. doi: 10.1208/s12248-011-9318-1.
- Tavelin S, Gråsjö J, Taipalensuu J, Ocklind G, Artursson P. 2002. Applications of epithelial cell culture in studies of drug transport. Methods Mol Biol. 188:233–272. doi: 10.1385/1-59259-185-X:233.
- van der Sandt IC, Blom-Roosemalen MC, de Boer AG, Breimer DD. 2000. Specificity of doxorubicin versus rhodamine-123 in assessing P-glycoprotein functionality in the LLC-PK1, LLC-PK1: MDR1 and Caco-2 Cell lines. Eur J Pharm Sci. 11(3):207–214. doi: 10.1016/S0928-0987(00)00097-X.
- Zolk O, Fromm MF. 2011. Transporter-mediated drug uptake and efflux: important determinants of adverse drug reactions. Clin Pharmacol Ther. 89(6):798–805. doi: 10.1038/clpt.2010.354.
- Zolnerciks JK, Booth-Genthe CL, Gupta A, Harris J, Unadkat JD. 2011. Substrate- and species-dependent inhibition of P-glycoprotein-mediated transport: implications for predicting in vivo drug interactions. J Pharm Sci. 100(8):3055–3061. doi: 10.1002/jps.22566.
