ABSTRACT
Boomplaas Cave in the Western Cape Province of South Africa is one of only a few African sites with inland archaeological deposits spanning Marine Isotope Stages 4–1. Work conducted half a century ago predicted Boomplaas to be a meagre plant-food location. We reassess this interpretation here by presenting updated lists of the current vegetation and foodplants growing within roughly a day’s foraging distance from the cave. By doing so, we increase the known foodplant species potentially available to Stone Age foragers by 356% and show that almost all the plant species/genera in the Boomplaas archaeobotanical assemblage still grow within a day’s range of the site. We present nutritional values for some of the plant foods, highlighting those richest in moisture, ash, protein, fat, fibre, carbohydrates and energy and suggesting that such foods may have been important staples in the dietary ecology of the Stone Age foragers who used the site. Lastly, we demonstrate that the Boomplaas Cave foodplant fitness landscape is relatively rich and varied compared to similar data from other Cape sites such as Klasies River Main Cave, Diepkloof Rock Shelter and Hollow Rock Shelter.
RÉSUMÉ
La grotte de Boomplaas, dans la province du Cap-Occidental d’Afrique du Sud, est l’un des rares sites de l’intérieur africain possédant des dépôts archéologiques couvrant les stades isotopiques marins 4 à 1. Des travaux menés il y a un demi-siècle prédisaient que Boomplaas aurait été un site pauvre en nourriture végétale. Nous réévaluons cette interprétation, présentant des listes actualisées de la végétation et des plantes alimentaires poussant présentement à une journée de distance environ de la grotte. Ce faisant, nous augmentons de 356% les espèces de plantes alimentaires connues qui étaient potentiellement disponibles aux chasseurs-cueilleurs de l’âge de pierre, et nous montrons que presque toutes les espèces/genres de plantes figurant dans l’assemblage archéobotanique de Boomplaas poussent encore aujourd’hui à moins d’une journée du site. Nous présentons les valeurs nutritionnelles de certains aliments végétaux, en mettant en évidence ceux qui sont les plus riches en humidité, cendres, protéines, graisses, fibres, glucides et énergie, et avançons que de tels aliments pourraient avoir été des éléments de base importants dans l’écologie alimentaire des chasseurs-cueilleurs de l’âge de pierre qui firent usage du site. Enfin, nous démontrons que le paysage de fitness des plantes alimentaires de la grotte de Boomplaas est relativement riche et varié par rapport à des données similaires provenant d’autres sites du Cap tels que Klasies River Main Cave, Diepkloof Rock Shelter et Hollow Rock Shelter.
Introduction
The southern Cape region of South Africa is one of only a few African landscapes with inland archaeological deposits spanning Marine Isotope Stages (MIS) 4–1. One of these sites is the limestone cave at Boomplaas in the Western Cape Province ∼80 km inland from the current Indian Ocean coastline (Pargeter and Faith Citation2020) (a). At 700 m above sea level in the foothills of the Swartberg Range, Boomplaas Cave overlooks the Cango Valley to the south (b). The Swartberg is a Fynbos-Albany Thicket landscape bordered to the north and south by the arid to semi-arid Succulent Karoo Biome (Mucina and Rutherford Citation2006). Current rainfall and evapotranspiration rates indicate a semi-arid environment for Boomplaas, which is prone to periodic aridity pulses (Chase et al. Citation2018). The Grobbelaars River provides ample permanent freshwater despite the general aridity of the area. Today, the vegetation of the Cango Valley consists mostly of bush and dense scrub, in which rock hyrax (Procavia capensis), baboons (Papio ursinus) and small antelopes represent the remaining wild mammal fauna amidst farming activities (Klein Citation1978; Faith Citation2013).
Figure 1. Boomplaas Cave and key finds: a) Map of the site’s location in the southern Cape of South Africa with other sites mentioned later in the text; b) view of the limestone cave from the valley (Justin Pargeter); c) Holocene (Member BLD) storage pits in the cave floor (supplied by Justin Pargeter from the H.J. Deacon Historical Archive, Iziko Museums); d) close-ups of an excavated Pappea capensis seed and Boophane disticha leaf remains (supplied by Justin Pargeter and Yvette van Wijk from the H.J. Deacon Historical Archive, Iziko Museums).
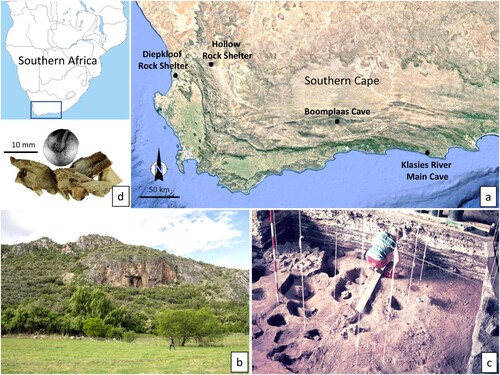
Hilary Deacon excavated Boomplaas Cave between 1974 and 1979 and it was initially the focus of several pioneering palaeoenvironmental studies (e.g. Klein Citation1978; Deacon Citation1979; Avery Citation1982; Scholtz Citation1986), complemented by stable isotope records from speleothems in the Cango Caves ∼3 km from the site (Talma and Vogel Citation1992). Current excavations focus on the site’s MIS 3 and MIS 2 deposits, dating from ∼45 kya to 13.7 kya (Pargeter et al. Citation2018; Pargeter and Faith Citation2020; Faith et al. Citation2024). Charcoal, faunal analyses, stable isotopes and sediment studies show that the major occupations at Boomplaas occurred alongside environmental changes (Webley Citation1978; Deacon et al. Citation1983; Deacon and Lancaster Citation1988; Talma and Vogel Citation1992), with decreased local environmental productivity and increased aridity by the end of MIS 2 (Faith Citation2013; Sealy et al. Citation2016; Chase et al. Citation2018; Faith et al. Citation2019).
Overall, the site’s palaeoenvironmental proxies suggest the expansion of C3 grassland vegetation and the increasing dominance of humid, winter rainfall-dominated conditions leading into MIS 2, followed by a transition towards increased C4 vegetation and more arid conditions, characteristic of the region’s current year-round rainfall zone (Sealy et al. Citation2016). Faith (Citation2013) suggested that more intense competition for resources, perhaps due to increasing population density, resulted in forager groups expanding into less favourable areas such as the Cango Valley (see also Pargeter and Faith Citation2020). The southern African Khoe-San genetic record suggests a gradual population decrease since 150 kya with a subsequent general increase over the last 25,000 years and a sharp rise at ∼2 kya (Schlebusch et al. Citation2020: Fig. 4).
The first paper published about Boomplaas Cave presented a list of 397 indigenous and 35 exotic plant species growing within ∼5 km of the site. In this, Moffett and Deacon (Citation1977: 127) emphasised possible plant use by pre-agricultural populations ‘to complement the archaeological and palaeo-ecological studies being undertaken in conjunction with the Boomplaas project’. They found that the Cango Valley around Boomplaas had a relatively high number of plant genera and species, probably resulting from its location in an ecotone with elements from both the moister Fynbos and more arid Karoo Biomes. Their surveys showed some 70 woody plants that people could have used for fuel, but only 35 foodplant species, one of which (Helichrysum serpyllifolium) is used as a tea, and four of which (Chenopodium murale [now Chenopodiastrum murale], Gnaphalium luteoalbum [now Pseudognaphalium luteoalbum], Foeniculum vulgare and Urtica urens) are not indigenous to southern Africa. They therefore concluded that:
‘available plant foods in the valley are negligible. Apart from the fruits of several tree species, which are known to be edible but at best could have provided for casual supplementary feeding, the only obvious plant food source is Hypoxis villosa, which is plentiful under the renosterbos [Elytropappus rhinocerotis] … .The prediction based on the survey is that while the valley may have served as a focus for hunting forays, it could not have supported other than very short-term visits’ (Moffett and Deacon Citation1977: 1933).
Our approach
A day’s foraging range represents the approximate distance that food-collecting groups typically travel from their home base before returning (Binford Citation1980, Citation1982; Grove Citation2009; Lombard and van Aardt Citation2023). These distances do not account for variation in movement energetics driven by topographic variability. Instead, they provide the broad foraging potential of a landscape. Layton et al. (Citation2012) listed daily foraging distances for hunter-gatherers from different biomes as ranging between 2 and 25 km, whereas Marlowe (Citation2005) suggested an average daily foraging range of between 9.5 and 14.1 km for African savanna hunter-gatherers. Yellen’s (Citation1976) analysis of Kalahari hunter-gatherer settlement patterns demonstrated that the longest geographical distance between camps was ∼17.5 km with an average distance of ∼9 km (Brown et al. Citation2007), that a single trip may include several overnight camping stops and that the maximum home-base foraging ranges varied between about 16 and 36 km.
Here, we focus on the species growing within roughly a day’s return trip around Boomplaas Cave. Plant foods harvested from this foraging range would have been eaten regularly by the people who used the site, either while foraging or at the cave where some surplus foods may have been stored (Moffet and Deacon Citation1977; Lombard and van Aardt Citation2023). The starting point of a foodplant fitness analysis constitutes an inventory of plant species known to grow on a landscape that used to be occupied by foragers, i.e. its current phyto-scape. We generated a list of plants growing roughly within a day’s foraging distance from Boomplaas by incorporating data from several sources:
Species recorded between 1879 and 2018 on the 3322CA 1: 50,000 grid map of South Africa as provided by the South African National Biodiversity Institute (SANBI);
Species from the Moffett and Deacon (Citation1977) surveys within ∼5 km from Boomplaas Cave;
Species listed by Mucina and Rutherford (Citation2006) and Dayaram et al. (Citation2019) as important for the various vegetation types represented within a ∼12.5 km radius of Boomplaas (a and b; ). Species lists, distinctive landscape features, broad vegetation characteristics, altitude, temperatures, rainfall, soil types and geology associated with each vegetation type can be downloaded from the SANBI VEGMAP database (http://bgis.sanbi.org/VegMap/Home).
Figure 2. Vegetation and topography in the environs of Boomplaas Cave: a) vegetation types (see for a key to the codes) within a ∼12.5 km radius of Boomplaas Cave (map adapted by Matt Caruana and Matt Lotter after Mucina and Rutherford Citation2006)); b) topography for roughly the same area; c) the Cango Valley in front of Boomplaas Cave (BPA) looking northwest with the Swartberg stretching into the distance and riparian vegetation marking the path of the Grobbelaars River (photograph Justin Pargeter).
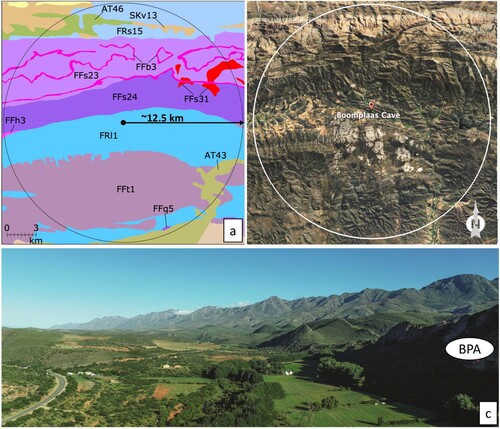
Table 1. Vegetation types within ∼12.5 km of Boomplaas (see http://bgis.sanbi.org/VegMap/Home for more details about each vegetation type).
By systematically comparing the Boomplaas checklist (the species inventory of plants currently growing in the specified forage-scape in SOM 1) against the foodplant checklist (Lombard and van Aardt Citation2023), we compiled an inventory of foodplant species currently known to grow around the site. The Lombard and van Aardt (Citation2023) foodplant checklist built on inventories reported by Peters et al. (Citation1992) for sub-Saharan Africa and by Welcome and van Wyk (Citation2019) for Botswana, eSwatini, Lesotho, Namibia and South Africa. From Peters et al. (Citation1992), we only included species listed in the official SANBI Plants of Southern Africa Checklist (https://posa.sanbi.org/sanbi/Explore) as indigenous or endemic to southern Africa. We retained information about baboon and chimpanzee plant foods because there is a well-established history of using primate diets to reconstruct hominin foraging and dietary behaviours (Dunbar Citation1976; Elton and Dunn Citation2020) and because they can presumably also be eaten raw by humans, at least in small quantities as fallback foods without severe side effects. Some of these foods may have served as famine foods for humans that published knowledge systems no longer maintain.
From Welcome and van Wyk (Citation2019), we eliminated naturalised, cultivated and exotic plants. We omitted species used for beverage preparation only (teas, alcoholic beverages or coffee substitutes), but included plants sourced for saps, nectars and gums as part of subsistence behaviours. We added species from other sources that are indigenous or endemic to southern Africa (see SOM 2 for references). Apart from the plants and their edible parts captured in the literature, we added botanical data about growth forms, photosynthetic pathways, toxicity, etc. (Lombard and van Aardt Citation2023). This approach provides the most complete potential foodplant checklist for Boomplaas to date (SOM 2), but comes with a few cautionary notes:
not all areas around the site have been surveyed equally, and our data do not account for landscape barriers, topographic variability and inter- and intra-species abundance. Our inventory thus represents a coarse-grained baseline of what might have been available to past foragers;
we verified nomenclature according to the SANBI checklist (2023: http://opus.sanbi.org/handle/20.500.12143/6880), but plant names change based on taxonomic opinions, identification details or the recognition of contextual synonymy (Peters et al. Citation1992). For example, Old World Rhus species now fall within the genus Searsia (Moffet Citation2007), African Acacia under either Vachellia or Senegalia (Kyalangalilwa et al. Citation2013). Creating foodplant lists without species duplication is therefore not always straightforward;
and, although the plant foods in the inventory have been recorded as edible, they should not be eaten without prior knowledge and toxicity testing. Several species may be harmful depending on the constitution of the consumer, quantity consumed, soil chemistry, growth phase, whether cooked or not and several other factors (Peters et al. Citation1992). We have cross-checked our list for potential toxicity against Neuwinger (Citation1994), von Koenen (Citation1996), van Wyk et al. (Citation2002) and Wink and van Wyk (Citation2008), but emphasise that we may not have identified all species that contain toxins.
Lastly, Moffet and Deacon (Citation1977) provided floristic density estimates for 14 quadrats of 50 m2 each, demarcated in different vegetation zones within 5 km of Boomplaas Cave. We use some of these data in combination with data for ‘dominant’ plants in the current vegetation types identified in a and (http://bgis.sanbi.org/VegMap/Home) to assess which of the potential foodplant staples may have been abundant on the Boomplaas foraging-scape and where people may have collected some of their key staple foods.
Results
The archaeobotany of Boomplaas and its overlap with the current species inventory
Moffet and Deacon (Citation1977) noted that the preservation of macroscopic plant remains, mainly seeds and leaves, is restricted to the most recent layers of the Boomplaas deposit. However, some of the site’s earlier stratigraphic units preserve pollen and other charred botanical remains (Deacon Citation1983; Deacon et al. Citation1984). Most of the macrobotanical remains come from a cluster of ≥40 storage pits dating to 4.2–1.4 kya (c) (Deacon Citation1983; Pargeter et al. Citation2018). These pits contained the remains of Pappea capensis fruit with oil-rich seeds and the corm bases of Hypoxis villosa and Watsonia (Deacon Citation1995). The pits seem to have been lined with the toxic leaves of Boophone disticha, perhaps to keep insects away from the stored plant foods (d) (Moffet and Deacon Citation1977). Hilary Deacon attributed the lack of additional plant foods in the pits to the limited availability of geophytes near the site (Moffet and Deacon Citation1977). Scholtz’s (Citation1986) study of charcoals from Boomplaas provides more information on past plant collection practices (), with Olea, Dodonea and Protea arborea the most abundant charcoal remains from MIS 3. Proteaceae, Compositae and Leucadendron species are more abundant during the site’s earlier MIS 2 phase, while by the end of MIS 2 Olea and Searsia species increase. Vachellia karroo remains characterise the MIS 1/Holocene assemblage alongside Searsia, Olea and other thicket species. Thus far, researchers have identified the remains of approximately 42 plant species/genera from Boomplaas’ archaeological deposits (Moffet and Deacon Citation1977; Deacon Citation1983; Scholtz Citation1986).
Table 2. Identified plant remains from Boomplaas Cave. All data compiled from Deacon (Citation1983) and Scholtz (Citation1986). Key: # = present within a day’s return trip of Boomplaas today, * = foodplant, ^ = genera including known foodplants.
We could not find local records for four plants, namely Exomis microphylla, Myrica sp., Searsia rehmanniana and Solanum tomentosum, growing in the immediate landscape around the site (). Ongoing field surveys may, however, provide more information as research at Boomplaas continues. Eight (19%) of the archaeobotanical species are known foodplants, while 21 (50%) represent genera that include known foodplants (Lombard and van Aardt Citation2023). Almost all the species or genera in the Boomplaas archaeobotanical assemblage still grow within a day’s foraging distance of the site (SOM 1). This pattern is valid for 86% (12 out of 14) of the species/genera identified for the MIS 3 deposits dating to 47.4–30.1 kya, 94% (17 out of 18) for those identified for MIS 2 with dates between 26.4 ka and 13.7 kya and 91% (29 out of 32) of the Holocene plants/genera older than 1.4 kya (). These statistics demonstrate that, despite climatic oscillation, it is reasonable to assume that a relatively large proportion of the plants or plant genera growing around Boomplaas Cave today may also have been present there in the past. It is therefore feasible to use the current species list as a proxy for hypothesising about past foraging potential, albeit with the understanding that the current phyto-scape is not a replica of the past, but that it indicates the capacity of the Boomplaas day-range foraging-scape.
The current Boomplaas foodplants and their edible parts
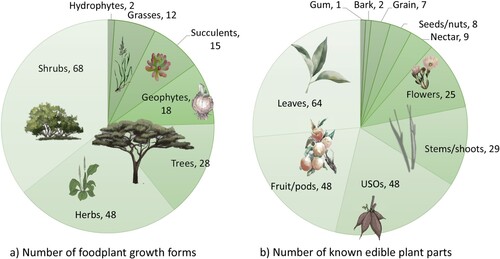
Our approach reveals that 137 foodplants in 51 genera with 242 edible parts grow within roughly a day’s foraging distance from Boomplaas Cave (; SOM 2), but ongoing/future surveys may increase these numbers. Shrubs (N = 68, 50%) and herbs (N = 48, 35%) dominate the foodplant growth forms (a). Trees or shrubs that may grow into trees (N = 28, 20%), geophytes (N = 18, 13%), succulents (N = 15, 11%) and grasses (N = 12, 9%) are all relatively well represented. Hydrophytes are, however, scarce, represented by only two plants, namely Berula thunbergia and Gunnera perpensa. There are no recorded sedges from which one could harvest plant foods. Most of the edible plant parts are leaves (N = 64, 47%), followed in equal representation by fruit/fruit pods (N = 48, 35%) and USOsFootnote1 (under-surface storage organs, N = 48, 35%; b). The number of species known to produce edible nectars (N = 9, 7%), seeds/nuts (N = 8, 6%) and grains (N = 7, 5%) is much fewer, but the grass grains especially may have been abundantly available. There are only two species (1.5%) on the immediate Boomplaas foraging-scape known to produce edible bark, namely Ilex mitis and Vachellia karroo, the latter of which also produces edible gum (b; ).
Table 3. Summative statistics for the foodplants and edible plant parts growing roughly within a day’s foraging from Boomplaas Cave. Totals do not always add up to 100% because of overlap in categories.
Figure 3. Proportional representation of the number of foodplants (a) and edible plant parts growing roughly within a day’s foraging from Boomplaas Cave (for percentages see ).
Nine known famine foodplants grow within a day’s foraging distance from Boomplaas Cave, including Cussonia spicata, Eragrostis curvula, Miscanthus ecklonii, Olea europaea, Phragmites australis, Portulacaria afra, Sisymbrium capense, Sporobolus fimbriatus and Zantedeschia aethiopica. Thirst quenchers, or plants from which the sap can be drunk as moisture, include Cussonia paniculata, C. spicata, Cyphia digitata, Dolichos angustifolius, Portulacaria afra and Vachellia karroo.
Possible plant-food staples around Boomplaas Cave
We found nutritional values for edible parts of about a third (N = 45, 33%) of the foodplant species growing within roughly a day’s foraging from Boomplaas Cave (SOM 3). Not all of these values are from South Africa and they do not reflect aspects such as the bioavailability of nutrients, growth phase or soil characteristics etc. We nevertheless suggest that these broad nutritional values are useful indicators for identifying plant foods that may have been staples on the site’s immediate foraging-scape. Plant foods that provide moisture become important staples in dry areas or during dry seasons and phases (Tanaka Citation1976). Fourteen plant foods within a day’s foraging around Boomplaas Cave have records of ≥80 g/100 g of moisture (a). Most are leaves (N = 7), followed by fruits (N = 6), USOs (N = 5) and stems (N = 2). Ash represents the total mineral content of our food and we need small amounts of the trace elements that it contains in our diet. The nine plant parts recorded with ≥10 g/100 g of ash around Boomplaas include mostly leaves (N = 4), followed by fruit (N = 2) and one each of USOs, seeds and stems (b).
Figure 4. Some of the highest nutritional values recorded for plant foods that grow within about a day’s foraging of Boomplaas Cave including a) moisture, b) ash, c) protein, d) fat, e) fibre, f) carbohydrates and g) energy. For further details see SOM 3.
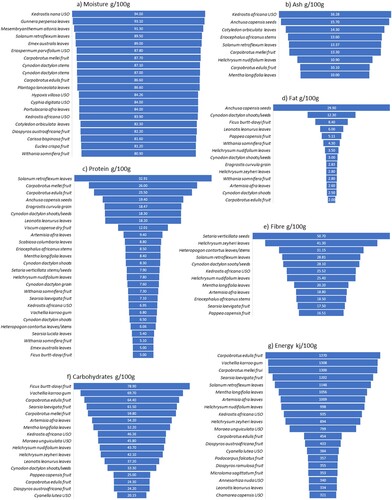
Protein is an important energy source, helping to build and/or maintain muscles, bones, hormones and enzymes. Kyriacou et al. (Citation2016) reported protein values of ∼5–22 g/100 g for a range of antelope tissues, representing a control for the amounts of protein obtained from hunting. Within a day’s foraging around Boomplaas Cave, 26 plant foods have recorded protein values of ≥5 g/100 g (c). Leaves (N = 8), fruits (N = 7) and stems/shoots (N = 6) represent most of these plant parts, followed by seeds (N = 3), grains (N = 2) and one USO and one gum. Plant foods that contain relatively high fat/lipid values contribute to the energy that humans may gain from eating them, provide essential fatty acids that our bodies cannot produce and enable the absorption of vitamins A, D, E and K. Ledger (Citation1968) calculated the fat content for the carcasses of several African bovid taxa at an average of ∼4.3% per carcass. The muscle meat of South African game usually contains <2 g/100 g of fat, with higher concentrations in marrow and organ meats (Kyriacou et al. Citation2016). Fourteen plant foods on the immediate Boomplaas foraging-scape have fat values of ≥2 g/100 g (d). Six of these have fat levels of ≥4.3%., i.e. Anchusa capensis seeds (29.9%), Cynodon dactylon shoots/seeds (12.3%), Ficus burtt-davyi fruits (8.4%), Leonotis leonurus leaves (6%), Pappea capensis fruits (5.11%) and Withania somnifera fruits (4.3%).
Fibre is important for the digestive system and a healthy gut microbiome (Fu et al. Citation2022). We found twelve plant foods within a day’s foraging from Boomplaas Cave with fibre levels of ≥15 g/100 g. Again, most of these are leaves (N = 6), followed by stems/shoots (N = 3), two fruits, two seeds and one USO (e). Foods with carbohydrate values of ≥30 g/100 g are considered above average (Ruiters-Welcome Citation2019) and thirteen plant foods currently growing around the cave have recorded carbohydrate values of ≥30 g/100 g, with four more having values of >20 g/100 g (f). Most of the carbohydrate-rich plant foods in our sample are fruits (N = 7), followed by leaves (N = 5), USOs (N = 3) and one each of gums, shoots and seeds. Singels (Citation2021) recorded a carbohydrate value of only 3.41 g/100 g for Hypoxis villosa USOs from the southern Cape (SOM 3). The eleven plant foods with recorded nutritional values from which humans can gain the most energy (∼1370–800 kj/100 g) on the immediate Boomplaas foraging-scape include Carpobrotus edulis fruits, Vachellia karroo gum, C. mellei fruits, Searsia laevigata fruits, Solanum retroflexum leaves, Mentha longifolia leaves, Artemisia afra leaves, Helichrysum nudifolium leaves, Kedrostis africana USOs, H. zeyheri leaves and Moraea unguiculata USOs. Nine more plant foods have relatively high energy values of ≥500 kj/100 g ( g).
Nineteen plant foods around Boomplaas have relatively high values in three or more nutritional categories (). We suggest that of the 45 Boomplaas foodplant species for which nutritional data are available (SOM 3), these 19 plant foods may reflect some of the key staple foods on the immediate foraging-scape around the cave. At the top of our list (each ticking six or five nutritional boxes in ) are eight plant foods, namely Carpobrotus edulis fruits, Helichrysum nudifolium leaves, Kedrostis africana USOs, Artemisia afra leaves, C. mellei fruits, Cynodon dactylon shoots/stems, Mentha longifolia leaves and Solanum retroflexum leaves (a–k). In terms of edible plant parts, fruits (N = 7) are at the top of the Boomplaas nutritional list, followed by leaves (N = 6), shoots/stems (N = 2) and one each of USOs, seeds, grains and gums ().
Table 4. Plant foods within about a day’s foraging around Boomplaas with relatively high values in three or more nutritional categories.
Location and density of some foodplants around Boomplaas Cave
Location and density data for the foodplants around Boomplaas Cave remain incomplete. We can, however, predict some aspects of past human use of its foraging-scape with the available information. For example, of the plants listed in Helichrysum nudifolium is an important species in the FFs24 and FFs31 vegetation types in the Swartberg just north of the site (see a and for locations relative to the cave and a key to vegetation codes). Here foragers may have collected the soft, new leaves of H. nudifolium during the spring months of September and October (d). Searsia laevigata is dominant in the FRl1 vegetation within which Boomplaas is located (a). Moffet and Deacon’s (Citation1977) survey also indicates it being very plentiful (12.5–25% coverage) to plentiful (2–5% coverage) on the undulating limestone hills between the Grobbelaars River and the Swartberg. The fruit of this shrub ( l), rich in protein, fibre and carbohydrates with a relatively high energy value, would thus have been opulently available during the summer and autumn months. Its leaves are also edible.
Figure 5. Examples of plant foods: a) Carpobrotus edulis fruit (Wendy June Norris CC BY-NC-ND iNaturalist); b) dried C. edulis fruit (with permission, Babylonstoren; c) Helichrysum nudifolium (Vathiswa Zikishe CC BY-NC iNaturalist); d) young H. nudifolium leaves (James Hallé CC BY-NC iNaturalist); e) Kedrostis africana climber (Andrew Hankey CC BY-SA iNaturalist); f) Kedrostis africana USO (Valentino Vallicelli free use); g) Artemisia afra leaves (Tony Rebelo CC BY-SA iNaturalist); h) Carpobrotus mellei flower and fruit (Felix Riegel CC BY-NC iNaturalist); i) Cynodon dactylon shoots/stems (Ricky Taylor CC BY-NC iNaturalist); j) Mentha longifolia leaves (Alan Lee CC BY-NC iNaturalist); k) young Solanum retroflexum leaf (Gigi Laidler CC BY-NC iNaturalist); l) Searsia laevigata fruit (Adriaan Grobler CC BY-NC iNaturalist); m) Diospyros austro-africana fruit (Brendan Cole CC BY-NC-ND iNaturalist); n) Eriocephalus africanus stem and leaves (Johan October CC BY-NC iNaturalist); o) Pappea capensis fruit (Magda stLucia CC BY-NC iNaturalist); p) Vachellia karroo gum (Tony Rebelo CC-BY-SA iNaturalist).

Diospyros austro-africana is an important species of the FRs15 vegetation north of the Swartberg and Moffet and Deacon (Citation1977) found it to be plentiful (2–5% coverage) on the lower slopes of the western side and in narrow strips on either side of the central part of the Cango Valley. It bears round, fleshy fruits from January to June that are high in moisture content and relatively high in carbohydrates and energy ( m). Its USOs and leaves are also edible. Eriocephalus africanus is also important in FRs15 vegetation and very plentiful (5–12.5% coverage) on the western lower slopes and in narrow strips on either side of the valley (Moffet and Deacon Citation1977). Young protein-rich stems and leaves would have been at their best during the spring and early summer (n). Moffet and Deacon (Citation1977) did not record Pappea capensis in their 5 km surveys, but Deacon (Citation1979) indicated that it grows within 10 km of Boomplaas Cave. It is a dominant species in the AT43 vegetation southeast of the site (a). Here, its protein-, fat-, carbohydrate- and energy-rich fruits could be harvested when it is produced from December to July (o). Deacon (Citation1979) reports how foragers can harvest 117–150 fruits within 60 seconds. People may have eaten its fruits with the seed and its leaves are also edible.
Vachellia karroo is a dominant species amongst the AT46 vegetation at the northwest edge of the Boomplaas day-range foraging-scape, as well as on the eastern side of the Cango Valley, where it occurs with 25–50% coverage in limited areas at the bottom of the steep limestone hills (Moffet and Deacon Citation1977). Its protein-rich gum with very high carbohydrate and energy values would have been a year-round treat for children and adults alike (p). This unassuming tree is, however, a versatile foodplant of which one can also eat the flower buds, fruit pods and bark. Additionally, its USOs can serve as a thirst quencher (SOM 2). We should thus not underestimate its prolific presence within a day’s foraging distance from Boomplaas Cave and the possibility that humans could have used it as a dependable year-round food source.
Discussion
The main outcome of this study is that we have increased the potential foodplant species growing around Boomplaas Cave from 30 to 137 — an increase of 356% — by extending the foraging range to roughly a day’s return trip and incorporating different observation records. Collectively, the 137 foodplants in our checklist for Boomplaas represent at least 242 edible plant parts. Using a similar approach, we found 161 foodplant species with 273 edible parts growing within a day’s foraging distance of Klasies River Main Cave ∼150 km southeast of Boomplaas along the south coast of South Africa (a; Lombard and van Aardt, Citation2023). This is a long-sequence Stone Age site generally seen as rich in plant foods (Deacon and Geleijnse Citation1988; Deacon Citation1995; van Wijk et al. Citation2017, Citation2019; Larbey et al. Citation2019) and with occupations that overlap with those of Boomplaas Cave. The day-range foraging-scape of Klasies River has only 17.5% more foodplants and 12.8% more edible plant parts than that of Boomplaas Cave.
Diepkloof Rock Shelter, ∼15 km inland from the Cape’s west coast and overlooking the Verlorenvlei River, is another site with occupations overlapping with Boomplaas Cave (a). Here, we found only 81 foodplant species with 159 edible parts within a day’s foraging distance (Lombard Citation2023), demonstrating that Boomplaas currently has 69.1% more foodplant species with 52.2% more edible parts on its day-range foraging-scape compared to Diepkloof. Hollow Rock Shelter, just east of the Cederberg Range (a), also has fewer foodplant species than Boomplaas with records for 109 foodplant species and 170 edible parts growing within ∼12.5 km of the site (Lombard and Högberg Citation2023), revealing that Boomplaas currently has 25.7% more foodplant species growing within a day’s foraging range and 42.4% more edible plant parts. The number of foodplant species and edible plant parts currently growing around Boomplaas Cave, and, where available, their nutritional values and density records, therefore demonstrate that — instead of being negligible or less favourable in terms of its foodplant foraging potential — the site and its immediate surroundings may have provided Stone Age foragers with a healthy and prolific plant-based diet during most of the year to complement or subsititute for their hunting endeavours.
The more than sixty storage pits in the central area of Boomplaas Cave are impressive (c), providing detailed insight into how Holocene foragers collected and stored their plant foods. According to Deacon (Citation1979), the pits represent evidence for harvesting and storing mainly Pappea capensis fruit. An early reference to hunter-gatherers eating the fruit and seeds of this species comes from the diary of Robert Jacob Gordon, a late eighteenth-century explorer. His entry for 30 September 1779 references a kouboom (P. capensis) in what is now South Africa’s Northern Cape Province west of Kakamas that also grows in the Eastern Cape (Skead Citation2009: 257), saying that ‘it has a small red core inside the green capsule; the pip tastes of almonds, and oil is made from it. They are very large here and the BushmenFootnote2 eat themselves fat on them’ (https://digitalcollections.lib.uct.ac.za/4th-journey-dutch). The bright-red fruit pulp has a delicious sweet-sour flavour (van Wyk and Gericke Citation2000), while a golden-yellow, edible, fragrant and non-drying oil can be extracted from the roasted seeds, although this has a somewhat purgative effect. The oil is also suitable for soap making and lubrication and people additionally use it to treat ringworm or skin infections caused by a fungus (Watt and Breyer Brandwyk Citation1962: 931–932). Teas, alcoholic beverages and preserves are also made from the fruits (Welcome and van Wyk Citation2019), so there may have been multiple reasons for collecting and storing them in the pits at Boomplaas. Nevertheless, considering the relatively low fat content of African bovid meat (Kyriacou et al. Citation2016), there is good reason to suggest that the Boomplaas foragers may have also targeted other available plant foods that are relatively fat-rich, such as Anchusa capensis and Cynodon dactylon seeds, as well as the fruit of Ficus burtt-davyi and Withania somnifera and Leonotis leonurus leaves.
Hilary Deacon (Citation1983) excavated P. capensis remains from most of the Boomplaas pits, attributing the general lack of other plant-food remains to their limited availability (Moffet and Deacon Citation1977). However, we suggest that the P. capensis remains perhaps represent a seasonal summer-autumn harvest after which the site’s inhabitants deserted the storage pits. Other storable foods available on the immediate Boomplaas foraging-scape that may have been similarly stored, perhaps after autumn-winter harvests, could include grains harvested from grasses such as Cynodon dactylon, Eragrostis curvula, Hypodiscus aristatus, Miscanthus ecklonii, Setaria verticillata, Sporobolus fimbriatus, Themeda triandra and seeds from plants such as Dodonaea viscosa, Lessertia frutescens, Leucadendron pubescens, Plantago lanceolata, Schotia afra and Vachellia karroo. Moffet and Deacon (Citation1977) also mention the remains of Hypoxis and Watsonia USOs in the storage pits. The current Boomplaas excavation team uses flotation to extract as much plant material as possible from the newly excavated deposits. Future work on such materials will be able to test whether the site’s inhabitants perhaps harvested and stored a much wider range of plant foods.
Previous researchers did not have the benefit of the syntheses of Peters et al. (Citation1992) and Welcome and van Wyk (Citation2019) that we were able to draw upon for building our foodplant checklist (Lombard and van Aardt Citation2023). Thus, many of the plants that Moffet and Deacon (Citation1977) listed were not recognised as potential Stone Age food sources available to the Boomplaas foragers. For example, species listed by them as ‘very plentiful’ or ‘plentiful’ within 5 km of the site, but not as food sources, include Diospyros austro-africana, Dodonaea viscosa, Eriocephalus africanus, Euclea undulata, Heteropogon contortus, Hyparrhenia hirta, Maytenus oleoides, Myrsine africana, Pelargonium peltatum, Searsia lucida, S. undulata, Tarchonanthus camphoratus and Themeda triandra.
While some tubers might be more valued for their protein, iron and iodine content (Schnorr et al. Citation2015), as well as the off-setting of nitrogen in protein where there is little or no animal fat available (Jones Citation2009; Speth Citation2010), notions about geophytes being the main plant-food staple of the Greater Cape Floristic Region (e.g. Deacon and Geleijnse Citation1988; De Vynck et al. Citation2016a, Citation2016b; Singels et al. Citation2016; Botha et al. Citation2019, Citation2020; Larbey et al. Citation2019) may have also skewed ideas about the staple value of other plant foods (cf. Tanaka Citation1976; Lombard and Högberg Citation2023). This skewing is evident when Moffet and Deacon (Citation1977) claim that the only obvious plant food around Boomplaas Cave is Hypoxis villosa and that the edible fruits could have provided ‘casual supplementary feeding’ only. Hilary Deacon (Citation1993: 89) later stated that ‘the only possible reason for occupying habitats in the Cape Fold Mountains was to gain access to fields of geophytes’. Such ideas may originate from Eurocentric notions about cooked starchy foods (e.g. potatoes and rice) being essential staples and the assumption that only foods brought to and cooked at the homebase would represent important staples.
To the contrary, Welcome and van Wyk (Citation2019) found that the most important plant foods for all 13 southern African groups in their survey (Herero, Kwangali, Manyo, Northern Sotho, Southern Sotho, Swati, Tsonga, Venda, Wambo, Xhosa, Zulu, Ju/’hoansi and Khoekhoen), were those eaten raw ‘as snacks’, often in situ while gathering them. Although the Khoekhoen prefer to collect USOs, fruits and leaves are the most important plant foods for all other groups, including Ju/’hoan hunter-gatherer descendants. The Khoekhoen are the descendants of incoming pastoralists who arrived ∼2 kya in southern Africa, with substantial subsequent genetic and cultural exchange between them and local hunter-gatherers (Breton et al. Citation2014; Schlebusch et al. Citation2017, Citation2020). This does not, however, imply that Stone Age foragers followed the subsistence patterns of the current Khoekhoe or Cape Coloured communities. Cape Malay people were brought as slaves to South Africa from Indonesia by the Dutch East India Company and their culinary heritage, along with Dutch and early trekboer food traditions, have all influenced Khoekhoe practices and vice versa. Instead, fruit and leaves form a substantial part of the plant-food staple package on the southern African foraging-scape (Welcome and van Wyk Citation2019), much more than ‘casual supplementary feeding’. The nutritional values of some fruits and leaves demonstrate that they can be considered staples by providing the main portion of a person’s daily energy and nutritional requirements (). We thus suggest that casting a wider net across the spectrum of available plant foods and their nutritional values may enrich thinking about the use of past southern Cape foraging-scapes, providing a more nuanced understanding of the foodplant fitness potential around Stone Age sites.
In conclusion
Moffet and Deacon’s (Citation1977) archaeobotanical work was ground-breaking for its time. Even today, we are unaware of similarly detailed vegetation data for southern African Stone Age sites. Nevertheless, field surveys provide only selected spatiotemporal samples, whereas our method allows us to also take in to account more inclusive data collected over a century or more by numerous observers. Both approaches bring different types of data to the foreground. When we integrate them alongside nutritional information — for example, our section on location and density — fine-grained hypotheses about how people may have used a foraging-scape in the past become possible. We plan to expand on this aspect with future work on the Boomplaas foraging-scape, creating a ‘foraging map’ correlated with seasonal foraging opportunities for day-range foraging. We also aim to explore the wider foraging ranges that required overnight camping to gather plant foods potentially consumed habitually by the Boomplaas foragers, with the understanding, of course, that the cave may have been one of several such camping-storage sites on an extended foraging-scape. In additional studies, we shall use the data presented in our SOM files to explore how varying climatic conditions may have affected the different foodplant species and to focus on the Boomplaas foodplants with toxins (SOM 2), identifying the parts that contain toxins, the types of toxins recorded, seasonal and/or growth phase differences in toxicity, the reduction of toxicity through cooking or drying and any health benefits associated with eating plant foods that contain toxins. Ongoing excavations at the site will draw on these data to facilitate macrobotanical identifications and to develop a more comprehensive palaeobotanical record for Boomplaas Cave.
Supplemental Material
Download MS Excel (17.8 KB)Supplemental Material
Download MS Excel (42.1 KB)Supplemental Material
Download MS Excel (95.5 KB)Acknowledgements
We thank Hanneli Snyman from SANBI for the data extraction, Matt Caruana and Matt Lotter from the University of Johannesburg for assistance with the vegetation map and two anonymous reviewers for their useful feedback. Estherna Pretorius provided the common names of the foodplants listed in SOM 2. No funding was received for the work reported on here and any mistakes or omisions remain our own.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Notes on contributors
Marlize Lombard
Marlize Lombard is Research Chair at the Palaeo-Research Institute of the University of Johannesburg in South Africa. She leads the inter-disciplinary Palaeo-TrACKS (Tracing Ancient Cognition and Knowledge Systems through the Stone Age/Palaeolithic) Research Programme that is geared towards generating knowledge about the biological, behavioural and cognitive evolution of Homo sapiens in Sub-Saharan Africa and how these aspects relate to each other.
Justin Pargeter
Justin Pargeter is an Assistant Professor in the Department of Anthropology at New York University, Director of the African Paleosciences Laboratory there and Senior Research Fellow at the University of Johannesburg. His research focuses on human biocultural evolution tracked by the relationships between lithic technology, cognition and environmental change in the archaeological record. His career focus has been on investigations of the later Pleistocene evolution of hunter-gatherer behaviour in sub-Saharan Africa through experimental archaeology, lithic analysis and the recovery of new field data.
Notes
1 The term ‘USOs’ does not equal geophytes. Geophytes only include species with specialised storage organs, whereas USOs also include other root systems. Moreover, foragers do not eat the USOs of all geophytes.
2 We reject any pejorative connotations that some may have imputed when using the term ‘Bushmen’ to refer to the descendants of southern African hunter-gatherer groups. Instead, we use the term here as employed by people to self-identify and by the original author.
References
- Avery, D.M. 1982. “Micromammals as palaeoenvironmental indicators and an interpretation of the late Quaternary in the Southern Cape Province, South Africa.” Annals of the South African Museum 85: 183–374.
- Binford, L.R. 1980. “Willow smoke and dogs’ tails: hunter-gatherer settlement systems and archaeological site formation.” American Antiquity 45: 4–20.
- Binford, L.R. 1982. “The archaeology of place.” Journal of Anthropological Archaeology 1: 5–31.
- Botha, M.S., Cowling, R.M., Esler, K.J., De Vynck, J.C., Cleghorn, N.E. and Potts, A.J. 2020. “Return rates from plant foraging on the Cape south coast: understanding early human economies.” Quaternary Science Reviews 235: 1–15.
- Botha, M.S., Cowling, R.M., Esler, K.J., De Vynck, J. and Potts, A.J. 2019. “Have humans living within the Greater Cape Floristic Region used the same plant species through time?” South African Journal of Botany 122: 11–20.
- Breton, G., Schlebusch, C.M., Lombard, M., Sjödin, P., Soodyall, H. and Jakobsson, M. 2014. “Lactase persistence alleles reveal partial East African ancestry of southern African Khoe pastoralists.” Current Biology 24: 852–858.
- Brown, C.T., Liebovitch, L.S. and Glendon, R. 2007. “Lévy flights in Dobe Ju/’hoansi foraging patterns.” Human Ecology 35: 129–138.
- Chase, B.M., Faith, J.T., Mackay, A., Chevalier, M., Carr, A.S., Boom, A., Lim, S. and Reimer, P.J. 2018. “Climatic controls on Later Stone Age human adaptation in Africa’s southern Cape.” Journal of Human Evolution 114: 35–44.
- Dayaram, A., Harris, L.R., Grobler, B.A., Van der Merwe, S., Rebelo, A.G., Ward Powrie, L., Vlok, J.H., Desmet, P.G., Qabaqaba, M., Hlahane, K.M. and Skowno, A.L. 2019. “Vegetation map of South Africa, Lesotho and Swaziland 2018: a description of changes since 2006.” Bothalia – African Biodiversity & Conservation 49: 1–11.
- Deacon, H.J. 1979. “Excavations at Boomplaas Cave: a sequence through the Upper Pleistocene and Holocene in South Africa.” World Archaeology 10: 241–257.
- Deacon, H.J. (ed.). 1983. Late Quaternary Environment and Culture Relationships in the Southern Cape: The Langkloof-Willowmore Archaeological Project and the Archaeology of the Cango Valley. Stellenbosch: Human Sciences Research Council.
- Deacon, H.J. 1993. “Planting an idea: an archaeology of Stone Age gatherers in South Africa.” South African Archaeological Bulletin 48: 86–93.
- Deacon, H.J. 1995. “Two late Pleistocene-Holocene archaeological depositories from the southern Cape, South Africa.” South African Archaeological Bulletin 50: 121–131.
- Deacon, H.J., Deacon, J., Scholtz, A., Thackeray, J.F., Brink, J.S. and Vogel, J. 1984. “Correlation of palaeoenvironmental data from the Late Pleistocene and Holocene deposits at Boomplaas Cave, southern Cape.” In Late Cainozoic Palaeoclimates of the Southern Hemisphere, edited by J.C. Vogel, 339–351. Rotterdam: A.A. Balkema.
- Deacon, H.J. and Geleijnse, V.B. 1988. “The stratigraphy and sedimentology of the main site sequence, Klasies River, South Africa.” South African Archaeological Bulletin 43: 5–14.
- Deacon, H.J., Scholtz, A. and Daitz, L.D. 1983. “Fossil charcoals as a source of palaeoecological information in the Fynbos region.” In Fynbos Palaeoecology: A Preliminary Synthesis, edited by H.J. Deacon, Q.B. Hendey and J.J.N. Lamprechts, 174–182. Pretoria: Council for Scientific and Industrial Research.
- Deacon, J. and Lancaster, N. 1988. Late Quaternary Palaeoenvironments of Southern Africa. Oxford: Clarendon Press.
- De Vynck, J.C., Cowling, R.M., Potts, A.J. and Marean, C.W. 2016a. “Seasonal availability of edible underground and aboveground carbohydrate resources to human foragers on the Cape south coast, South Africa.” PeerJ 4: e1679.
- De Vynck, J.C., van Wyk, B.E. and Cowling, R.M. 2016b. “Indigenous edible plant use by contemporary Khoe-San descendants of South Africa’s Cape South Coast.” South African Journal of Botany 102: 60–69.
- Dunbar, R.I. 1976. “Australopithecine diet based on a baboon analogy.” Journal of Human Evolution 5: 161–167.
- Elton, S. and Dunn, J. 2020. “Baboon biogeography, divergence, and evolution: morphological and paleoecological perspectives.” Journal of Human Evolution 145: 102799.
- Faith, J.T. 2013. “Taphonomic and paleoecological change in the large mammal sequence from Boomplaas Cave, Western Cape, South Africa.” Journal of Human Evolution 65: 715–730.
- Faith, J.T., Chase, B.M. and Avery, D.M. 2019. “Late Quaternary micromammals and the precipitation history of the southern Cape, South Africa.” Quaternary Research 91: 848–860.
- Faith, J.T., Chase, B.M. and Pargeter, J. 2024. “The Last Glacial Maximum climate at Boomplaas Cave, South Africa.” Quaternary Science Reviews 329: 108557.
- Fu J., Zheng Y., Gao Y. and Xu W. 2022. “Dietary fiber intake and gut microbiota in human health.” Microorganisms 10: 2507.
- Grove, M. 2009. “Hunter–gatherer movement patterns: causes and constraints.” Journal of Anthropological Archaeology 28: 222–233.
- Jones, M. 2009. “Moving north: archaeobotanical evidence for plant diet in Middle and Upper Palaeolithic Europe.” In The Evolution of Hominin Diets: Integrating Approaches to the Study of Palaeolithic Subsistence, edited by J.J. Hublin and M.P. Richards, 171–180. New York: Springer.
- Klein, R.G. 1978. “A preliminary report on the larger mammals from the Boomplaas Stone Age cave site, Cango Valley, Oudtshoorn District, South Africa.” South African Archaeological Bulletin 33: 66–75.
- Kyalangalilwa, B., Boatwritht, J.S., Daru, B.H., Maurin. O. and van der Bank, M. 2013. “Phylogenetic position and revised classification of Acacia s.l. (Fabaceae: Mimosoideae) in Africa, including new combinations in Vachellia and Senegalia.” Botanical Journal of the Linnean Society 172: 500–523.
- Kyriacou, K., Blackhurst, D.M., Parkington, J.E. and Marais, A.D. 2016. “Marine and terrestrial foods as a source of brain-selective nutrients for early modern humans in the southwestern Cape, South Africa.” Journal of Human Evolution 97: 86–96.
- Larbey, C., Mentzer, S.M., Ligouis, B., Wurz, S. and Jones, M.K. 2019. “Cooked starchy food in hearths ca. 120 kya and 65 kya (MIS 5e and MIS 4) from Klasies River Cave, South Africa.” Journal of Human Evolution 131: 210–227.
- Layton, R., O’Hara, S. and Bilsborough, A. 2012. “Antiquity and social functions of multilevel social organization among human hunter-gatherers.” International Journal of Primatology 33: 1215–1245.
- Ledger, H.P. 1968. “Body composition as a basis for a comparative study of some East African mammals.” Symposium of the Zoological Society of London 21: 289–310.
- Lombard, M. 2022. “Sedge foodplants growing in the Cradle of Humankind, South Africa, and Cyperus esculentus tubers (Patrysuintjies) as a C4 superfood.” Open Quaternary 8: 1–21.
- Lombard, M. 2023. “The Diepkloof Rock Shelter foodplant fitness landscape, Western Cape, South Africa.” Azania: Archaeological Research in Africa 58: 214–234.
- Lombard, M. and Högberg, A. 2023. “The foodplant fitness landscape of Hollow Rock Shelter, Western Cape, South Africa.” Journal of Archaeological Science: Reports 49: 103997.
- Lombard, M. and van Aardt, A. 2023. “Method for generating foodplant fitness landscapes: with a foodplant checklist for southern Africa and its application to Klasies River Main Site.” Journal of Archaeological Science 149: 105707.
- Marlowe, F.W. 2005. “Hunter-gatherers and human evolution.” Evolutionary Anthropology 14: 54–67.
- Moffett, R.O. 2007. “Name changes in the Old World Rhus and recognition of Searsia (Anacardiaceae).” Bothalia 37: 165-175.
- Moffett, R.O. and Deacon, H.J. 1977. “The flora and vegetation in the surrounds of Boomplaas Cave: Cango Valley.” South African Archaeological Bulletin 32: 127–145.
- Mucina, L. and Rutherford, M.C. 2006. The Vegetation of South Africa, Lesotho And Swaziland. Pretoria: South African National Biodiversity Institute.
- Neuwinger, H.D. 1994. African Ethnobotany: Poisons and Drugs: Chemistry, Pharmacology, Toxicology. London: Chapman & Hall Press.
- Pargeter, J. and Faith, J.T. 2020. “Lithic miniaturization as adaptive strategy: a case study from Boomplaas Cave, South Africa.” Archaeological and Anthropological Sciences 12: 225.
- Pargeter, J., Loftus, E., Mackay, A., Mitchell, P.J. and Stewart, B. 2018. “New ages from Boomplaas Cave, South Africa, provide increased resolution on late/terminal Pleistocene human behavioural variability.” Azania: Archaeological Research in Africa 53: 156–184.
- Peters, C.R., O’Brien, E.M. and Drummond, R.B. 1992. Edible Wild Plants of Sub-Saharan Africa. London: Kew Publishing.
- Ruiters-Welcome, A.K. 2019. “Food plants of southern Africa.” PhD diss., University of Johannesburg.
- Schlebusch, C.M., Malmström, H., Günther, T., Sjödin, P., Coutinho, A., Edlund, H., Munters, A.R., Vicente, M., Steyn, M., Soodyall, H. and Lombard, M. 2017. “Southern African ancient genomes estimate modern human divergence to 350,000 to 260,000 years ago.” Science 358: 652–655.
- Schlebusch, C.M., Sjödin, P., Breton, G., Günther, T., Naidoo, T., Hollfelder, N., Sjöstrand, A.E., Xu, J., Gattepaille, L.M., Vicente, M. and Scofield, D.G. 2020. “Khoe-San genomes reveal unique variation and confirm the deepest population divergence in Homo sapiens.” Molecular Biology and Evolution 37: 2944–2954.
- Scholtz, A. 1986. “Palynological and palaeobotanical studies in the southern Cape.” MA diss., University of Stellenbosch.
- Schnorr, S.L., Crittenden, A.N., Venema, K., Marlowe, F.W. and Henry, A.G. 2015. “Assessing digestibility of Hadza tubers using a dynamic in-vitro model.” American Journal of Physical Anthropology 158: 371–385.
- Sealy, J.C., Lee-Thorp, J.A., Loftus, E., Faith, J.T. and Marean, C.W. 2016. “Late Quaternary environmental change in the Southern Cape, South Africa, from stable carbon and oxygen isotopes in faunal tooth enamel from Boomplaas Cave.” Journal of Quaternary Science 31: 919–927.
- Singels, E. 2021. “The role of geophytes in Stone Age hunter-gatherer subsistence and human evolution in the Greater Cape Floristic Region.” PhD diss., University of Cape Town.
- Singels, E., Potts, A.J., Cowling, R.M., Marean, C.W., De Vynck, J. and Esler, K.J. 2016. “Foraging potential of underground storage organ plants in the southern Cape, South Africa.” Journal of Human Evolution 101: 79–89.
- Skead, C.J. 2009. Historical Plant Incidence in Southern Africa: A Collection of Early Travel Records in Southern Africa. Pretoria: South African National Biodiversity Institute.
- Speth, J.D. 2010. The Paleoanthropology and Archaeology of Big-game Hunting, Interdisciplinary Contributions to Archaeology. New York: Springer.
- Talma, A. and Vogel, J.C. 1992. “Late Quaternary paleotemperatures derived from a speleothem from Cango caves, Cape Province, South Africa.” Quaternary Research 37: 203–213.
- Tanaka J. 1976. “Subsistence ecology of Central Kalahari San.” In Kalahari Hunter-Gatherers: Studies of the !Kung San and Their Neighbors, edited by R.B. Lee and I. DeVore, 98–119. Cambridge: Harvard University Press.
- van Wijk, Y.E., Tusenius, M.L., Rust, R., Cowling, R.M. and Wurz, S. 2017. “Modern vegetation at the Klasies River archaeological sites, Tsitsikamma coast, south-eastern Cape, South Africa: a reference collection.” Plant Ecology and Evolution 150: 13–34.
- van Wijk, Y.E., Rust, R., Uithaler, E.M. and Wurz, S. 2019. “Ethnobotanical research at Klasies River linking past, present, and future.” Ethnobotany Research and Applications 18: 1–24.
- van Wyk, B.E. and Gericke, N. 2000. People's Plants: A Guide to Useful Plants of Southern Africa. Pretoria: Briza Publications.
- van Wyk, B.E., Heerden, F.V. and Oudtshoorn, B.V. 2002. Poisonous Plants of South Africa. Pretoria: Briza Publications.
- Von Koenen, E. 1996. Medicinal, Poisonous and Edible Plants in Namibia (Volume 2). Göttingen: Klaus Hess Publishers.
- Watt, J.M. and Breyer-Brandwijk, M.G. 1962. The Medicinal and Poisonous Plants of Southern and Eastern Africa Being an Account of their Medicinal and Other Uses, Chemical Composition, Pharmacological Effects and Toxicology in Man and Animal. London: E. & S. Livingstone Ltd.
- Webley, L. 1978. “Analysis of sediment samples obtained from Boomplaas Cave.” BA (Hons) diss., University of Stellenbosch.
- Welcome, A.K. and van Wyk, B.E. 2019. “An inventory and analysis of the food plants of southern Africa.” South African Journal of Botany 122: 36–179.
- World Health Organisation and United Nations University. 2007. Protein and Amino Acid Requirements in Human Nutrition. Geneva: World Health Organization.
- Wink, M. and van Wyk, B.E. 2008. Mind-Altering and Poisonous Plants of The World. Portland: Timber Press.
- Yellen. J.E. 1976. “Settlement patterns of the !Kung: an archaeological perspective.” In Kalahari Hunter-Gatherers, edited by R.B. Lee and I. DeVore, 47–76. Cambridge: Harvard University Press.
