ABSTRACT
The advantage for camouflage of variation in colour forms (binary, multi, or continuous) is a topic of increasing interest, but there are few long-term studies. We found that the ratios of colour forms of 337 polymorphic Manuka moths (Declana floccosa), recorded at one location, did not change over 42 years. This unexpected stability, in the face of probable bird predation against commoner forms and genetic drift, might result from the moth’s continuous variation that prevents predators forming a specific search image, or an unchanged food supply for the larvae. The flight period change from summer to winter, apparently the first recorded, was possibly driven by wasp (Vespula vulgaris) predation after their arrival about 1980.
Introduction
How colour polymorphism in cryptic prey species evolved, and the selection pressures exerted on both predators and prey to maintain it, have been the subject of much research. Even though it may affect 40% of some moth taxa, confirming that it is a result of frequency-dependent selection has proved difficult (Bond Citation2007, Franks & Oxford Citation2009). There is also a linguistic difficulty that ‘polymorphic’ by definition means having ‘two or more, genetically determined, distinct forms within a population’ (Owen Citation1980, p.148). New Zealand insects seem to differ from much of the world in having many species with continuous variation, rather than, or within, distinct forms. An example is the New Zealand grasshopper (Phaulacridium marginale Walker, 1870) with two basic colours, brown and green; and patterns, striped and unstriped (Early Citation2009); but, on one small piece of bark, all 23 brown striped individuals had different colours and patterns (Flux Citation2019).
The problem of understanding continuous variation (‘discrete or indiscrete’, Davison et al. (Citation2019); ‘massive polymorphism’, Whiteley et al. (Citation1997); or ‘exuberant visible polymorphisms’, Franks and Oxford (Citation2009)) is compounded by predators belonging to different orders seeing ‘colours’ of different wavelengths, if they see colour at all; this is well illustrated by predation on the snail Cepaea nemoralis by rodents at night and birds by day (Rosin et al. Citation2011). The current theory that an underlying supergene acts to stabilise colour forms in C. nemoralis may require revision (Davison et al. Citation2019); but, in cichlid fishes, cross-breeding experiments have demonstrated a genetic basis for continuous variation (Albertson et al. Citation2014). Several strategies for avoiding predators may co-exist; e.g. the bivalve Donacilla cornea uses masquerade (mimicry) and crypsis (camouflage) against some backgrounds, or the selective advantage of being different from each other (continuous variation) in other places (Whiteley et al. Citation1997). Fortunately, in our study it was possible to bypass these difficulties by using pattern, rather than colour, to define separate forms.
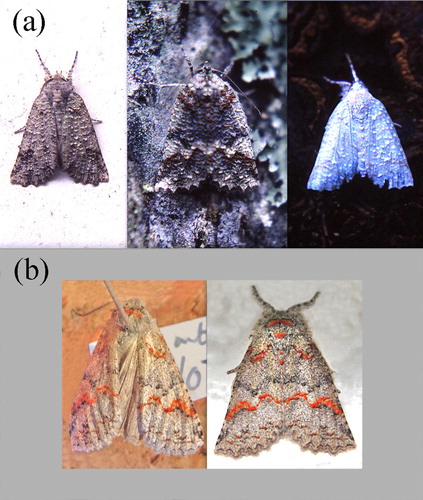
Of the many species of polymorphic moths in New Zealand, the Manuka moth (Declana floccosa) is probably the most variable. Hudson (Citation1888) describes seven typical forms, but adds that all forms intergrade in colour and markings, and all may have tufts of orange-yellow scales. Some of the more striking examples are illustrated in .
These nocturnal moths are attracted to light and land on house walls, where they attach themselves head up, flat against the surface to avoid shadows (Owen Citation1980, p.46). If disturbed they drop, or shuffle on landing and flatten again, often remaining there all the following day, despite being at risk of attack by house sparrows (Passer domesticus), chaffinches (Fringilla coelebs) and fantails (Rhipidura fuliginosa). There is no evidence that D. floccosa selects appropriate colour backgrounds, as some moths do (Sargent Citation1968; Owen Citation1980; Grant & Howlet Citation1988; Kang et al. Citation2015); indeed one white D. floccosa was found spending the day on a black tree-fern stump (a).
Figure 2. (a) Above, day sites chosen by free-living moths include a white wall and black log. (b) Below, the same moth alive (right), and faded after 12 years in the collection.
Clearly, a distinction must exist between moths which are camouflaged to match a specific background and search for it (Grant & Howlet Citation1988; Kang et al. Citation2015), and D. floccosa, which has no known ‘normal’ matching background to select. Also, human alterations to the environment have positive and negative effects on both the animal’s background and food supply, and those of its predators (Rosin et al. Citation2017). These changes are as marked in New Zealand as in Europe; but have taken place far more recently, giving moths less time to adapt to new backgrounds and new predators.
There are few long-term studies of changes in the ratios of polymorphic forms over time such as those of Ford (Citation1967, Citation1981), Kettlewell (Citation1973), and Cook et al. (Citation2012); so the opportunity was taken to collect every specimen found from 1974 to 2016 at one suburban house in Belmont, Lower Hutt, Wellington (41 11 S, 174 55 E).
Because D. floccosa adopts a camouflage strategy apparently designed to prevent bird predators developing a specific search image (Tinbergen Citation1960), our hypothesis was that there would be no stabilising selection to maintain the existing ratios of colour forms. Either disruptive selection against common forms (Bond & Kamil Citation2002; Bond Citation2007), or random genetic drift, should result in the proportions of colour forms changing over time. There was also an opportunity to study population changes, sex ratios, and emergence times.
Methods
The study area was a 0.5 ha garden, with favoured trees for D. floccosa larvae (J. Dugdale, personal comm. 2018): Lawson cypress (Chamaecyparis lawsoniana) and Monterey cypress (Cupressus macrocarpa), planted in 1953 as garden hedges 50 and 30 m in length before the study began, but increasing greatly in size over 42 years. The lawsonia hedge was gradually felled for firewood from 2000, but one large tree remained in 2016. An 11 ha forest block of mixed Pinus radiata and Cupressus macrocarpa, planted about 1940, containing an understory of native vegetation regrowth, adjoined the garden on one side; and there was open grassland for sheep, cattle, and horses on the other three.
A moth light-trap was not used because, if run nightly for many years, it might have caused significant changes in the local moth population. There was no street lighting and the nearest visible house was 150 m away. In all, 337 D. floccosa were collected. Some were caught as they landed on the windows at night; most were picked off the white painted weatherboard walls in the morning, while looking for animals killed by cats (Flux Citation2007, Citation2017); and a few found on vegetation in the garden. They were caught directly into plastic vials to avoid loss of scales, and placed in a chest freezer at –18 degrees Celsius. A day later they were pinned in a fairly natural position, and transferred to insect-proof wooden storage boxes when set. Although these boxes were completely dark, some colours faded (b); but the patterns remained distinct.
In 2016, each moth was photographed at a constant distance from the same cork tile background in tungsten lighting, with a white label alongside to allow colours to be standardised against white in Adobe Photoshop (a few required correction for slight overexposure of dark moths or underexposure of pale ones). They were then printed commercially at twice life size, and the 70 × 90 mm prints could be handled like a pack of cards to compare different arrangements by colour form, sex, and date of capture.
Based on Hudson’s (Citation1888, Citation1928) written descriptions and colour illustrations, one typical moth of each of his seven forms was chosen (), and the rest aligned alongside their closest model. They retained their allotted form number throughout subsequent analyses. The forms described by Hudson are mainly based on pattern, because the colours are more variable and tend to follow the underlying pattern. He describes them as: 1 Greyish white with many darker streaks; 2 ‘Several’ round spots on a paler background (he illustrates four spots, and this is the normal form); 3 Numerous black spots; 4 Two stripes crossing the wing, one near the thorax, one two-thirds out; 5 Like form 4, with stripes joined with two lines parallel to the hind margin; 6 Curved lines and small black spots; 7 A broad band crossing the middle of the wing.
Figure 3. Typical models representing the seven Declana floccosa forms described by Hudson (Citation1888), but not in the same numerical sequence.

We used Chi-square tests to assess differences between summer versus winter emergence, and morph frequencies between seasons and years, by decades. All statistical probabilities quoted are calculated from Fisher’s exact two-tail Chi square tests based on the original data.
Results
Population change
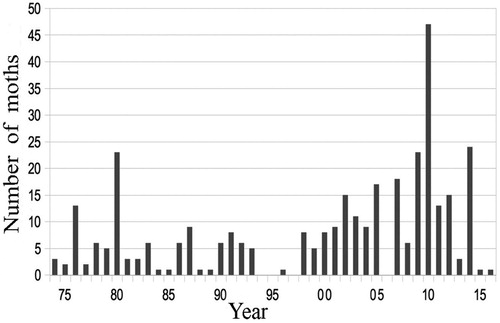
The number of D. floccosa caught each year () was very variable, but showed a significant decline after 1980–85. Only one moth was caught during 1994–97. Thereafter numbers increased somewhat erratically to a peak of 47 in 2010, becoming the commonest moth. Other moths, like Wiseana spp., Pseudocoremia leucelaea, Orocrambus flexuosellus, and Phrissogonus laticostatus, which had been far more common, outnumbering D. floccosa 100:1, did not recover. Unfortunately, these moths were too numerous to count, but one evening in 1966 there were 73 (mainly Wiseana spp.) on the kitchen window at once, and 10 a day were fed to a pet magpie (Gymnorhina tibicen). After 2000, the total for other moths over the whole summer would not have exceeded 50.
Flight period
The sample sizes are fairly similar: 1974–99, 111; 2000–2009, 113; 2010–2016, 101; and for comparison gives these samples as percentages. Based on the original data, the decline in summer from 1974–99 to 2000–2009 is significant (Chi square = 4.399, df = 1, P = 0.023) and the increase in winter more so (Chi square = 9.826, df = 1, P = 0.0031): combining these, the cross-over from summer to winter emergence is very significant (Chi square = 28.889, df = 2, P = 0.0001).
Figure 5. Percentage of Declana floccosa caught each season, showing change from summer to winter emergence between 1974–99 and 2000–09. n = 111, 113, 101 per time period.
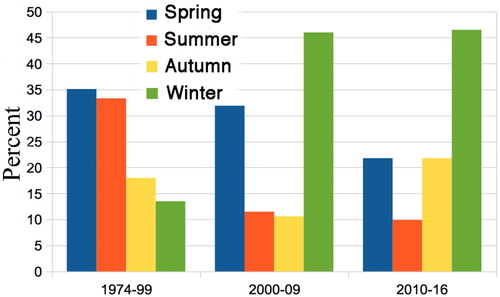
There is no significant difference in seasonality between 2000–2009 and 2010–2016.
Sex ratio
The sex ratio of the moths caught appeared to change with time. By decades, the ratios were: 1970s, 19 male:10 female (66% male); 1980s, 30:21 (59% male); 1990s, 21:15 (58% male); 2000s, 55:55 (50% male); and 2010s, 53:47 (53% male); but this trend was not statistically significant (Chi-square = 2.06, df = 4, P = 0.1515).
Seasonally, the sex ratio varied: spring (August-October) 46 male:49 female; summer 40:18; autumn 30:26; winter 56:54. The preponderance of males in summer is significant (Chi square = 4.328, df = 1, P = 0.0375).
Colour forms
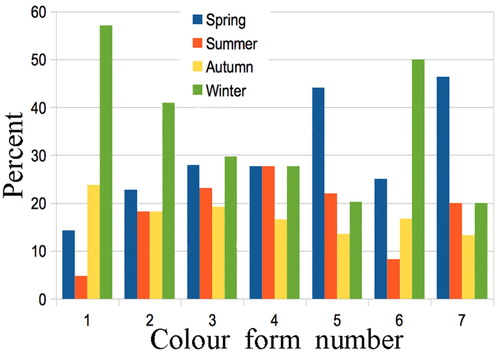
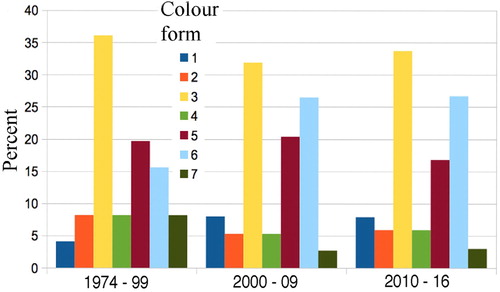
With captures of only 31, 54, and 37 in the three decades 1970s, 1980s, 1990s, breakdown into seven colour forms was impractical, so they were merged to compare the1974–1999 total against the 2000–2009 and 2010–2016 samples, each then having over 100 moths. The percentage distribution of the seven colour forms () showed no significant differences with time; indeed they look remarkably similar. Even the largest difference, between forms 5 and 6 in 1974–1999 and 2010–2016, is not significant (Chi square = 0.845, df 1, P = 0.485).
Figure 6. Percentage of Declana floccosa colour forms in each time period do not vary significantly, even for forms 5 and 6 between 1974–99 and 2010–16 (see text). n = 111, 113, 101 per time period.
Colour forms showed differences in season of emergence, with forms one, two, and six being twice as common in winter compared with other seasons. Because of small sample sizes (21, 22, 72 respectively) only the winter emergence of form 6 is significantly higher than in summer (Chi square = 10.698, df = 1, P = 0.001) and autumn (Chi square = 5.378, df = 1, P = 0.020). Forms five and seven were twice as common in spring, but the differences were not significant at P = 0.05; and forms three and four were evenly distributed seasonally (). The sex ratio within six colour forms did not differ significantly from 50:50; but colour form 3 was highly skewed toward males, 80:30 (Chi square = 11.043, df = 1, P = 0.0008) and this held over the three time periods: 32:9, 24:11, 24:10.
Discussion
The most surprising result was the highly significant change in the seasonal emergence of adult moths from summer to winter. According to Hudson (Citation1928) D. floccosa flies from September until the end of April, probably with several broods in a season; and because some were found in mid winter he suggested they hibernated. From the mint condition and pale greenish veins of some winter specimens, we suspect many may be emerging from pupae. Other authors give flight periods of August, November, and from March to June for Christchurch (Meyrick Citation1883); and ‘throughout the year’ (Gaskin Citation1966). In an earlier paper (Flux & Meads Citation2003), we found a marked difference in flight period between Belmont (continuous throughout the year, with peak in spring, (September-October)) and the Orongorongo Valley, 19 km east of Belmont (major peak in summer, (January-February), minor peak in October). No convincing explanation has been put forward for this difference.
The additional information presented here, from twice the number of moths (337 v. 160), shows that the Belmont population was in the process of changing from a summer emergence to a winter one. John Dugdale (personal comm. 2018) suggested that the shift from summer to winter emergence might be a response to escape wasp predation, and the increased population of D. floccosa could be due to a release from competition or a better food supply for the larvae. Vespula vulgaris arrived in Wellington around 1980 (Donovan Citation1984), increasing rapidly in the southern North Island from 1987–1991 (Clapperton et al. Citation1994). It became a significant pest of our 35 fruit trees, with 1–3 colonies killed annually from 2010. The timing agrees with the decline in moth abundance (), but is, of course, only correlation. Vespula germanica was present earlier, but takes fewer lepidoptera larvae (Harris & Oliver Citation1993).
Wasps can certainly find adult Declana by day (), and seem unlikely to be doing this only by sight. If female Declana attract males by releasing pheromones, and these are picked up by wasps as Hendrichs et al. (Citation1994) found for fruit fly pheromones, it could explain their scarcity compared to males in summer; but why colour form 3 was differentially selected remains a mystery.
Figure 8. Live female Declana feredayi attacked by common wasp, Mole Tops, Nelson Lakes, 0920 h, 21.1.08. Note the copious, loose, elongated scales which make the moth hard to grip. The moth was dismembered in 1 min 40 s, and the wasp flew off with the thorax. This is typical of the way wasps tackle bees (Free Citation1970).

The relative importance of predation at different stages of a moth’s life history is extremely difficult to measure. The proportions of eggs, larvae, and adults eaten by even a single top predator species like V. vulgaris will vary with weather, and between years; other predators may specialise on different stages, and in turn be subject to wasp predation. In general, predation against adults, the least abundant stage, will be critical but depend on the levels of other available prey. As Toft and Beggs (Citation1995) suggest for crane flies, species with emergence times at the peak of the wasp season will be most at risk.
Better survival of D. floccosa, compared with other moths, may be a result of its apparently normal extended emergence period, which gives late season emerging moths a selective advantage against wasp predation. Caterpillars at the peak of the wasp season ‘have virtually no chance … of surviving to adults’ (Beggs & Rees Citation1999). Apart from the pine processionary (Thaumetopoea pityocampa) in Portugal, where a genetic mutant in 1997 apparently started a summer breeding race which can no longer interbreed with the normal winter population (Santos et al. Citation2013), we are unaware of any other moth that has changed emergence times. However, the great reduction in moth numbers since the 1960s may have concealed this effect. Overseas there is widespread concern at the decline of moths in North America, Europe, and the UK (see review by Fox Citation2013) in parallel with the universal decline in all insects (Dirzo et al. Citation2014).
Butterfly-Conservation (Citation2013) reports that the Light Emerald moth (Campaea margaritata) has substantially extended its summer flight period since the 1970s, with a prominent second generation now also in autumn, which they attribute to climate change; this double-brooding is also being found in European birds (Both et al. Citation2019). Temperatures in New Zealand as yet show little change: here, starlings (Sturnus vulgaris) have continued to nest at the same time for the past 40 years (Flux & Flux Citation2015), while those in Europe, over the same period, now nest nine days earlier (Thellesen Citation2017). The high quantities of the insecticide DDT, used from the 1950s until banned in 1968, do not correlate with the decline in moths, although DDT levels remain 16 times higher than in most other countries (Eems et al. Citation2013).
The polymorphism shown by D. floccosa is more extreme than in many insects in that no two individuals are alike. Hence the usual hypothesis that it is maintained as a result of visual predators selecting the most abundant form (Bond Citation2007) seems weak. Possibly with multiple colour forms, rather than a few, visual predators may not be able to form any search image, giving a selective advantage from being different (Whiteley et al. Citation1997). And predators may avoid the prey even when found because of neophobia, and the risk of it being unpalatable (Barnett et al. Citation2014). As Franks and Oxford (Citation2009) point out, ‘dietary wariness is a critical factor missing from earlier models’ and might explain the evolution of continuous variation. This could be combined with protective colouration as a form of distance-dependent defensive colouration (Barnett & Cuthill Citation2014).
Our second hypothesis, that the proportions of colour forms would be subject to random genetic drift, was not supported. Hudson’s (Citation1928) work offers a plausible explanation. He observed that the larvae are different colours, matching different food plants; and from his feeding trials this seemed to be a genetic character. If these pigments, or pigment precursors, are retained by the adults, as they are in many insects (Shamim et al. Citation2014), the ratios of the colour forms could reflect the vegetation in and around the garden, which did not change over the 42 years of the study.
Acknowledgements
We are very grateful to family members, and our gardener, Kevin Priest, for alerting us to moths they found. Dr John Dugdale gave us a list of larval food plants, and suggested that wasps may have caused the shift in flight season. Dr Greg Holwell and two reviewers provided much helpful advice on earlier drafts.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Albertson RC, Powder KE, Hu Y, Coyle KP, Roberts RB, Parsons KJ. 2014. Genetic basis of continuous variation in the levels and modular inheritance of pigmentation in cichlid fishes. Molecular Ecology 23: 5135–5150. doi: 10.1111/mec.12900
- Barnett CA, Bateson M, Rowe C. 2014. Better the devil you know: avian predators find variation in prey toxicity aversive. Biology Letters 10(11). doi: 10.1098/rsbl.2014.0533
- Barnett JB, Cuthill IC. 2014. Distance-dependent defensive coloration. Current Biology 24, 24, R1157–R1158. doi:10.1016j.cub.2014.11.015 doi: 10.1016/j.cub.2014.11.015
- Beggs JR, Rees JS. 1999. Restructuring of Lepidoptera communities by introduced Vespula wasps in a New Zealand beech forest. Oecologia 119: 565–571. doi: 10.1007/s004420050820
- Bond AB. 2007. The evolution of color polymorphism: crypticity, searching images, and apostatic selection. Annual Review of Ecology, Evolution, and Systematics 38: 489–514. doi: 10.1146/annurev.ecolsys.38.091206.095728
- Bond AB, Kamil AC. 2002. Visual predators select for crypticity and polymorphism in virtual prey. Nature 415: 609–613. doi: 10.1038/415609a
- Both C, Ubels R, Ravussin P-A. 2019. Life-history innovation to climate change: can single-brooded migrant birds become multiple breeders? Journal of Avian Biology 2019: e01951. doi: 10.1111/jav.01951
- Butterfly-Conservation. 2013. The state of Britain’s larger moths. http://www.butterfly-conservation.org/files/state-of-britains-larger-moths PDF file.
- Clapperton BK, Tilley JAV, Beggs JR, Moller H. 1994. Changes in the distribution and proportions of Vespula vulgaris (L.) and Vespula germanica (Fab.) (Hymenoptera: Vespidae) between 1987 and 1990 in New Zealand. New Zealand Journal of Zoology 21: 295–303. doi: 10.1080/03014223.1994.9517998
- Cook LM, Grant BS, Saccheri IJ, Mallet J. 2012. Selective bird predation on the peppered moth: the last experiment of Michael Majerus. Biology Letters 8: 609–612. doi: 10.1098/rsbl.2011.1136
- Davison A, Jackson HJ, Murphy EW, Reader T. 2019. Discrete or indiscrete? Redefining the colour polymorphism of the land snail Cepaea nemoralis. Heredity, 123, 162–175. doi: 10.1038/s41437-019-0189-z
- Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJB, Collen B. 2014. Defaunation in the anthropocene. Science 345: 401–406. doi: 10.1126/science.1251817
- Donovan BJ. 1984. Occurrence of the common wasp, Vespula vulgaris (L.) (Hymenoptera: Vespidae) in New Zealand. New Zealand Journal of Zoology 11: 417–427. doi: 10.1080/03014223.1984.10428256
- Early J. 2009. Know your New Zealand … Native Insects & Spiders. New Holland Publishers, Auckland. 176 p.
- Eems M, Jaspers VLB, Van den Steen E, Bateson M, Carere C, Clergeau P, et al. 2013. Can starling eggs be useful as a biomonitoring tool to study organohalogenated contaminants on a worldwide scale? Environment International 51: 141–149. doi: 10.1016/j.envint.2012.11.003
- Flux JEC. 2007. Seventeen years of predation by one suburban cat in New Zealand. New Zealand Journal of Zoology 34: 289–296. doi: 10.1080/03014220709510087
- Flux JEC. 2017. Comparison of predation by two suburban cats in New Zealand. European Journal of Ecology 3: 85–90. doi: 10.1515/eje-2017-0009
- Flux JEC. 2019. Illustrate Ecology: Also available in pink. New Zealand Ecological Society Newsletter No. 167: 2.
- Flux JEC, Flux MM. 2015. The fertility clinic: a bird's-eye view of our future. New Zealand Journal of Zoology 42: 284–289. doi: 10.1080/03014223.2015.1099549
- Flux JEC, Meads MJ. 2003. Variation in the flight period of Declana floccosa. The Weta 26: 17–19.
- Ford EB. 1967. Moths. The New Naturalist. Collins, London. 266 p.
- Ford EB. 1981. Taking Genetics into the Countryside. Weidenfeld & Nicholson, London, 150 p.
- Fox R. 2013. The decline of moths in Great Britain: a review of possible causes. Insect Conservation and Diversity 6: 5–19. doi: 10.1111/j.1752-4598.2012.00186.x
- Franks DW, Oxford GS. 2009. The evolution of exuberant visible polymorphisms. Evolution 63: 2697–2706. doi: 10.1111/j.1558-5646.2009.00748.x
- Free JB. 1970. The behaviour of wasps (Vespula germanica L. and V. vulgaris L.) when foraging. Insectes Sociaux 17: 11–19. doi: 10.1007/BF02223769
- Gaskin DE. 1966. The Butterflies and Common Moths of New Zealand. Whitcombe and Tombs, Wellington, 219 p.
- Grant B, Howlet RJ. 1988. Background selection by the peppered moth (Biston betularia Linn.): individual differences. Biological Journal of the Linnean Society 33: 217–232. doi: 10.1111/j.1095-8312.1988.tb00809.x
- Harris RJ, Oliver EH. 1993. Prey diets and population densities of the wasps Vespuls vulgaris and V. germanica in scrubland-pasture. New Zealand Journal of Ecology 17: 5–12.
- Hendrichs J, Katsoyannos BI, Wornoayporn V, Hendrichs MA. 1994. Odour-mediated foraging by Yellowjacket wasps (Hymenoptera: Vespidae): predation on leks of pheromone-calling Mediterranean fruit fly males (Diptera: Tephritidae). Oecologia 99: 88–94. doi: 10.1007/BF00317087
- Hudson GV. 1888. On the varieties of a common moth (Declana floccosa). Transactions and Proceedings of the Royal Society of New Zealand 21: 190–193.
- Hudson GV. 1928. Butterflies and Moths of New Zealand. Ferguson & Osborn, Wellington. 386 p.
- Kang C, Stevens M, Moon J, Lee S, Jablonski PG. 2015. Camouflage through behavior in moths: the role of background matching and disruptive coloration. Behavioral Ecology 26: 45–54. doi: 10.1093/beheco/aru150
- Kettlewell B. 1973. The Evolution of Melanism: The Study of a Recurring Necessity; With Special Reference to Industrial Melanism in the Lepidoptera. Clarendon Press, Oxford. 423 p.
- Meyrick E. 1883. A monograph of the New Zealand Geometrina. Transactions of the New Zealand Institute 16: 49–113.
- Owen D. 1980. Camouflage and Mimicry. Oxford University Press, Oxford. 158 p.
- Rosin ZM, Olborska P, Surmacki A, Tryjanowski P. 2011. Differences in predatory pressure on terrestrial snails by birds and mammals. Journal of Bioscience 36: 691–699. doi: 10.1007/s12038-011-9077-2
- Rosin ZM, Lesicki A, Kwiecinski Z, Skorka P, Tryjanowski P. 2017. Land snails benefit from human alterations in rural landscapes and habitats. Ecosphere 8: e01874. doi: 10.1002/ecs2.1874
- Santos HM, Paiva M-R, Rocha S, Kerdelhue C, Branco M. 2013. Phenotypic divergence in reproductive traits of a moth population experiencing a phenological shift. Ecology and Evolution 3: 5098–5108. doi: 10.1002/ece3.865
- Sargent, TD. 1968. Cryptic moths: effects on background selections of painting the circumocular scales. Science 159: 100–101. doi: 10.1126/science.159.3810.100
- Shamim G, Ranjan SK, Pandey DM, Ramani R. 2014. Biochemistry and biosynthesis of insect pigments. European Journal of Entomology 111: 149–164. doi: 10.14411/eje.2014.021
- Thellesen PV. 2017. Common starling Sturnus vulgaris clutch size, brood size and timing of breeding during 1971-2015 in Southwest Jutland, Denmark. Danish Ornithological Society Journal 111: 87–95.
- Tinbergen L. 1960. The natural control of insects on pinewoods 1. Factors influencing the intensity of predation by songbirds. Archives Neerlandaises de Zoologie 13: 265–343. doi: 10.1163/036551660X00053
- Toft RJ, Beggs JR. 1995. Seasonality of crane flies (Diptera: Tipulidae) in South Island beech forest in relation to the abundance of Vespula wasps (Hymenoptera: Vespidae). New Zealand Entomologist 18: 37–43. doi: 10.1080/00779962.1995.9722000
- Whiteley DAA, Owen DF, Smith DAS. 1997. Massive polymorphism and natural selection in Donacilla cornea (Poli, 1791) (Bivalvia: Mesodesmatidae). Biological Journal of the Linnean Society 62: 475–494.