Abstract
Objective. To further characterize time-to-first pain relief, effect size, correlations between various outcome measures and durability of relief for single-tablet naproxen 500 mg/esomeprazole 20 mg (NAP/ESO) given twice daily and celecoxib (CEL) (200 mg) given once daily versus placebo in knee osteoarthritis (OA). Methods. Unpublished data from two double-blind, double-dummy, placebo-controlled trials in which patients aged ≥50 years with knee OA were randomized to NAP/ESO (n = 487), CEL (n = 486) or placebo (n = 246) were pooled (NCT00664560 and NCT00665431). Acute response endpoints: 1) Time to first significant pain response, 2) Western Ontario and McMaster Osteoarthritis Index (WOMAC) pain subscale and 3) American Pain Society Patient Outcome Questionnaire (APS-POQ) scores. Sustainability endpoints: 1) Routine Assessment of Patient Index Data (RAPID3) and 2) WOMAC Stiffness, Pain and Total scores; and Patient Global Assessment (PGA) at 6 and 12 weeks. Effect sizes for all measures were calculated. Rescue pain medication use also was analyzed, as was the correlation of WOMAC to RAPID3. Results. NAP/ESO produced statistically significant decreases in WOMAC Pain on Days 2–7 and at Weeks 6 and 12 (all p < 0.05); most APS-POQ pain assessments with NAP/ESO were significantly improved on Days 2–7 compared with placebo (all p < 0.05). A good or excellent response occurred in a median of 6 days. RAPID3 and WOMAC total/stiffness/function/PGA scores decreased significantly at Weeks 6 and 12 (all p < 0.05). Placebo-adjusted WOMAC pain effect sizes were 0.44, 0.34 and 0.25 at Day 7, week 6 and week 12, respectively. RAPID3 to WOMAC total and WOMAC pain to RAPID3: Pain scores were highly correlated at 6 and 12 weeks (correlation coefficients >0.80). No significant differences in overall responses were found between CEL and NAP/ESO. Conclusion. Naproxen/esomeprazole produced a significant absolute moderate early pain response, which was maintained for 12 weeks. RAPID3 was found to be highly correlated with the typical OA measure (WOMAC) and might be a useful clinical tool for measuring NSAID response. NCT00664560: https://clinicaltrials.gov/ct2/show/NCT00664560, NCT00665431: https://www.clinicaltrials.gov/ct2/show/NCT00665431.
Introduction
Osteoarthritis (OA), a progressive disease characterized by joint pain accompanied by functional limitations and reduced quality of life, is estimated to affect approximately 250 million people worldwide [Citation1-3]. More than 40% of patients with OA report having substantial knee symptoms every day [Citation4], and 54% of them have inadequate pain relief [Citation5] NSAIDs are an effective and well-established treatment for managing the signs and symptoms of OA [Citation6,7], and naproxen is one of the most commonly prescribed therapeutic agents for knee OA worldwide [Citation8]. However, non-selective NSAIDs such as naproxen may induce upper gastrointestinal ulceration [Citation9-11]. To reduce the risk of serious gastrointestinal complications, physicians may prescribe gastroprotective therapies, such as esomeprazole, with non-selective NSAIDs or prescribe COX-2 selective NSAIDs, such as celecoxib (CEL) [Citation12].
Although there is a lack of consensus among evidence-based pharmacologic treatment guidelines for the management of knee OA, naproxen is recommended either as a first- or second-line pharmacologic agent [Citation13-20]. Additionally, some guidelines recommend naproxen for advanced pharmacological management of pain in patients at an increased cardiovascular risk [Citation15], because observational studies suggest that there is an increased risk of cardiovascular events in patients taking NSAIDs [Citation21]. A recently published meta-analysis suggests that naproxen might have a favorable cardiovascular safety profile compared with other NSAIDs [Citation22]. Because OA continues to progress with increasing age [Citation4] and cardiovascular risk increases with age [Citation23], naproxen may be an appropriate option for a large number of patients with OA. Previously published data have demonstrated the comparable efficacy of naproxen 500 mg taken twice daily (BID) and CEL 200 mg/day and the superiority of both to placebo (PBO) in the management of knee OA [Citation24,25].
In some practice guidelines, acetaminophen is recommended as a first-line analgesic with oral NSAIDs as second-line therapy, owing to the potentially serious gastrointestinal adverse events associated with NSAIDs [Citation15,26,27]. However, a prospective, non-interventional, epidemiologic study in patients with knee or hip OA evaluated therapeutic effectiveness of pain management medication for OA as measured by patient satisfaction and the proportion of patients who switched to an alternate therapy during the 3-month observational period [Citation28]. Of the 5976 patients evaluated, 75% were treated with acetaminophen at baseline and 60% were treated with an NSAID. At the end of follow-up, the number of patients treated with acetaminophen decreased to 24% (p < 0.001) and the number of patients treated with an NSAID increased to 86% (p < 0.001). The limited efficacy of acetaminophen is supported by a network meta-analysis published in 2015, which determined that acetaminophen was the least efficacious treatment for knee OA pain as compared with commonly used NSAIDs and intra-articular injections [Citation29].
Despite the recommendation of concomitant administration of gastroprotective agents with NSAIDs to prevent NSAID-associated ulcer development, the combined use of these agents remains suboptimal owing to low physician co-prescription and poor patient adherence [Citation30-32]. A single-tablet enteric-coated combination of naproxen 500 mg/immediate release esomeprazole 20 mg (hereafter referred to as NAP/ESO) is a potential solution for appropriate patients. NAP/ESO significantly reduced the incidence of endoscopic ulcers compared with PBO and improved upper gastrointestinal tolerability compared with enteric-coated naproxen alone in previous trials [Citation24,33,34]. No new or emerging safety issues, including the predefined cardiovascular endpoints, were observed in long-term NAP/ESO trials [Citation35]. The objective of the present analysis was to further characterize time-to-first pain relief, effect size, correlations between various outcome measures, and durability of pain relief for oral NAP/EPO (500 mg/20 mg BID) versus matching PBO (BID), with oral CEL (200 mg once daily) as an active control.
Methods
Study design and patient sample
Two identical, 12-week, randomized, double-blind, parallel-group, PBO-controlled, multicenter Phase 3 trials (NCT00664560 and NCT00665431) were conducted across 157 centers in the US from April to December 2008 [Citation24,33]. The original primary objective was to demonstrate, in the two independent trials, that a fixed-dose combination of enteric-coated NAP/ESO BID was non-inferior to CEL 200 mg once daily for the treatment of the signs and symptoms of OA of the knee. The changes from baseline for both active treatments were compared to outcomes of PBO treatment. In the current analysis, data from the two studies were pooled to compare the efficacy of 12-week treatment with NAP/ESO, CEL and PBO for the treatment of patients with OA of the knee with a wide array of acute and chronic OA outcome measures.
Patient inclusion and exclusion criteria have been described previously [Citation24,33]. In brief, eligible patients were aged ≥50 years with a 6-month history of OA of the knee (ACR functional class rating I, II or III) and had been receiving a stable dose of NSAIDs, COX-2-selective inhibitors or other oral analgesic therapy for ≥6 weeks. Oral analgesic therapy was withdrawn at screening. After a 7- to 14-day washout period, eligible patients returned for a baseline visit, once they had experienced a flare of OA pain. A flare was defined as a Western Ontario and McMaster Osteoarthritis Index (WOMAC) pain score ≥40 mm at baseline, mean change in WOMAC pain score from screening to baseline ≥15 mm, and worsening of Patient Global Assessment (PGA) by ≥1 point, as assessed within 7–14 days of washout. Patients who met the criteria were then scheduled for the baseline visit. Patients who did not meet the criteria within this timeline were not enrolled in the trial. Gastroprotective agents or other NSAIDs (ie, other than the study medications) were not allowed at any time during the study. Allowed concomitant analgesics included low-dose aspirin (LDA; ≤325 mg/day) as well as rescue acetaminophen. Concomitant use of oral prednisone (≤7.5 mg/day) and antiplatelet agents (non-concomitant with LDA) was allowed during the study. Supplemental use of antacid (6 tablets per day) and rescue use of acetaminophen (≤3 g/day), except within 48 h before efficacy assessments, also were permitted.
Both studies were reviewed and approved by an independent ethics committee (Copernicus Group Institutional Review Board), and all patients gave written, informed consent in accordance with the 1996 Declaration of Helsinki [Citation24,33].
Endpoints
The co-primary efficacy outcomes of the two studies were mean change from baseline in WOMAC pain and function subscores, and PGA ofOA using a visual analog scale (PGA-VAS; 0–100 mm) [Citation24]. Data from both studies were pooled to assess the primary efficacy outcomes, acute onset of pain relief, and analgesic sustainability.
Acute response endpoints were measured during the first 7 days of treatment using the WOMAC 100-mm VAS score, the American Pain Society Patient Outcome Questionnaire (APS-POQ), and the PGA of OA. The WOMAC is a validated, self-administered, patient-reported health status scale that assesses pain, stiffness and physical function in patients with hip and/or knee OA [Citation36-38]. The WOMAC consists of 24 items divided into three subscales: pain (5 items: during walking, using stairs, in bed, sitting or lying and standing), stiffness (2 items: after first waking and later in the day) and physical function (17 items: descending stairs, ascending stairs, rising from sitting, standing, bending to floor, walking on flat surface, getting in/out of a car, shopping, putting on socks, rising from bed, taking off socks, lying in bed, getting in/out of bath, sitting, getting on/off toilet, heavy household duties and light household duties). The APS-POQ consists of a series of questions that measure the intensity and duration of pain and interference with daily activities [Citation39]. In the PGA of OA, patients were asked “Considering all the ways your arthritis affects you, how well are you doing?” and responses were scored on 100-mm VAS anchored by Very Poor (far left) and Excellent (far right) [Citation36].
Analgesic sustainability was measured at weeks 6 and 12 with the WOMAC index, the Multidimensional Health Assessment Questionnaire (MDHAQ9, version R780-NP, physical function, pain and PGA) [Citation40], the Outcome Measures in Rheumatoid Arthritis Clinical Trials-Osteoarthritis Research Society International (OMERACT-OARSI) Responder Index [Citation41], and the Routine Assessment of Patient Index Data 3 (RAPID3) [Citation42]. The MDHAQ is a survey in which patients self-report their current health status. RAPID3 is a subset of this assessment which captures three areas: functional ability (10 items: difficulties associated with dressing, getting in and out of bed, lifting a full cup, walking on flat ground, washing and drying oneself, bending down to pick up clothing from the floor, turning faucets on and off, getting in and out of vehicles, walking 2 miles, and participating in recreational activities and sports); pain (10-point VAS) and global health assessment (10-point VAS). Responses from each section are scored on a scale of 0 to 10 (0 = least functional difficulty, least pain, or best global health state; 10 = greatest functional difficulty, greatest pain, or poorest global health state). The sum of the three subscores composes the RAPID3 score; scores range from 0 to 30 (0 = best health status; 30 = poorest health status) [Citation42-45]. RAPID3 has been utilized most often in measuring responses to treatment in patients with rheumatoid arthritis [Citation42]. RAPID3 can be scored in as little as 5 s on the MDHAQ form [Citation46]. The OMERACT-OARSI Responder Index is a questionnaire used to classify patients with OA as treatment responders or nonresponders based on domains of pain, function, and PGA of disease state [Citation41]. The percentage of patients who used rescue acetaminophen and the percentage of days during the trial that acetaminophen was used were analyzed. Safety endpoints have been reported previously [Citation33]. Assessments, including WOMAC and RAPID3 pain, were made in the morning prior to the first dose of randomized treatment for that day; therefore, these assessments correspond with the trough concentrations of pain medication.
Data analysis
The analyses were performed using the modified intent-to-treat population, defined as all randomized patients who received at least 1 dose of study medication and provided at least 1 post-baseline efficacy evaluation with last observation carried forward imputation. Changes from baseline differences versus PBO were analyzed using analysis of covariance models with baseline values as covariates; least squares (LS) means, CIs and p-values were generated. Hedges’ g effect size estimates (without correction for small sample size) were used to describe the magnitude of difference between two groups and calculated as: (Active treatment change from baseline LS mean − PBO change from baseline LS mean)/Root mean squared error.
A negative effect size indicates improvement versus placebo. Spearman and Pearson correlation coefficients were calculated to assess the strength of the relationships between WOMAC pain and RAPID3: pain and WOMAC total and RAPID3 at 6 and 12 weeks.
Results
Baseline demographics and clinical characteristics
Baseline characteristics for patients in the three treatment groups in each study have been described previously [Citation24,33]. As in the individual studies, demographic and disease characteristics for the treatment groups in the pooled data were evenly matched () [Citation24,33]. At baseline, patients in the NAP/ESO, CEL and PBO treatment groups had mean ages of 32.6, 33.1 and 32.8 years, respectively. In the NAP/ESO, CEL and PBO treatment groups, females accounted for 65.3%, 61.9% and 64.6% of patients, respectively (). Baseline pain scores were similar between groups and indicated moderate-to-severe pain ().
Table 1. Patient demographics and disease characteristics at baseline (modified ITT population)a.
Primary efficacy outcomes
From baseline to weeks 6 and 12, mean improvements in the co-primary outcomes of WOMAC pain, WOMAC function and PGA-VAS scores were generally similar in patients who received NAP/ESO or CEL (). Patients who received NAP/ESO had significantly greater improvements (all p ≤ 0.003) for all co-primary outcomes at weeks 6 and 12 versus patients who received PBO (). Patients treated with CEL showed greater improvements (all p ≤ 0.03 vs PBO) for all primary outcomes, except for week-6 PGA-VAS (). CEL did not separate from PBO in the PGA-VAS at week 6 (CEL − PBO = +2.9; 95% CI: −1.1, 6.8; p = 0.1523); however, patients treated with CEL had significantly greater PGA-VAS improvements by week 12 (CEL − PBO = +5.7; 95% CI: 1.57, 9.82; p = 0.0068).
Figure 1. Co-primary outcomes of WOMAC pain, WOMAC function, and PGA-VAS. (A) After 6 weeks of treatment. (B) After 12 weeks of treatment.
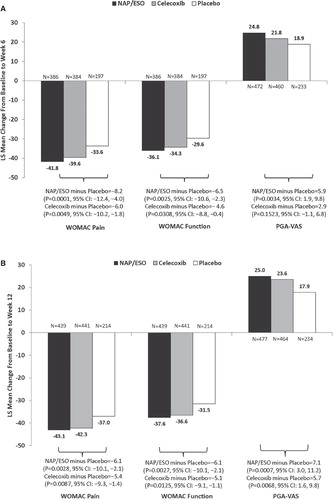
When compared directly, NAP/ESO and CEL were not significantly different from each other in any outcome at any time point; however, at week 6 there was a trend toward greater improvement in PGA-VAS with NAP/ESO treatment than with CEL (; NAP/ESO − CEL = –3.0; 95% CI: –6.2, 0.2; p = 0.0674). Absolute effect sizes were similar for CEL and NAP/ESO (all >1). Placebo-adjusted (standardized) effect sizes for NAP/ESO are summarized in .
Table 2. NAP/ESO effect sizes (Hedges’ g) for efficacy endpoints (modified ITT population with LOCF).
Acute onset of pain relief
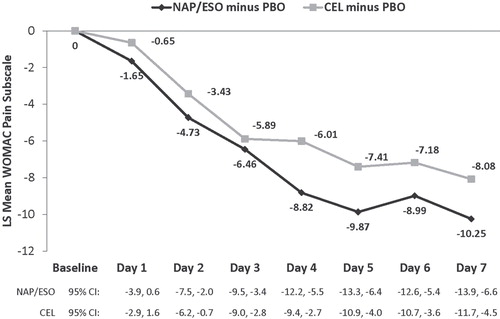
WOMAC pain subscale responses during the first 7 days of NAP/ESO and CEL therapy indicated that both active therapies resulted in early significant pain relief (); 95% CIs were -13.9 to -6.6 at day 7 for NAP/ESO and -11.7 to -4.5 for CEL. This response was evident by Day 2 of treatment (all p < 0.05) (). The magnitude of the early response was slightly more favorable to NAP/ESO than CEL (). Effect sizes ranged from 0.12 (Day 1) to 0.45 (Day 5, ). In both active treatment groups, the median time to good or excellent responses on the PGA was 6 days.
Acute APS-POQ pain assessments also demonstrated that patients treated with NAP/ESO or CEL experienced early pain relief. By Day 2, patients who received either NAP/ESO or CEL reported significantly greater improvements than patients who received PBO (all p < 0.05). Standardized (placebo-adjusted) effect sizes for NAP/ESO on Day 7 ranged from 0.28 (How has pain interfered with your relations with people?) to 0.55 (How has pain interfered with your general activity?). Patients treated with either active regimen reported significant APS-POQ improvements through Days 2–7 (all p < 0.05). At Day 7, the mean APS-POQ pain assessment scores were 62% higher for patients who received NAP/ESO (vs PBO); patients treated with CEL had 53% higher APS-POQ scores (vs PBO). No significant differences between NAP/ESO and CEL were found in any of the questions at any time during the 7-day evaluation. The differential absolute effect sizes between the two active treatments were greatest in response to the question “How has pain interfered with your general activity?” The differential responses were lowest in response to “How has pain interfered with your mood?” Standardized effect sizes were generally similar between the active treatments, although they trended lower with CEL.
Analgesic sustainability–other measures
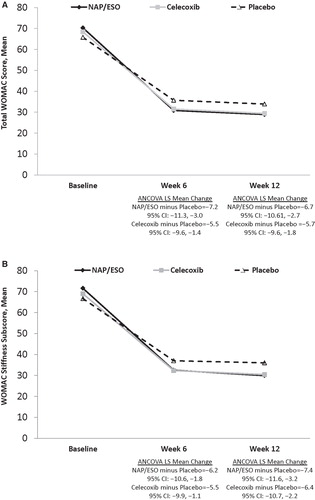
WOMAC total scores () and stiffness () subscores for patients treated with either NAP/ESO or CEL improved significantly (all p < 0.05) from baseline to week 6 and week 12. Neither of these outcomes differed significantly between treatment groups at any time point.
Figure 3. WOMAC scores at baseline and treatment weeks 6 and 12. (A) WOMAC total scores. (B) WOMAC stiffness subscores.
The analgesic sustainability of NAP/ESO treatment was confirmed by significant (all p < 0.05) findings of improvement in the results from other measures, including RAPID3 and its Pain and PGA components (,,). The RAPID3: Physical Function scores decreased slightly in the NAP/ESO group, but not statistically (). However, the responses of the CEL group were not significantly different (p > 0.05) from those of the PBO group in RAPID3 or any of its components (,,,). Furthermore, CEL did not differ statistically significantly from NAP/ESO at any time point for these measures (data not shown). For both treatments, effect sizes were minimally clinical meaningful when compared with change from placebo (≥0.20, ), with the exception of physical function for CEL at weeks 6 and 12. Effect sizes were smaller in all measures and at all measurement points with CEL compared with NAP/ESO.
Table 3. RAPID3 at week 6 and week 12 (modified ITT population with LOCF).
Table 4. RAPID3: pain at week 6 and week 12 (modified ITT population with LOCF).
Table 5. RAPID: patient global scores at week 6 and week 12 (modified ITT population with LOCF).
Table 6. RAPID: physical function at week 6 and week 12 (modified ITT population with LOCF).
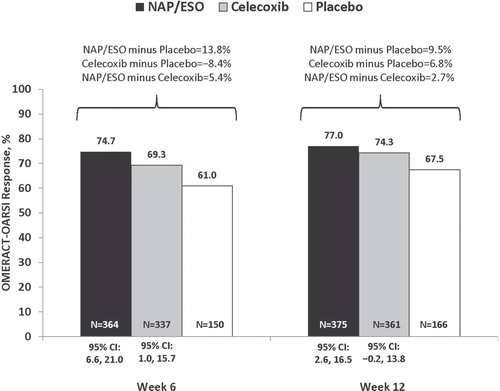
At weeks 6 and 12, OMERACT-OARSI response was achieved by a greater percentage of patients treated with NAP/ESO than those treated with PBO despite a PBO response rate of >60% (). In the NAP/ESO group, the OMERACT-OARSI response was statistically significantly different from PBO at weeks 6 (95% CI: 6.6, 21.0) and 12 (95% CI: 2.6, 16.5); in the CEL group, this response was significantly different from PBO only at week 6 (95% CI: 1.0, 15.7) ().
Figure 4. Percentage of patients who attained OMERACT-OARSI response after 6 or 12 weeks of treatment.
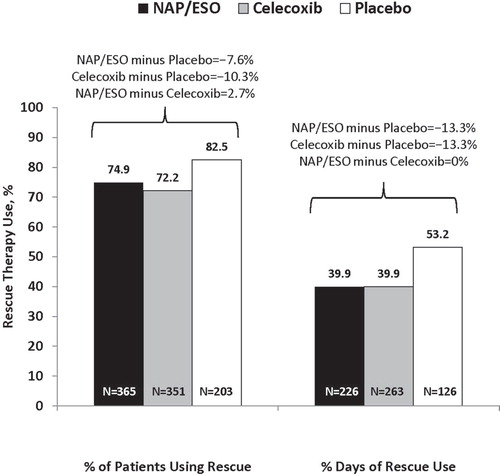
Acetaminophen rescue was used by a greater percentage of patients in the PBO group than in either of the active treatment groups (). However, the time to first use of acetaminophen rescue therapy was not significantly different between the groups (1 day in all groups). Patients in the PBO group used rescue therapy for a greater percentage of the study than did patients in either active treatment group (53.2% vs 39.9%, respectively; p = 0.0006; ). There were no differences between CEL and NAP/ESO in regard to time to first use, or the percentage, of patients using acetaminophen rescue.
Correlation of WOMAC and RAPID3 scores
WOMAC pain and RAPID3-pain assessments were highly correlated with each other at each visit, and WOMAC total score was also highly coordinated with the RAPID3 score at each visit ().
Table 7. Correlation of WOMAC and RAPID3 scores (modified ITT population)
Discussion
Efforts to understand the relative efficacy of oral NSAIDs for the treatment for knee OA have been ongoing since CEL was launched in 1999. Effect sizes are one method for comparing data across trials, and several meta-analyses have reported effect sizes for WOMAC pain response with various analgesics [Citation29,47-49]. In a 2004 study, effect sizes ranging from 0.27 to 0.37 were reported for randomized controlled trials involving NAP [Citation47]. Similarly, effect sizes ranging from 0.20 to 0.45 were reported for CEL trials [Citation47]. Pooled effect sizes for pain reduction with NSAIDs overall were 0.32 (0.24–0.39) across all trials and 0.23 (0.15–0.31) in 10 trials in which non-responders were not excluded [Citation47]. In another meta-analysis based on a minimum clinically significant threshold of WOMAC pain improvement (defined as 9.7 mm), pooled NSAID response overall was 10.2 mm; however, no therapies had effect sizes that exceed the mean threshold for minimally clinical important improvement in the short-term or long-term [Citation48]. From Stam et al. [Citation49], meta-analyses results demonstrated that naproxen 1000 mg/day (the dose used in these studies) had an effect size of 0.39 (0.26–0.53) relative to placebo for pain response [Citation49]. In the most recently published network meta-analysis of pain response with various pharmacologic interventions, the Hedges’ g WOMAC pain effect sizes at 12 weeks for NAP and CEL versus placebo were 0.38 and 0.33, respectively [Citation29].
Data from the pooled NAP/ESO trials in the present analysis confirm that maximal NSAID effectiveness occurs early in therapy and refute some prior studies suggesting that the pain effect of oral NSAIDs is limited to the first 2 to 3 weeks [Citation47,48]. WOMAC pain effect sizes in the pooled NAP/ESO analyses generally were similar to those reported in other meta-analyses. Although the magnitude of change for NAP/ESO, as measured by effect size, decreased slightly over time, pain response remained clinically and significantly improved for up to 12 weeks. Further, there was some evidence for tachyphylaxis with CEL in the present study; OMERACT/OARSI responders, RAPID3 and its individual component scores for CEL were not different from placebo at 12 weeks. Others have reported nonsignificant responses with CEL, including Bannuru et al. [Citation29] With an effect size of 0.15 (0.00–0.30) versus acetaminophen, CEL was superior to placebo but not acetaminophen for pain response, whereas naproxen (effect sizes of 0.38 vs placebo and 0.2 vs acetaminophen) was significant in both comparisons [Citation29]. In general, comparisons of effect size from different study results should be interpreted with caution because the numerical value of the effect size can vary by method of calculation; methods that pool SDs for each measure (eg, Hedges’ g) typically producing higher values than those that use the SD of the baseline measure (eg, Glass’ Δ). However, if the SD for baseline measures is smaller than post baseline measures, which is not unexpected, the effect size based on Hedges’ g will be smaller than that based on Glass’ Δ. In addition, analyses with very large samples (like Bannuru’s analysis) likely have relatively small SDs, which in turn can lead to larger effect size estimates. Finally, it is not entirely clear from many reports in the literature when the pain assessments were made. In our studies, assessments were made just before the next treatment administration in the morning, generally the time of maximal discomfort but the lowest drug concentration. Therefore, our assessment times represent a conservative estimate of treatment effect.
Limited data exist on the acute and long-term longitudinal responses to NSAIDs for patients with OA. In addition to pain response, the present study examined a wide array of acute and long-term outcomes, including RAPID3. An absolute decrease of 17 mm in VAS for knee OA pain and a 15 mm decrease in function have been reported as clinically meaningful changes [Citation50], and these thresholds were met or exceeded in the present trial. Furthermore, Tubach [Citation50] has proposed a patient acceptable symptom state (PASS) to address the concept of a partial symptomatic remission from NSAID treatment of knee OA; and these PASS thresholds were met or exceeded in the present analysis at 6 and 12 weeks with NAP/ESO treatment, with the exception of PGA response. The RAPID3 has shown utility as a patient-reported outcome in rheumatoid arthritis and other rheumatic diseases, including OA, potentially decreasing the need for joint counts, radiographic assessments and longer health assessment questionnaires [Citation40,44,45,51]. Our study supports the contention that RAPID3 could be useful in the routine assessment of pain response in knee OA patients, as the correlation between RAPID3 and WOMAC was strong, both acutely and over time.
Limitations
The data in these studies were developed from patients who had symptomatic knee OA, as demonstrated by a flare before randomization; were at least 50 years of age; and had a history of at least 6 months of OA of the knee. Eligible patients also were required to be taking a stable dose of NSAIDs, COX-2 inhibitors, or other oral analgesic therapy for at least 6 weeks prior to screening. Therefore, the results may not apply to OA patients with different clinical and demographic characteristics. Further, our patients had high absolute baseline pain VAS scores (approximately 8) and clinically meaningful absolute decreases (approximately 50%); thus, the results may be different in those with lower baseline pain.
Safety
Safety results of these studies, including adverse events and upper gastrointestinal tolerability, have been published in detail previously [Citation24,33]. Serious AEs were reported in ≤2% of patients receiving active treatment, and only 1 event was considered possibly treatment-related (anaphylactic reaction to CEL) [Citation24]. There were no deaths in either of the studies. Patients treated with NAP/ESO reported significantly more heartburn-free days than those treated with CEL [Citation33]. Both active treatment groups were antacid-sparing compared with PBO, although the percentages of patients using antacid rescue was not different between the active treatment groups.
Conclusions
The fixed combination of NAP/ESO produced an absolute moderate and statistically significant early pain response in patients with knee OA, which was observed as early as 2 days after initiating therapy and was maintained for 12 weeks. Results for NAP/ESO were generally comparable to CEL, but there was some evidence of tachyphylaxis with CEL in some measures by Week 12 compared with placebo, with lower effect sizes than NAP/ESO. Acute responses were generally similar across the pain measures evaluated, with differences favoring NAP/ESO versus CEL noted by Day 4 based on the WOMAC pain subscale. WOMAC pain standardized effect sizes remained stable over the 12-week study for NAP/ESO, and other measures’ effect sizes essentially remained stable from Week 6 to Week 12. RAPID3 and RAPID3 Pain were found to be highly correlated with the typical OA measures, WOMAC total and pain, suggesting that RAPID3 might be a useful clinical tool for measuring NSAID response in OA patients.
Acknowledgments
The authors would like to thank Jana Steinmetz of Premier Research, Inc, Naperville, IL, and Ying Zhang of POZEN, Inc, Chapel Hill, NC, for statistical support. Medical writing services were provided by Cathryn M. Carter, MS, and Tonya Goodman, of Arbor Communications, Inc; this support was funded by Horizon Pharma USA, Inc.
Declaration of interest
POZEN Inc. sponsored the original studies. The pooled analysis was funded by Horizon Pharma USA, Inc. John G Fort is an employee of POZEN, Inc and current stock and stock option holder. Jeffrey D Kent and Amy Y Grahn are employees of Horizon Pharma USA, Inc and current stock and stock option holders. Robert J Holt has served as a consultant for POZEN, Inc and Horizon Pharma USA, Inc. Alfonso E Bello has been a consultant for Horizon Pharma USA, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Notes
References
- National Clinical Guideline Center. London: National Institute for Health and Care Excellence (UK). 2014. Available from http://www.nice.org.uk/guidance/cg177/resources/guidance-osteoarthritis-pdf. Last accessed 24 January 2015
- Fernandes L, Hagen KB, Bijlsma WJ, Andreassen O, Christensen P, Conaghan PG, et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis 2013;72:1125–35
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163–96
- Jordan J, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and caucasians: the johnston county osteoarthritis project. J Rheumatol 2007;34:172–80
- Conaghan PG, Peloso PM, Everett SV, Rajagopalan S, Black CM, Mavros P, et al. Inadequate pain relief and large functional loss among patients with knee osteoarthritis: evidence from a prospective multinational longitudinal study of osteoarthritis real-world therapies. Rheumatology (Oxford) 2015;54:270–7
- American College of Rheumatology Ad Hoc Group on Use of Selective and Nonselective Nonsteroidal Antiinflammatory Drugs. Recommendations for use of selective and nonselective nonsteroidal antiinflammatory drugs: an American College of Rheumatology white paper. Arthritis Rheum 2008;59:1058–73
- Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American college of rheumatology subcommittee on osteoarthritis guidelines. Arthritis Rheum 2000;43:1905–15
- McGettigan P, Henry D. Use of non-steroidal anti-inflammatory drugs that elevate cardiovascular risk: an examination of sales and essential medicines lists in low-, middle-, and high-income countries. PLoS Med 2013;10:e1001388
- Larkai EN, Smith JL, Lidsky MD, Graham DY. Gastroduodenal mucosa and dyspeptic symptoms in arthritic patients during chronic nonsteroidal anti-inflammatory drug use. Am J Gastroenterol 1987;82:1153–8
- Massó González EL, Patrignani P, Tacconelli S, García Rodríguez LA. Variability among nonsteroidal antiinflammatory drugs in risk of upper gastrointestinal bleeding. Arthritis Rheum 2010;62:1592–601
- García Rodríguez LA, Barreales Tolosa L. Risk of upper gastrointestinal complications among users of traditional NSAIDs and COXIBs in the general population. Gastroenterology 2007;132:498–506
- Lanza FL, Chan FK, Quigley EMM. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol 2009;104:728–38
- Balmaceda CM. Clinical trial data in support of changing guidelines in osteoarthritis treatment. J Pain Res 2014;7:211–18
- Balmaceda CM. Evolving guidelines in the use of topical nonsteroidal anti-inflammatory drugs in the treatment of osteoarthritis. BMC Musculoskelet Disord 2014;15:27
- Bruyère O, Cooper C, Pelletier JP, Branco J, Luisa Brandi M, Guillemin F, et al. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: a report from a task force of the european society for clinical and economic aspects of osteoporosis and osteoarthritis (ESCEO). Semin Arthritis Rheum 2014;44:253–63
- Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: The Chronic Osteoarthritis Management Initiative of the U.S. Bone and Joint Initiative. Semin Arthritis Rheum 2014;43:701–12
- Rosemont IL. Treatment of osteoarthritis of the knee evidence-based guideline, 2nd edition. J Am Acad Orthop Surg 2013;21:571–6
- Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology. American college of rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64:465–74
- McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 2014;22:363–88
- Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JW, Dieppe P, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003;62:1145–55
- Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA; American Heart Association. Use of nonsteroidal antiinflammatory drugs, an update for clinicians: a scientific statement from the American Heart Association. Circulation 2007;115:1634–42
- Coxib and traditional NSAID Trialists’ (CNT) Collaboration. Bhala N, Emberson J, Merhi A, Abramson S, Arber N, et al. Vascular and upper GI effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomized trials. Lancet 2013;382:769–79
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 2014;129:e28–e292
- Hochberg MC, Fort JG, Svensson O, Hwang C, Sostek M. Fixed-dose combination of enteric-coated naproxen and immediate-release esomeprazole has comparable efficacy to celecoxib for knee osteoarthritis: two randomized trials. Curr Med Res Opin 2011;27:1243–53
- Essex MN, Bhadra P, Sands GH. Efficacy and tolerability of celecoxib versus naproxen in patients with osteoarthritis of the knee: a randomized, double-blind, double-dummy trial. J Int Med Res 2012;40:1357–70
- Richy F, Bruyere O, Ethgen O, et al. Time dependent risk of gastrointestinal complications induced by non-steroidal anti-inflammatory drug use: a consensus statement using a meta-analytic approach. Ann Rheum Dis 2004;63:759–66
- Hippisley-Cox J, Coupland C. Risk of myocardial infarction in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis. Br Med J 2005;330:1336
- Gimenez S, Armada B, Iturralde Iriso J, Ginel Mendoza L, Fernández-Morales B. Clinical management of patients with hip and knee osteoarthritis: patient satisfaction with treatment switch. Rheumatol Int 2014;34:823–32
- Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis. Ann Intern Med 2015;162:46–54
- Abraham NS, El-Serag HB, Johnson ML, Hartman C, Richardson P, Ray WA, Smalley W. National adherence to evidence-based guidelines for the prescription of nonsteroidal anti-inflammatory drugs. Gastroenterol 2005;129:1171–8
- Goldstein JL, Howard KB, Walton SB, McLaughlin TP, Kruzikas DT. Impact of adherence to concomitant gastroprotective therapy on nonsteroidal-related gastroduodenal ulcer complications. Clin Gastroenterol Hepatol 2006;4:1337–45
- van Soest EM, Sturkenboom MC, Dieleman JP, Verhamme KM, Siersema PD, Kuipers EJ. Adherence to gastroprotection and the risk of NSAID-related upper gastrointestinal ulcers and haemorrhage. Aliment Pharmacol Ther 2007;26:265–75
- Cryer BL, Sostek MB, Fort JG, Svensson O, Hwang C, Hochberg MC. A fixed-dose combination of naproxen and esomeprazole magnesium has comparable upper gastrointestinal tolerability to celecoxib in patients with osteoarthritis of the knee: results from two randomized, parallel-group, placebo-controlled trials. Ann Med 2011;43:594–605
- VIMOVO® prescribing information. Deerfield, IL: Horizon Pharma USA, Inc.; 2014. Available from http://www.vimovo.com/pi/. Last accessed 29 January 2015]
- Sostek MB, Fort JG, Estborn L, Vikman K. Long-term safety of naproxen and esomeprazole magnesium fixed-dose combination: phase III study in patients at risk for NSAID-associated gastric ulcers. Curr Med Res Opin 2011;27:847–54
- Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833–40
- Bellamy N, Campbell J, Stevens J, Pilch L, Stewart C, Mahmood Z. Validation study of a computerized version of the Western Ontario and McMaster Universities VA3.0 Osteoarthritis Index. J Rheumatol 1997;24:2413–15
- Bellamy N. WOMAC: a 20-year experiential review of a patient-centered self-reported health status questionnaire. J Rheumatol 2002;29:2473–6
- Moskowitz RW, Sunshine A, Brugger A, Lefkowith JB, Zhao WW, Geis GS. American pain society pain questionnaire and other pain measures in the assessment of osteoarthritis pain: a pooled analysis of three celecoxib pivotal studies. Am J Ther 2003;10:12–20
- Castrejón I, Bergman MJ, Pincus T. MDHAQ/RAPID3 to recognize improvement over 2 months in usual care of patients with osteoarthritis, systemic lupus erythematosus, spondyloarthropathy, and gout, as well as rheumatoid arthritis. J Clin Rheum 2013;19:169–74
- Pham T, van der Heijde D, Lassere M, Altman RD, Anderson JJ, Bellamy N. Outcome variables for osteoarthritis clinical trials: the OMERACT-OARSI set of responder criteria. J Rheumatol 2003;30:1648–54
- Pincus T. Pain, function, and RAPID scores: vital signs in chronic diseases, analogous to pulse and temperature in acute diseases and blood pressure and cholesterol in long-term health. Bull NYU Hosp Jt Dis 2008;66:155–65
- Pincus T, Yazici Y, Bergman M. A practical guide to scoring a multi-dimensional health assessment questionnaire (MDHAQ) and routine assessment of patient index data (RAPID) scores in 10-20 seconds for use in standard clinical care, without rulers, calculators, websites or computers. Best Pract Res Clin Rheumatol 2007;21:755–87
- Pincus T, Askanase AD, Swearingen CJ. A multi-dimensional health assessment questionnaire (MDHAQ) and routine assessment of patient index data (RAPID3) scores are informative in patients with all rheumatic diseases. Rheum Dis Clin North Am 2009;35:819–27
- Pincus T, Castrejón I. MDHAQ/RAPID3 scores: quantitative patient history data in a standardized “scientific” format for optimal assessment of patient status and quality of care in rheumatic diseases. Bull NYU Hosp Jt Dis 2011;69:201–14
- Pincus T. RAPID3, an index of only 3 patient self-report core data set measures, but not ESR, recognizes incomplete responses to methotrexate in usual care of patients with rheumatoid arthritis. NYU Hosp Jt Dis 2013;71:17–20
- Bjordal JM, Ljunggren AE, Klovning A, Slørdal L. Non-steroidal anti-inflammatory drugs, including cyclo-oxygenase-2 inhibitors, in osteoarthritic knee pain: meta-analysis of randomised placebo controlled trials. BMJ 2004;329:1317
- Bjordal JM, Klovning A, Ljunggren AE, Slørdal L. Short-term efficacy of pharmacotherapeutic interventions in osteoarthritic knee pain: A meta-analysis of randomised placebo-controlled trials. Eur J Pain 2007;11:125–38
- Stam WB, Jansen JP, Taylor SD. Efficacy of etoricoxib, celecoxib, lumiracoxib, non-selective NSAIDs, and acetaminophen in osteoarthritis: a mixed treatment comparison. Open Rheum J 2012;6:6–20
- Tubach F, Ravaud P, Baron G, Falissard B, Logeart I, Bellamy N, et al. Evaluation of clinically relevant states in patient reported outcomes in knee and hip osteoarthritis: the patient acceptable symptom state. Ann Rheum Dis 2005;64:34–7
- Pincus T, Yazici Y, Castrejón I. Pragmatic and scientific advantages of MDHAQ/RAPID3 completion by all patients at all visits in routine clinical care. Bull NYU Hosp Jt Dis 2012;70:30–6