ABSTRACT
Background
Despite the numerous health benefits of distance running, it is also associated with the development of ‘gradual onset running-related injuries’ (GORRIs) one of which is Iliotibial Band Syndrome (ITBS). Novel risk factors associated with a history of ITBS (hITBS) have not been described in a large cohort of distance runners.
Objective
To identify risk factors associated with hITBS in distance runners.
Design
Descriptive cross-sectional study.
Setting
21.1 km and 56 km Two Oceans Marathon races (2012–2015).
Participants
106 743 race entrants completed the online pre-race medical screening questionnaire. A total of 1 314 runners confirmed an accurate hITBS diagnosis.
Methods
Selected risk factors associated with hITBS explored included: demographics (race distance, sex, age groups), training/running variables, history of existing chronic diseases (including a composite chronic disease score) and history of any allergy. Prevalence (%) and prevalence ratios (PR; 95% CI) are reported (uni- & multiple regression analyzes).
Results
1.63% entrants reported hITBS in a 12-month period. There was a higher (p < 0.0001) prevalence of hITBS in the longer race distance entrants (56 km), females, younger entrants, fewer years of recreational running (PR = 1.07; p = 0.0009) and faster average running speed (PR = 1.02; p = 0.0066). When adjusted for race distance, sex, age groups, a higher chronic disease composite score (PR = 2.38 times increased risk for every two additional chronic diseases; p < 0.0001) and a history of allergies (PR = 1.9; p < 0.0001) were independent risk factors associated with hITBS.
Conclusion
Apart from female sex, younger age, fewer years of running and slower running speed, two novel independent risk factors associated with hITBS in distance runners are an increased number of chronic diseases and a history of allergies. Identifying athletes at higher risk for ITBS can guide healthcare professionals in their prevention and rehabilitation efforts.
Introduction
Regular participation in moderate-to high-intensity physical activity for >150 min/week can reduce the burden of non-communicable diseases [Citation1–3]. Mass community-based participation in sport and endurance events such as distance running has increased in popularity due to its affordability and extensive health benefits [Citation3,Citation4]. Running is a repetitive high-impact exercise and long-distance runners are prone to gradual onset injuries, especially in the lower extremities [Citation5–7]. The impact of long-distance running on lower limb muscle fatigue, symmetry, gait deviations, and joint mechanics/kinematics has been studied, highlighting the potential for increased injury susceptibility [Citation8–11]. Iliotibial Band Syndrome (ITBS) is one of the most common gradual onset running-related injuries (GORRIs) with an annual incidence of 7% to 14% [Citation5,Citation6,Citation12–16].
ITBS affects the knee and classically presents with lateral knee discomfort but can radiate along the length of the iliotibial band, presenting as hip or thigh pain [Citation17]. Historically it has been suggested that ITBS resulted from friction of the iliotibial band (ITB) on the lateral femoral condyle [Citation18–20]. However, a more recent review of arthroscopic, cadaveric, diagnostic imaging, histologic and biomechanical studies concluded that the pathology of ITBS is not related to friction but suggests that it is an impingement of a fat pad deep to the distal ITB, resulting in para-inflammation and pain [Citation21].
Prevention of running injuries is a priority and a clear understanding of the risk factors associated with running injuries is important to plan intervention strategies to reduce the risk of ITBS. Several studies report various extrinsic/intrinsic and modifiable/non-modifiable risk factors associated with ITBS in distance runners, which suggests a multifactorial etiology [Citation6,Citation12,Citation13,Citation22,Citation23]. Several proposed extrinsic risk factors, such as running on angled surfaces including downhill running, excessive training (sudden increase in mileage and frequency) and poorly fitting running footwear are reported [Citation6,Citation16,Citation24].
Historically, multiple intrinsic risk factors including anatomical and biomechanical risk factors such as discrepancy in leg length, prominence of the lateral femoral epicondyles, and diminished flexibility of the ITB have been identified [Citation16,Citation24–28]. It has also been suggested that running-related biomechanical risk factors include greater hip adduction and knee internal rotation [Citation24,Citation29] and that runners may develop ITBS as a result of weak gluteal muscles, particularly the gluteus medius [Citation29–33]. However, the underlying anatomical and biomechanical factors causing ITBS in distance runners are still not well understood and many of these historical intrinsic risk factors for ITBS have been challenged [Citation21]. A recent review concluded that ITBS is likely related to a complex relationship between the ITB and mechanical function of the in-series hip musculature (gluteus maximus and the tensor fascia lata) [Citation34].
Modifiable factors such as training intensity and volume play a significant role in the development of ITBS. High weekly mileage, interval training, and muscular weakness of knee extensors, flexors, and hip abductors have been identified as potential risk factors for the development of ITBS [Citation35]. Furthermore, running speed and exhaustion might lead to an alteration in the biomechanics that influence the development of ITBS [Citation36]. Therefore, appropriate training modifications and targeted strengthening exercises for the hip abductors and lower limb muscles are important in preventing and managing ITBS.
Non-modifiable factors such as sex, age, and chronic diseases may also influence the development of ITBS. For instance, female runners with ITBS have been found to present with specific biomechanical risk factors [Citation28]. These findings suggest that non-modifiable factors can also contribute to the ITBS. A comprehensive approach that addresses both modifiable and non-modifiable factors is essential in the prevention and management of ITBS.
Currently, the mainstay of correcting intrinsic risk factors is to decrease pain and improve function in runners with this condition is to focus on: 1) correction of abnormal running biomechanics that increase either ITB strain or compression of lateral knee structures [Citation34], and 2) rehabilitation targeting neuromuscular control, endurance, and strength of the hip-abductor and external-rotator muscles [Citation21].
Recently, novel intrinsic risk factors associated with running-related injuries have been identified. Of particular interest is that recent studies show that a history of chronic disease and allergies may be related to any GORRIs in runners [Citation37–39], and cyclists [Citation40]. However, the possible association between chronic medical conditions, medication use or allergies and specific running-related injuries such as ITBS, has not been reported.
This study aims to identify selected independent risk factors associated with a history of ITBS (hITBS) in distance runners entering the 2012 – 2015 Two Oceans Marathon races (21.1 km and 56 km). The specific risk factors to be considered in multiple regression analysis are demographics (race distance, sex, age groups), training-related variables (years of recreational running, weekly running distance, running speed), and a history of chronic disease and allergies. Despite the growing body of literature on ITBS, there remains a notable gap in our understanding of specific risk factors contributing to its development and persistence. The relationship between hITBS in distance runners and a history of chronic disease or allergies has not been reported.
Material and methods
Study design and ethical concerns
This descriptive cross-sectional study forms part of a series of ongoing SAFER (Strategies to reduce Adverse medical events For the ExerciseR) studies. Ethics approval was obtained from both the Research Ethics Committees of (REC 009/2011) and the (REC 433/2015 and 700/2019) before the onset of the study.
Participants and demographics
All race entrants of the 21.1 km & 56 km Two Oceans Marathon for each year from 2012–2015 were included. Of the total 106 743 race entrants over the 4 years, 76 654 (71.8%) runners (44 042 males: 32 612 females) gave informed consent that their data may be used for research purposes.
Online pre-race medical questionnaire
Participants completed a mandatory online pre-race medical screening questionnaire at the time of registration for the Two Oceans Marathons. The questionnaire is based on guidelines by the European Association for Cardiovascular Prevention and Rehabilitation (EACPR) for cardiovascular evaluation in distance runners [Citation41–43]. We previously reported details on the development of the questionnaire and the main questions included [Citation44,Citation45]. In summary, all entrants were requested to provide details about personal medical history, questions on training over the last 12 months, injury history and history of chronic diseases or allergies. There were specific questions related to the following: runner demographics (race distance, sex and age), running training/racing history (years of recreational running, average weekly training/running distance in the last 12 months, and average training speed, history of chronic disease (risk factors for CVD, history of CVD, symptoms of CVD, endocrine disease, respiratory disease, gastrointestinal disease, nervous system/psychiatric disease, kidney/bladder disease, hematological/immune disease and cancer), and history of any allergies [Citation38].
Gradual onset injuries are classified as injuries that do not have a specific abrupt, precipitating event as the onset of injury, and which are the result of a series of interactions between the agent (transfer of kinetic energy); host(athlete); and environment [Citation7]. In this study we asked the participants the following specific question on running injuries: ‘Do you or did you suffer from any symptoms of a running injury (muscles, tendons, bones, ligaments or joints) in the past 12 months or currently?.’ The classification of an injury was: ‘Only if the injury is/was severe enough to interfere with or require treatment e.g. use medication, or require you to seek medical advice from a health professional)?’ If a runner responded ‘yes,’ additional questions asked included: whether the injury was experienced at present or during the previous 12 months, unilateral to the left or right or bilateral, as well as whether the injury was specifically ITBS. Responses to these questions were subsequently used to define the study groups [Citation44,Citation45].
Defining the study groups
Control group
If a runner responded ‘no’ to the question related to a running injury in the past 12 months, they were in the non-injured control group (CON group). Of the 76 654 consenting entrants, 60 635 (34 506 males: 26 129 females) reported no running injury in the past 12 months and this was the control group.
ITBS group
If a runner responded ‘yes’ to the question related to a running injury in the past 12 months, they were asked to select the specific common running injury from the following list: ‘patellofemoral pain, iliotibial band (ITB), plantar fasciitis, Achilles tendon injury, lower back pain, hip muscle injury (including gluteus/buttock muscles), hamstring injury, quadriceps muscle injury, calf muscle injury, shin splints (bone), shin splints (muscle/tendon), lower leg compartment syndrome, foot pain, heel pain, or other’ injury. A total of 1466 entrants who specified they had a running injury in the past 12 months selected ‘iliotibial band (ITB).’ These participants were considered for possible inclusion in the hITBS group. Participants reporting other specific injuries were excluded from the analyzes.
To improve the accuracy of the self-reported diagnosis of the injury, we included a question on the treatment of ITBS. Runners were asked to select the treatment modalities for their injury from a list that included rest, tablets, stretches, physiotherapy, cortisone injection, other injection, surgery, orthotics, strengthening exercises and equipment change. Only rest, stretches, and equipment change are interventions that can be self-prescribed and self-applied. If a runner selected one or more treatment modalities that could only be administered by a health professional, the self-reported diagnosis of the specific injury was considered to be verified. Of the 1 466 entrants that selected ‘iliotibial band (ITB)’ as the injury, the diagnosis of hITBS in 1 314 (89.6%) runners was considered more accurate and these entrants were the hITBS group. A total of 152 entrants with a self-reported diagnosis of hITBS were considered non-verified and excluded from analyzes.
Main outcome and independent variables reported
The primary outcome for this study was hITBS in the past 12 months among race entrants. The following main categories of variables of interest were explored as factors associated with hITBS in a multivariate model: (1) runner demographics, (2) training-related variables (years of recreational running, weekly running distance, running speed), (3) history of chronic disease, and (4) history of any allergies [Citation38,Citation46].
We calculated an additional chronic disease composite score by combining the 10 chronic disease variables (risk factors for CVD, history of CVD, symptoms of CVD, endocrine disease, respiratory disease, gastrointestinal disease, nervous system/psychiatric disease, kidney/bladder disease, hematological/immune disease and cancer) to present a single score of the associated risk of an increase in the number of chronic diseases [Citation38].
Statistical data analysis
The race participant’s questionnaire data were entered into an Excel spreadsheet (Microsoft 2010) and analyzed using the SAS v9.4 statistical software. Only data from the consenting runners were utilized for statistical analysis.
Prevalence ratios (PR) were calculated as the measure of association. Univariate unadjusted prevalence (% and 95% CI) and PR were reported for sex, race distance and age groups, running experience, training/running history, training speed, history of chronic disease and history of any allergies. The total sample (n = 76 654) was used to estimate the overall prevalence. Runners reporting other specific injuries and non-verified hITBS were excluded from the analyzes, resulting in a sample of 61 949. A multiple regression model was performed to determine independent risk factors associated with hITBS.
The categorical variables entered into the model included the demographics, history of chronic disease and a history of any allergy. A recurring statement was included to account for the exchangeable correlation structure as one runner could report more than one injury per year. The training and running variables were entered into the model as continuous variables. The prevalence of hITBS (% and 95% CI) was reported at the first quartile, median and third quartile for these variables. The chronic disease composite score was entered into the multiple regression model rather than the individual’s chronic diseases to provide a more parsimonious and robust model without the confounding effect of multi-collinearity. The multiple regression model included all the significant univariate risk factors, and the results for the final model only included the retained significant risk factors. The statistical significance level was 5% unless otherwise specified.
Results
Profile of all race entrants and study participants
The demographic profile (race distance, sex, age groups) of all race entrants in both the 21.1 km & 56 km Two Oceans Marathon for 2012–2015 were compared with the consenting running participants in this study ().
Table 1. The profile by race distance, sex and age groups of all race entrants, and consenting participants.
In our study population, there were significantly more race entrants in the 21.1 km race distance (p = 0.0011) compared to all race entrants than in the 56 km race distance. There were no significant differences when comparing sex and age groups between all race entrants and consenting race entrants.
Risk factors associated with hITBS (univariate analysis)
Runner demographics (race distance, sex, age) (univariate analysis)
The period prevalence of hITBS (n = 1 314) among all consenting race entrants (n = 76 654) in the past 12 months was 1.63% (95% CI 1.53–1.73). The number (n), prevalence (%; with 95% CI) and prevalence ratio (PR; with 95% CI) of runners with hITBS by race distance, sex and age group are depicted in .
Table 2. The number (n), prevalence (95% CI) and prevalence ratio (PR; 95% CI) of participating race entrants (n = 61 949) and entrants with hITBS (n = 1314) by race distance, sex and age group (univariate analysis).
There was a significantly higher prevalence of hITBS in the 56 km vs. 21.1 km race participants (PR = 1.40; p < 0.0001) and in female vs. male runners (PR = 1.40; p < 0.0001). Compared to the >50 years age group, a significantly higher prevalence of hITBS is seen in the ≤30 years (PR = 3.02; p < 0.0001), 31–40 years (PR = 3.00; p < 0.0001) and 41–50 years (PR = 2.01, p < 0.0001) age groups.
Training-related variables (years of recreational running, weekly running distance, running speed) (univariate analysis)
The prevalence (%; 95% CI) and unadjusted prevalence ratio (PR; 95% CI) of runners reporting hITBS by running, training/racing history are shown in .
Table 3. The prevalence (%; with 95% CI) and prevalence ratio (PR; with 95% CI) of runners reporting hITBS by running, training/racing history (univariate analysis, unadjusted).
Fewer number of years as a recreational runner were associated with a higher prevalence of hITBS (PR = 1.07, a 7% increase in risk for every 5-years fewer in number of years as a recreational runner; p = 0.0009). A slower average running speed was associated with a higher prevalence of hITBS (PR = 1.02, a 2% increase in risk for every 1 km/hr decrease in average running speed; p = 0.0066). Average weekly training/running distance in the last 12 months was not associated with hITBS (p = 0.80). There was no significant interaction between years as a recreational runner and running speed and the association with the prevalence of hITBS (p = 0.07).
History of underlying chronic disease and allergies (univariate analysis)
The number (n), prevalence (%; 95% CI) and unadjusted prevalence ratio (PR; 95% CI) of runners with hITBS by main categories of chronic disease and allergies are shown in .
Table 4. The number (n), prevalence (%; 95% CI) and prevalence ratio (PR; with 95% CI) of distance runners with hITBS by history of chronic disease and allergies (univariate analysis, unadjusted).
In the univariate analysis, several specific chronic diseases are significantly associated with an increased prevalence of hITBS in distance runners. In decreasing order of PR, those with a PR above 2 include: any GIT disease (PR = 3.11; p < 0.0001); any hematological/immune disease (PR = 2.79; p = 0.0038); any kidney/bladder disease (PR = 2.56; p = 0.0002); any nervous system/psychiatric disease (PR = 2.25; p < 0.0001); any respiratory disease (PR = 2.23; p < 0.0001); and any symptoms of CVD (PR = 2.16; p = 0.0106). In addition, distance runners with a history of any allergies (PR = 2.43; p < 0.0001) were significantly associated with a higher prevalence of hITBS.
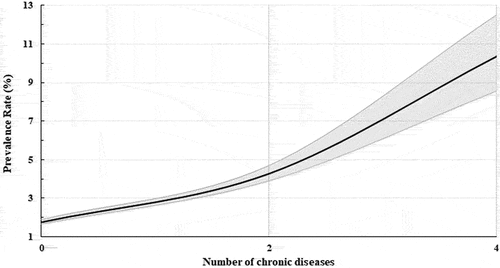
The relationship between the prevalence of hITBS and the number of chronic diseases (chronic disease composite score) is shown in . For every two additional chronic diseases, the prevalence of hITBS increases 2.42 times (p < 0.0001).
Independent risk factors associated with hITBS (multiple regression analysis)
The multiple regression analysis included all univariate significant risk factors to determine the independent risk factors associated with hITBS. The adjusted prevalence ratio (PR; with 95% CI) of the multiple regression analysis is depicted in .
Table 5. The prevalence ratio (PR; with 95% CI) of significant independent risk factors (adjusted for race distance, sex, and age) associated with hTBS in distance runners (multiple regression analysis).
The independent risk factors (adjusted for race distance, sex, and age) associated with a higher prevalence of hITBS were runners with a higher chronic disease composite score (PR = 2.38 times increased risk for every 2 additional chronic diseases; p < 0.0001), and a history of any allergies (PR = 1.90; p < 0.0001).
Discussion
This study aimed to identify selected independent risk factors associated with a history of ITBS (hITBS) in distance runners entering the 2012–2015 Two Oceans Marathon races (21.1 km and 56 km). The first main finding of this study was that a significantly higher prevalence of hITBS was associated with participation in longer race distance (56 km) race entrants, female sex, younger age, fewer number of years of recreational running, slower average running speed. A second main finding is that independent risk factors associated with hITBS in distance runners (multiple regression analysis adjusting for age, sex, and race distance) were a higher chronic disease composite score and a history of any allergies.
Race distance, running experience, running speed, sex, age and hITBS
In this study there was a significantly higher prevalence of hITBS in the 56 km vs. 21.1 km race participants entrants (PR = 1.40; p < 0.0001). Common contributors to the development of hITBS are training-related factors such as a sudden increase in training load [Citation27] and running experience. There is limited evidence supporting that a sudden change in training load is linked with an increased risk of a running-related injury [Citation47]. In our study, we noted that the average weekly training/running distance in the last 12 months was not associated with a higher prevalence of hITBS (p = 0.80).
It is suggested that novice runners who increased their running distance by more than 30% over a 2-week period appear to be more vulnerable to distance-related injuries such as ITBS compared with runners that increase their running distance by less than 10% [Citation48]. A meta-analysis concluded that novice runners have a significantly greater risk of injury per 1000 hours of running than recreational runners [Citation15]. In our study we noted that fewer years of recreational running experience was related to a higher prevalence of hITBS, with a 6% increase in risk for every 5 years of less experience as a recreational runner. Research has shown that experienced runners’ past experiences and knowledge play a significant role in shaping their preventive behaviors [Citation49]. It was also shown that the risk of injury is highest for less experienced runners who run at a slower speed [Citation50]. We report that a slower average running speed was also associated with a higher prevalence of hITBS, with a 2% increase in risk for every 1 km/hr decrease in average running speed. Slow running may reduce the angle of knee flexion at foot strike [Citation28], and a runner’s state of exhaustion may also be contributing factors in the development of ITBS [Citation36]. It has also been documented that for a given running distance, slow-speed running decreases knee joint loads per stride but, conversely, increases the cumulative load at the knee joint compared to faster running [Citation51]. The primary reason proposed for the increase in cumulative load at slower speeds is the increase in the number of strides needed to cover the same distance [Citation51]. Although speculative, the cumulative load on the ITB could also potentially be increased by slower running for a given distance. Another study concluded that small decreases in step width can substantially increase ITB strain as well as strain rates [Citation52]. The existing body of research consistently demonstrates that increasing cadence within the range of 5% to 30% from the habitual cadence can confer protective mechanical benefits against injury. Several studies collectively support the notion that cadence modification is a valuable approach for mitigating the risk of musculoskeletal injuries during physical activity [Citation53–55]. There was no significant interaction found between years as a recreation runner and running speed (p = 0.07).
In our study we show that a higher prevalence of hITBS was observed among female vs. male runners (PR = 1.4; p < 0.0001). Multiple studies have highlighted the biomechanical risk factors associated with ITBS in runners, with a particular focus on female athletes. A systematic review provided quantitative evidence about the biomechanical risk factors associated with ITBS in runners, indicating that there is evidence that females diagnosed with ITBS display increased hip adduction and knee internal rotation angles [Citation28,Citation29]. As a result, this may exert an additional strain on the hip abductor muscles eccentrically, causing compression of the ITB against the greater trochanter or lateral femoral condyle, potentially leading to a greater prevalence of symptoms among female runners [Citation56]. Furthermore, another study showed different alterations in running kinematics and attributed a greater strain on the iliotibial band (ITB) in female runners to their greater peak hip adduction angle and knee internal rotation angle [Citation57].
Chronic disease history and hITBS
With physical activity being included in lifestyle modification, the popularity of distance running is increasing, and more older runners are entering races. Older runners are also likely to have a higher prevalence of chronic medical conditions such as cardiovascular diseases, diabetes and others. Recent publications report the relationship between chronic diseases (and allergies) and recurrent injuries [Citation58], as well as between chronic diseases (and allergies) and all GORRIs [Citation37–39,Citation59]. More specifically, recently published cross-sectional studies on endurance athletes in cohorts of 29 585 distance runners [Citation38], 2 824 trail runners [Citation37] and 21 824 recreational road cyclists [Citation40] report that GORRIs were associated with a history of multiple chronic diseases and any allergies. Results in recent cross-sectional studies conducted among distance runners concluded chronic diseases and allergies are associated risk factors for Medial Tibial Stress Syndrome (MTSS) [Citation39] and Patellofemoral Pain Syndrome (PFPS) [Citation59].
In this study, we show that hITBS is associated with a higher chronic disease composite score and a history of any allergies, independent of other risk factors. For every additional two chronic diseases, the risk of hITBS increases 2.4 times. A history of any allergies is associated with a 1.9 increase in the risk of hITBS. The specific chronic disease variables associated with the highest prevalence of hITBS in distance runners (in univariate) were those with any GIT disease (PR = 3.1), hematological/immune disease (PR = 2.8), kidney/bladder disease (PR = 2.6), nervous system/psychiatric disease (PR = 2.3), respiratory disease (PR = 2.2) and any symptoms of CVD (PR = 2.2).
As this is a descriptive cross-sectional study, we cannot draw any inferences on the cause-and-effect relationship between hITBS and any of the identified independent risk factors such as a history of chronic diseases or allergies. There is no obvious direct link between a history of ITBS in runners and chronic diseases or allergies, and in this study we did not explore any potential mechanisms to explain such a link. However, there is some evidence that chronic diseases may be related to an increased risk of musculoskeletal injury in runners [Citation38]. Chronic diseases may affect bony and soft tissue structures, either directly due to the underlying disease process and/or indirectly, through the use of certain chronic medication [Citation38]. There is evidence from several studies that a wide range of chronic diseases are associated with an increased risk of musculoskeletal injuries including bone stress injuries (chronic obstructive pulmonary disease (COPD) [Citation60] and tendinopathy (obesity, hypercholesteremia and diabetes mellitus) [Citation61–64]. There is also evidence that several medications used to treat chronic diseases are associated with an increased risk of musculoskeletal pathology, such as osteoporosis (corticosteroids) [Citation65], osteopenia (proton pump inhibitors, corticosteroids) [Citation65], tendon ruptures (corticosteroids) [Citation66], myopathy (statins and corticosteroids) [Citation67,Citation68] and tendinopathy (fluoroquinolones [Citation69], statins [Citation64,Citation70], corticosteroids [Citation66], aromatase inhibitors [Citation71], and isotretinoin [Citation38]. [Citation72] These potential musculoskeletal side effects of commonly used chronic medication could theoretically cause alteration in the biomechanics that could influence the development of ITBS.
For example, current thinking is that the pathology of ITBS is related to a complex relationship between the ITB and mechanical function of the in-series hip musculature (gluteus maximus and the tensor fascia lata) [Citation34] and that rehabilitation should target neuromuscular control, endurance, and strength of the hip-abductor and external-rotator muscles [Citation21]. Given that both chronic disease and medication used to treat chronic disease may negatively affect, for example muscle adaptation to load and healing, this can potentially increase the risk of developing GORRIs such as ITBS [Citation38]. However, this is speculative and further study is required to explore these possible mechanisms. At this stage, clinicians that manage runners with ITBS by implementing injury prevention and rehabilitation programs, should just be aware of the possible association between ITBS and chronic diseases.
Allergy history and hITBS
We show that a history of allergies was an independent risk factor associated with hITBS (PR = 2.43 p < 0.0001). These findings are similar to those reported for any GORRIs, MTSS and PFPS in distance runners [Citation38,Citation39,Citation59] and trail runners [Citation37]. Available literature suggests there are significant numbers of ultramarathon runners who have had allergies and hay fever, with a point prevalence that varies between 25% and 42% [Citation73,Citation74]. As with chronic diseases, the association between a history of allergies and ITBS may be directly due to the underlying disease process and/or indirectly, through the use of medication to treat allergies. There is evidence linking allergies to low bone mineral density and osteoporosis among adults and children, possibly due to chronic inflammation [Citation75,Citation76]. Furthermore, common treatments for allergies are histamine receptor antagonists (antihistamines) and corticosteroids, both of which can negatively affect muscle adaptation to load and healing, thereby indirectly be associated with hITBS [Citation77–80]. Again, this is speculative and further study is required to explore these possible mechanisms and at this stage, clinicians should just be aware of the possible association between ITBS and allergies.
Strengths and limitations
One of the strengths of this descriptive cross-sectional study is that it is a very large study investigating selected risk factors associated with hITBS in distance runners in a multiple regression analysis. Over a 4-year period, comprehensive data were collected on all race entrants who registered and started the race. In addition, we report a high race participant consenting cohort (71.8%). The multiple regression analysis and the large sample size allow for valid results with narrow confidence limits. The study also has several limitations. The diagnosis of ‘iliotibial band syndrome (ITBS)’ relied on self-reporting by participants and injury data are also limited by recall bias. We did implement measures to increase the accuracy of this diagnosis by including only race entrants who also consulted a health professional that could verify their diagnosis. It would be of interest to explore whether the identified risk factors are different between the 21 km vs 56 km race entrants however because of sample size we could not run analysis separately for the two race distances. We acknowledge that by postulating that these variables are potential risk factors associated with a hITBS, we cannot make any causal inference to specifically guide injury prevention and treatment interventions. We use the term ‘associated with’ to refer to the relationship between these potential risk predictors and a hITBS, but we recognize the association does not imply a cause-effect relationship [Citation81]. We also acknowledge that the magnitude of this statistical association (about 2X higher risk of chronic disease and allergies in runners with a history of ITBS) does not necessarily reflect the same magnitude when it relates to clinical significance, and this requires further study.
Conclusions
The prevalence of hITBS was significantly higher in the following runners: longer race distance (56 km), female sex, younger age, fewer years of recreational running experience and runners with a slower average running speed. Furthermore, distance runners with multiple chronic diseases and a history of allergies, who enter a mass community-based ultra-marathon event (56 km and 21.1 km), are at higher risk of hITBS. The risk factors associated with ITBS are complex; therefore, besides a comprehensive history of extrinsic risk factors and a detailed biomechanical evaluation focussing on the hip muscle complex, clinicians should be aware that chronic diseases and allergies are risk factors associated with a hITBS in runners. Although speculative, underlying chronic disease and allergies may affect the response and adaptation of tissue (e.g. muscle) to loading, and this has implications when prescribing exercise for patients with chronic diseases and during rehabilitation of patients presenting with ITBS. However, further research is needed to determine the cause-effect of these novel risk factors associated with ITBS in distance runners. Future targeted and prospective studies should be able to improve risk factor knowledge and result in the implementation of effective preventive measures.
What are the new findings?
There was a significantly higher (p < 0.0001) prevalence of hITBS in the longer race distance entrants (56 km), females, younger entrants, fewer years of recreational running (PR = 1.07; p = 0.0009) and slower average running speed (PR = 1.02; p = 0.0066).
Runners with a higher chronic disease composite score (PR = 2.4 times increased risk for every 2 additional chronic diseases) are at higher risk of reporting a history of ITBS.
Distance runners with a history of any allergies are 1.90 times more likely to report a history of ITBS.
How it might impact clinical practice in the near future?
Clinicians should be aware that chronic diseases and allergies are risk factors associated with hITBS in distance runners. Although speculative, underlying chronic disease; allergies and potentially the medication to treat chronic disease or allergies may affect the response and adaptation of tissue (e.g. muscle) to loading, and this has implications when prescribing exercise for patients with chronic diseases and during rehabilitation of patients presenting with ITBS.
Author contributorship
Jandre V. Marais (JVM): study concept, study planning, data interpretation, manuscript (first draft), manuscript editing
Audrey Jansen van Rensburg (AJvR): study concept, study planning, data interpretation, manuscript (first draft), manuscript editing
Martin P. Schwellnus (MS): responsible for the overall content as guarantor, study concept, study planning, data interpretation, manuscript, manuscript editing, facilitating funding
Esme Jordaan (EJ): data cleaning, study planning, data analysis including statistical analysis, data interpretation, manuscript editing
Pieter Boer (PB): data analysis including statistical analysis, data interpretation, manuscript editing
Ethical approval statement
Ethics approval was obtained from both the Research Ethics Committees of the University of Cape Town (REC 009/2011) and the University of Pretoria (REC 433/2015 and 700/2019) before the onset of the study.
Submission statement
Our manuscript represents original work and has not been previously published or simultaneously submitted elsewhere for publication. All authors have approved the manuscript submission to The Physician and Sportsmedicine journal.
Acknowledgements
The authors would like to thank the Two Oceans Marathon medical staff and the Two Oceans Marathon race organizers for their support. A special thanks to all the runners participating in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
No additional data is available.
Additional information
Funding
References
- Khan KM, Thompson AM, Blair SN, et al. Sport and exercise as contributors to the health of nations. Lancet. Jul 7 2012;380(9836):59–64. doi: 10.1016/s0140-6736(12)60865-4
- Kohl HW 3rd, Craig CL, Lambert EV, et al. The pandemic of physical inactivity: global action for public health. Lancet. Jul 21 2012;380(9838):294–305. doi: 10.1016/s0140-6736(12)60898-8
- World Health Organization Guidelines Approved by the Guidelines Review Committee. Global recommendations on physical activity for health. World Health Org. WHO Guidelines Approved by the Guidelines Review Committee; 2010. https://iris.who.int/bitstream/handle/10665/44399/9789241599979_eng.pdf?sequence=1.
- Hills AP, Street SJ, Byrne NM. Physical activity and health: “what is old is new again”. Adv Food Nutr Res. 2015;75:77–95. doi: 10.1016/bs.afnr.2015.06.001
- van Gent RN, Siem D, van Middelkoop M, et al. Incidence and determinants of lower extremity running injuries in long distance runners: a systematic review. Br J Sports Med. 2007 Aug;41(8):469–480. discussion 480. doi: 10.1136/bjsm.2006.033548
- van der Worp MP, van der Horst N, de Wijer A, et al. Iliotibial Band Syndrome in Runners. Sports Med. 2012;42(11):969–992. doi: 10.1007/BF03262306
- Bahr R, Clarsen B, Derman W, et al. International olympic committee consensus statement: methods for recording and reporting of epidemiological data on injury and illness in sport 2020 (including STROBE Extension for Sport Injury and Illness Surveillance (STROBE-SIIS)). Br J Sports Med. 2020;54(7):372–389. doi: 10.1136/bjsports-2019-101969
- Winter SC, Gordon S, Brice SM, et al. A multifactorial approach to overuse running injuries: a 1-year prospective study. Sports Health. 2020 May;12(3):296–303. doi: 10.1177/1941738119888504
- Gao Z, Mei Q, Fekete G, et al. The effect of prolonged running on the symmetry of biomechanical variables of the lower limb joints. Symmetry. 2020;12(5):720. doi: 10.3390/sym12050720
- Gao Z, Fekete G, Baker JS, et al. Effects of running fatigue on lower extremity symmetry among amateur runners: from a biomechanical perspective. Front physiol. 2022;13:899818. doi: 10.3389/fphys.2022.899818
- Dempster J, Dutheil F, Ugbolue UC. The prevalence of lower extremity injuries in running and associated risk factors: a systematic review. Physical Activity And Health. 2021;5(1):133–145. doi: 10.5334/paah.109
- Strauss EJ, Kim S, Calcei JG, et al. Iliotibial band syndrome: evaluation and management. J Am Acad Orthop Surg. 2011 Dec;19(12):728–736. doi: 10.5435/00124635-201112000-00003
- van der Worp MP, ten Haaf DS, van Cingel R, et al. Injuries in runners; a systematic review on risk factors and sex differences. PLOS ONE. 2015;10(2):e0114937. doi: 10.1371/journal.pone.0114937
- Tonoli DC, Cumps E, Aerts I, et al. Incidence, risk factors and prevention of running related injuries in long-distance running: a systematic review. Sport & Geneeskunde. 2010;43(5):12–18.
- Videbaek S, Bueno AM, Nielsen RO, et al. Incidence of running-related injuries per 1000 h of running in different types of runners: a systematic review and meta-analysis. Sports Med. 2015 Jul;45(7):1017–1026. doi: 10.1007/s40279-015-0333-8
- Taunton JE, Ryan MB, Clement DB, et al. A retrospective case-control analysis of 2002 running injuries. Br J Sports Med. 2002;36(2):95–101. doi: 10.1136/bjsm.36.2.95
- Pegrum J, Self A, Hall N. Iliotibial band syndrome. BMJ. 2019;l980. doi: 10.1136/bmj.l980
- Fairclough J, Hayashi K, Toumi H, et al. Is iliotibial band syndrome really a friction syndrome? J Sci Med Sport. 2007 Apr;10(2):74–76. discussion 77-8. doi: 10.1016/j.jsams.2006.05.017
- Renne JW. The iliotibial band friction syndrome. J Bone Joint Surg Am. 1975 Dec;57(8):1110–1111. doi: 10.2106/00004623-197557080-00014
- Noble CA. The treatment of iliotibial band friction syndrome. Br J Sports Med. 1979 Jun;13(2):51–54. doi: 10.1136/bjsm.13.2.51
- Geisler PR. Iliotibial band pathology: synthesizing the available evidence for clinical progress. J Athl Train. 2020;56(8):805–815. doi: 10.4085/1062-6050-548-19
- Geisler PR, Lazenby T. Iliotibial band impingement syndrome: an evidence-informed clinical paradigm change. Int J Athl Train. 2017;22(3):1–11. doi: 10.1123/ijatt.2016-0075
- Baker RL, Souza RB, Fredericson M. Iliotibial band syndrome: soft tissue and biomechanical factors in evaluation and treatment. PM & R: J Injury, Function, and Rehabilitation. 2011 Jun;3(6):550–561. doi: 10.1016/j.pmrj.2011.01.002
- Orchard JW, Fricker PA, Abud AT, et al. Biomechanics of iliotibial band friction syndrome in runners. Am J Sports Med. 1996 May;24(3):375–379. doi: 10.1177/036354659602400321
- Fredericson M, Weir A. Practical management of iliotibial band friction syndrome in runners. Clin J Sport Med. 2006;16(3):261–268. doi: 10.1097/00042752-200605000-00013
- Fredericson M, Wolf C. Iliotibial band syndrome in runners: innovations in treatment. Sports Med. 2005;35(5):451–459. doi: 10.2165/00007256-200535050-00006
- Louw M, Deary C. The biomechanical variables involved in the aetiology of iliotibial band syndrome in distance runners – a systematic review of the literature. Phys Ther Sport. 2014;15(1):64–75. doi: 10.1016/j.ptsp.2013.07.002
- Aderem J, Louw QA. Biomechanical risk factors associated with iliotibial band syndrome in runners: a systematic review. BMC Musculoskelet Disord. Nov 16 2015;16(1):356. doi: 10.1186/s12891-015-0808-7
- Noehren B, Davis I, Hamill J. ASB clinical biomechanics award winner 2006: prospective study of the biomechanical factors associated with iliotibial band syndrome. Clin Biomech (Bristol, Avon). 2007;22(9):951–956. doi: 10.1016/j.clinbiomech.2007.07.001
- Fredericson M, Cookingham CL, Chaudhari AM, et al. Hip abductor weakness in distance runners with iliotibial band syndrome. Clin J Sport Med. 2000 Jul;10(3):169–175.
- MacMahon JM, Chaudhari AM, Andriacchi TP. Biomechanical injury predictors for marathon runners: striding towards iliotibial band syndrome injury prevention. In Hong, editor. Proceedings of XVIII International symposium on biomechanics in sports, Hong Kong; 2000 Jun 25–30; Symposium 2000, Hong Kong, China. Department of Sports Science and Physical Education The Chinese University of Hong Kong, c2000; 2000. p. 456–459. https://ojs.ub.uni-konstanz.de/cpa/issue/view/ISBS2000
- Ferber R, Noehren B, Hamill J, et al. Competitive female runners with a history of iliotibial band syndrome demonstrate atypical hip and knee kinematics. J Orthop Sports Phys Ther. 2010 Feb;40(2):52–58. doi: 10.2519/jospt.2010.3028
- Niemuth PE, Johnson RJ, Myers MJ, et al. Hip muscle weakness and overuse injuries in recreational runners. Clin J Sport Med. 2005 Jan;15(1):14–21.
- Hutchinson LA, Lichtwark GA, Willy RW, et al. The iliotibial band: a complex structure with versatile functions. Sports Med. 2022 May 01;52(5):995–1008.
- Beals C, Flanigan D. A review of treatments for iliotibial band syndrome in the athletic population. J Sports Med. 2013 Oct 02;2013: 367169. doi: 10.1155/2013/367169
- Chen S, Wang Y, Bing F, et al. Effects of running speeds and exhaustion on iliotibial band strain during running. Bioengineering. 2023;10(4):417. doi: 10.3390/bioengineering10040417
- Viljoen CT, Sewry N, Schwellnus MP, et al. Independent risk factors predicting gradual onset injury in 2824 trail running race entrants: SAFER XVIII study. Wilderness Environ Med. 2021;32(3):293–301. doi: 10.1016/j.wem.2021.04.002
- Mokwena PL, Schwellnus MP, Van Rensburg AJ, et al. Chronic disease, allergies, and increased years of running are risk factors predicting gradual onset running-related injuries in ultramarathon runners—SAFER XIX study in 29 585 race entrants. Clin J Sport Med. 2021;32(4):e422–e429. doi: 10.1097/jsm.0000000000000949
- Boer P-H, Schwellnus MP, Jordaan E. Chronic diseases and allergies are risk factors predictive of a history of medial tibial stress syndrome (MTSS) in distance runners: SAFER study XXIV. Phys Sportsmed. 2022;51(2):1–9. doi: 10.1080/00913847.2021.2021597
- du Toit F, Schwellnus M, Wood P, et al. History of chronic disease is a novel intrinsic risk factor associated with gradual onset injuries in recreational road cyclists: a cross-sectional study in 21,824 cyclists – SAFER XIV. Phys Ther Sport. 2020 Nov;46:137–144. doi: 10.1016/j.ptsp.2020.08.008
- Borjesson M, Urhausen A, Kouidi E, et al. Cardiovascular evaluation of middle-aged/senior individuals engaged in leisure-time sport activities: position stand from the sections of exercise physiology and sports cardiology of the European association of cardiovascular prevention and rehabilitation. Eur J Cardiovasc Prev Rehabil. 2011 Jun;18(3):446–458.
- Rotunno A, Schwellnus MP, Swanevelder S, et al. Novel factors associated with analgesic and anti-inflammatory medication use in distance runners: pre-race screening among 76 654 race entrants—SAFER Study VI. Clin J Sport Med. 2018 Sep;28(5):427–434. doi: 10.1097/jsm.0000000000000619
- Schwabe K, Schwellnus M, Swanevelder S, et al. Leisure athletes at risk of medical complications: outcomes of pre-participation screening among 15,778 endurance runners – SAFER VII. Phys Sportsmed. 2018 Nov;46(4):405–413.
- Schwellnus M, Swanevelder S, Derman W, et al. Prerace medical screening and education reduce medical encounters in distance road races: SAFER VIII study in 153 208 race starters. Br J Sports Med. 2019 May;53(10):634–639. doi: 10.1136/bjsports-2018-099275
- Sewry N, Schwellnus M, Borjesson M, et al. Pre-race screening and stratification predicts adverse events-A 4-year study in 29585 ultra-marathon entrants, SAFER X. Scand J Med Sci Sports. 2020 Jul;30(7):1205–1211. doi: 10.1111/sms.13659
- Viljoen CT, Janse van Rensburg DC, Verhagen E, et al. Epidemiology of injury and illness among trail runners: a systematic review. Sports Med. 2021 May;51(5):917–943. doi: 10.1007/s40279-020-01418-1
- Damsted C, Glad S, Nielsen RO, et al. Is there evidence for an association between changes in training load and running-related injuries? a systematic review. Int J Sports Phys Ther. 2018 Dec;13(6):931–942.
- Nielsen RO, Parner ET, Nohr EA, et al. Excessive progression in weekly running distance and risk of running-related injuries: an association which varies according to type of injury. J Orthop Sports Phys Ther. 2014 Oct;44(10):739–747. doi: 10.2519/jospt.2014.5164
- Evert V, Marit W, Caroline Silveira B. ‘I just want to run’: how recreational runners perceive and deal with injuries. BMJ Open Sport Exerc Med. 2021;7(3):e001117. doi: 10.1136/bmjsem-2021-001117
- Damsted C, Parner ET, Sørensen H, et al. ProjectRun21: Do running experience and running pace influence the risk of running injury—A 14-week prospective cohort study.J Sci Med Sport. 2019 [Mar/1/2019];22(3):281–287. doi: 10.1016/j.jsams.2018.08.014
- Petersen J, Sørensen H, Nielsen RØ. Cumulative loads increase at the knee joint with slow-speed running compared to faster running: a biomechanical study. J Orthop Sports Phys Ther. 2015 Apr 01;45(4):316–322. doi: 10.2519/jospt.2015.5469
- Meardon SA, Campbell S, Derrick TR. Step width alters iliotibial band strain during running. Sports Biomech. 2012;11(4):464–472. doi: 10.1080/14763141.2012.699547
- Schubert AG, Kempf J, Heiderscheit BC. Influence of stride frequency and length on running mechanics: a systematic review. Sports Health. 2014 May;6(3):210–217. doi: 10.1177/1941738113508544
- Heiderscheit BC, Chumanov ES, Michalski MP, et al. Effects of step rate manipulation on joint mechanics during running. Med Sci Sports Exerc. 2011 Feb;43(2):296–302.
- Lenhart RL, Thelen DG, Wille CM, et al. Increasing running step rate reduces patellofemoral joint forces. Med Sci Sports Exerc. 2014 Mar;46(3):557–564.
- Charles D, Rodgers C. aliterature review and clinical commentary on the development of iliotibial band syndrome in runners. Int J Sports Phys Ther. 2020;15(3):460–470. doi: 10.26603/ijspt20200460
- Suárez Luginick B, Rueda Ojeda J, Collazo García C, et al. Kinematics of recreational runners with iliotibial band injury. J Human Sport Exercise. 2018;13(3). doi: 10.14198/jhse.2018.133.19
- Swanevelder S, Sewry N, Schwellnus M, et al. Predictors of multiple injuries in individual distance runners: a retrospective study of 75,401 entrants in 4 annual races–SAFER XX. J Sport Health Sci. 2021 Nov 18;11(3):339–346.
- Marandure TT, Schwellnus MP, Grant C, et al. Patellofemoral pain syndrome is associated with chronic disease and allergies in 60 997 distance runner race entrants: SAFER XXX Study. Clin J Sport Med. 2023;33(6):603–610. doi: 10.1097/JSM.0000000000001166
- Graat-Verboom L, Spruit MA, van den Borne BE, et al. Correlates of osteoporosis in chronic obstructive pulmonary disease: an underestimated systemic component. Respir med. 2009 Aug;103(8):1143–1151.
- Abate M, Schiavone C, Salini V, et al. Occurrence of tendon pathologies in metabolic disorders. Rheumatology (Oxford). 2013 Apr;52(4):599–608. doi: 10.1093/rheumatology/kes395
- Applegate KA, Thiese MS, Merryweather AS, et al. Association between cardiovascular disease risk factors and rotator cuff tendinopathy: a cross-sectional study. J Occup Environ Med. 2017 Feb;59(2):154–160.
- Lin TT, Lin CH, Chang CL, et al. The effect of diabetes, hyperlipidemia, and statins on the development of rotator cuff disease: a nationwide, 11-year, longitudinal, population-based follow-up study. Am J Sports Med. 2015 Sep;43(9):2126–2132. doi: 10.1177/0363546515588173
- Marie I, Delafenêtre H, Massy N, et al. Tendinous disorders attributed to statins: a study on ninety-six spontaneous reports in the period 1990-2005 and review of the literature. Arthritis Rheum. Mar 15 2008;59(3):367–372. doi: 10.1002/art.23309
- Rice JB, White AG, Scarpati LM, et al. Long-term systemic corticosteroid exposure: a systematic literature review. Clin Ther. 2017 Nov;39(11):2216–2229.
- Blanco I, Krähenbühl S, Schlienger RG. Corticosteroid-associated tendinopathies: an analysis of the published literature and spontaneous pharmacovigilance data. Drug Saf. 2005;28(7):633–643. doi: 10.2165/00002018-200528070-00005
- Mammen AL, Longo DL. Statin-associated autoimmune myopathy. N Engl J Med. Feb 18 2016;374(7):664–669. doi: 10.1056/NEJMra1515161
- Gupta A, Gupta Y. Glucocorticoid-induced myopathy: pathophysiology, diagnosis, and treatment. Indian J Endocrinol Metab. 2013 Sep;17(5):913–916. doi: 10.4103/2230-8210.117215
- Mandell L, Tillotson G. Safety of fluoroquinolones: an update. Can J Infect Dis. 2002 Jan;13(1):54–61. doi: 10.1155/2002/864789
- Mansi I, Frei CR, Pugh MJ, et al. Statins and musculoskeletal conditions, arthropathies, and injuries. JAMA Intern Med. 2013;173(14):1318–1326. doi: 10.1001/jamainternmed.2013.6184
- Vuillemin V, Guerini H, Bard H, et al. Stenosing tenosynovitis. J Ultrasound. 2012 Feb;15(1):20–28. doi: 10.1016/j.jus.2012.02.002
- Kirchgesner T, Larbi A, Omoumi P, et al. Drug-induced tendinopathy: from physiology to clinical applications. Joint Bone Spine. 2014 Dec;81(6):485–492. doi: 10.1016/j.jbspin.2014.03.022
- Robson-Ansley P, Howatson G, Tallent J, et al. Prevalence of allergy and upper respiratory tract symptoms in runners of the London marathon. Med Sci Sports Exerc. 2012 Jun;44(6):999–1004.
- Hoffman MD, Krishnan E, Lucia A. Health and exercise-related medical issues among 1,212 ultramarathon runners: baseline findings from the Ultrarunners Longitudinal TRAcking (ULTRA) Study. PLOS ONE. 2014;9(1):e83867–e83867. doi: 10.1371/journal.pone.0083867
- Barrick BJ, Jalan S, Tollefson MM, et al. Associations of self-reported allergic diseases and musculoskeletal problems in children: a US population-based study. Ann Allergy Asthma Immunol. 2017 Aug;119(2):170–176.
- Sirufo MM, Suppa M, Ginaldi L, et al. Does allergy break bones? Osteoporosis and its connection to allergy. Int J Mol Sci. 2020;21(3):712. doi: 10.3390/ijms21030712
- Wolak M, Bojanowska E, Staszewska T, et al. Histamine augments collagen content via H1 receptor stimulation in cultures of myofibroblasts taken from wound granulation tissue. Mol Cell Biochem. 2021 Feb;476(2):1083–1092. doi: 10.1007/s11010-020-03974-6
- Nacht S, Garzon P. Effects of corticosteroids on connective tissue and fibroblasts. Adv Steroid Biochem Pharmacol. 1974 Jan 1;4:157–187. doi: 10.1016/B978-0-12-037504-2.50007-9
- Crowther CL. The effects of corticosteroids on the musculoskeletal system. Orthop Nurs. 2001 Nov;20(6):33–7; quiz 37–9. doi: 10.1097/00006416-200111000-00008
- Stone S, Malanga GA, Capella T. Corticosteroids: review of the history, the effectiveness, and adverse effects in the treatment of joint pain. Pain Physician. 2021 Jan;24(S1):S233–s246.
- Nielsen RO, Simonsen NS, Casals M, et al. Methods matter and the ‘too much, too soon’ theory (part 2): what is the goal of your sports injury research? Are you describing, predicting or drawing a causal inference? Br J Sports Med. 2020;54(22):1307–1309. doi: 10.1136/bjsports-2020-102144