?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In this work, we present the results from a study of the speciation of the ternary systems formed by copper(II),2-(benzo(d)oxazol-2-yl)nicotinic acid (Oxa, HL) as the main ligand, and the amino acids Serine (Ser, A–), Threonine (Thr, A–), Methionine (Met, A–), Phenylalanine (Phe, A–), Aspartate (Asp, A2–), Glutamate (Glu, A2–), and Histidine (His, A–) as secondary ones. The potentiometric data was analyzed using the computational program LETAGROP for systems in aqueous solution at 25 °C and 1.0 M KNO3 as the ionic medium. The ternary complexes [Cu(L)(HA)]+, [Cu(L)(A)], [Cu(L)2(A)]–, [Cu(L)(A)2]–, [Cu(L)(A)(OH)]– and [Cu(L)(A)(OH)2]2– were detected. The relative stability of the ternary complexes was compared to the binary ones considering the values of ΔlogK and logχ (defined in text of results and analysis). Subsequently using the formation constants, the species distribution diagrams were generated and are briefly discussed. The UV–visible spectra of the ternary complexes show a maximum at 610–654 nm indicating that the Cu2+ is penta-coordinated.
1. Introduction
The N,N-donor aromatic ligand 2,2′-bipyridine (Bipy) is an important ligand whose chemistry has become of great interest for medicinal chemistry due to the variety of medicinal activities derived from its structural properties. One of these properties is the ability to be a strong chelating agent capable to bind in a bidentate way to biologically essential metallic trace elements, showing preference for metal ions that are harder than softer acids. When the ligand is coordinated to a metal ion, its rigid planar aromatic structure causes the complex intercalation between the planar base of DNA and RNA, inducing a desired cytotoxic activity [Citation1–6].
Copper is an essential element present in all living beings playing a major role in their biochemistry [Citation7–9]. Several copper-based complexes exhibit antibacterial activity [Citation10–14]. Our group has reported the antibacterial activity of ternary complexes formed by Cu(II), 2,2-bipyridyne and several aminoacids [Citation15], and several research papers have reported numerous Cu(II)-based complexes with cytotoxic activities [Citation16–19].
The ligand 2-(benzo[d]oxazol-2-yl) nicotinic acid (L–) it’s a compound that is structurally analogous to 2,2′-bipyridine. As seen in , both ligands are expected to coordinate in a similar way by the two nitrogen atoms forming 5-membered chelate rings. As evidence of this is the fact that all binary species in the Cu(II)–L system had their analogous counterpart in the Cu(II)–Bipy system and also with similar formation constants [Citation20]. Because of this is expected that L mimic Bipy high capacity to strongly coordinate several kinds metal ions as also its capacity to induce desired medicinal activities like antibacterial and cytotoxicity. When a ligand is introduced into the body to be coordinated to Cu(II) and form a complex with a desired medicinal activity (induced or increased by the ligand) [Citation1,Citation2], it is necessary to consider the presence of molecules that will compete with the main ligand for coordination sites on the metal nucleus. This favors the formation of ternary complexes leading to a possible change in the medicinal activity [Citation2,Citation13]. Due to the great competition between the ligands to form complexes, it is difficult to determine precisely the speciation of the system. To address this issue, speciation is studied in the system formed by the Cu(II), Oxa as main ligand and one of the possible ligands in blood, in a medium very similar to blood serum (usually aqueous medium). In this work we extended the study to include the amino acids Serine (Ser, A–), Threonine (Thr, A–), Methionine (Met, A–), Phenylalanine (Phe, A–), Aspartate (Asp, A2–), Glutamate (Glu, A2–), and Histidine (His, A–). The findings from this work contribute to the understanding of the mixed-ligand copper(II) complexes with heterocyclic ligands and amino acids in aqueous solution.
2. Experimental
2.1. General information
Cu(NO3)2·3H2O (Merck p.a.) and the amino acids Serine (Ser, A–), Threonine (Thr, A–), Methionine (Met, A–), Phenylalanine (Phe, A–), Aspartate (Asp, A2–), Glutamate (Glu, A2–), and Histidine (His, A–) all Merck p.a. commercial products were used without further purification. HNO3 and KOH solutions were prepared using 100.0 mM Titrisol Merck ampoules. The KOH solution was standardized against potassium hydrogen phthalate (Merck p.a.) using phenolphthalein as indicator, and the HNO3 solution was standardized with the KOH solution of known concentration. The solutions were prepared using triply glass-distilled water previously boiled to remove any dissolved CO2. The Cu(II) stock solution was standardized using a Na2EDTA·2H2O (Merck p.a.) solution (0.01 mol dm−3) in a buffer media (pH = 10) using murexide (Merck p.a.) as indicator. The heterocyclic ligand 2-(benzo(d)oxazol-2-yl)nicotinic acid (L–) was synthesized according to a method previously reported [Citation20].
Potentiometric measurements were carried out in aqueous solution using 1.0 mol dm−3 KNO3 as ionic medium and nitrogen free of CO2.
2.2. Potentiometric methods
The measurements were performed using a total metal concentration MT = 2–3 mM and molar ratios R = 1:1:1, 1:1:2 and 1:2:1, respectively.
The Cu2+-L-Amino Acids (A) systems were studied according to the reaction scheme:(1)
(1) where A–n represents the amino acids Serine (Ser, A–), Threonine (Thr, A–), Methionine (Met, A–), Phenylalanine (Phe, A–), Aspartate (Asp, A2–), Glutamate (Glu, A2–), and Histidine (His, A–). [Cuq(OH)p(L)r(A)s]2q–r–sn–k are the ternary (p, q, r, s) complexes, where βkqrs denote the respective stability constants.
The potentiometric data was analyzed using the program LETAGROP [Citation21, Citation22] with the objective to minimize the ZB = (H-h)/MT function, where ZB denotes the average number of moles of H+ associated per mol of metal, H is the total (analytical) concentration of H+, h is the H+ equilibrium concentration and MT represents the total (analytical) concentration of Cu(II).
Equilibria corresponding to the formation of both the hydroxy Cu(II) complexes and the binary systems Cu(II)-An– and Cu(II)-L at the same experimental conditions were considered in the calculation of the stability constants of the ternary complexes, all they were previously studied by our group [Citation20,Citation23] and are showed in .
Table 1. Reported stability constants of the hydroxo complexes of Cu(II) at 25 °C and KNO3 1 M as ionic medium [Citation23].
Table 2. Reported stability constants of the binary complexes formed in the systems Cu(II)–HSer, Cu(II)–HMet, Cu(II)–HPhe and Cu(II)–HThr at 25 °C and KNO3 1 M as ionic medium [Citation23].
Table 3. Reported stability constants of the binary complexes formed in the systems Cu(II)–H2Asp, Cu(II)–H2Glu and Cu(II)–HHis at 25 °C and KNO3 1 M as ionic medium [Citation23].
Table 4. Reported stability constants of the binary complexes formed in the system Cu(II)– HOxa at 25 °C and KNO3 1 M as ionic medium [Citation20].
The stability constants of the Cu(II) hydroxy complexes, the protonation constants of the ligands and the stability constants of the binary complexes were kept fixed during the analysis.
The aim was to find a complex or set of complexes that resulted in the lowest sum of squared errors, The fittings were done by testing different (p, q, r) and (p, q, r, s) combinations.
The species distribution diagrams were generated using the HySS computer program [Citation24] yielding the βpqrs values summarized in and .
Table 5. Ternary species with corresponding formation constants for the H+-Cu(II)-L-Ser, H+-Cu(II)-L-Thr, H+-Cu(II)-L-Met and H+-Cu(II)-L-Phe systems at KNO3 1.0 M and 25 °C.
Table 6. Ternary species with corresponding formation constants for the H+-Cu(II)-L-Asp, H+-Cu(II)-L-Glu and H+-Cu(II)-L-His systems at KNO3 1.0 M and 25 °C.
2.3. Spectrophotometric measurements
Spectrophotometric measurements in the UV–vis range were performed using a Shimadzu UV-1601 PC spectrophotometer and a quartz cell (cell pass ℓ = 1 cm). The species distribution diagrams calculated from the analysis of potentiometric data were used to perform the measurements. The pH zones were chosen based on where the majority species were formed in each case. 50 mL flasks were used to prepare the different blends. Each mixture contained a concentration of 2 mM Cu(II), 2 mM 2-(benzo(d)oxazol-2-yl)nicotinic acid and 2 mM amino acid solution along with a specific amount of KOH to reach the desired pH value. The prepared mixtures are replicates of the potentiometric measurements considering the pH zone of formation of the most abundant species.
3. Results and discussion
3.1. Potentiometric analysis
The formation constants of the ternary species in the Cu(II)-L-A–n systems were determined using LETAGROP program. A–n represents the amino acids used. The experiments were carried at KNO3 1.0 M and 25.0 ± 0.1° C. The results are summarized in and .
In and the formation constants of the studied systems are compared with their analogous counterparts detected in the Cu(II)-L-amino acid systems where the main ligand L is the sulfur analogue of HOxa and the amino acids employed were the same that those used for this research. The complexes with HOxa exhibited stability constants that were higher than those with the benzothiazole variant, which is product of HOxa higher basicity [Citation2,Citation20,Citation25].
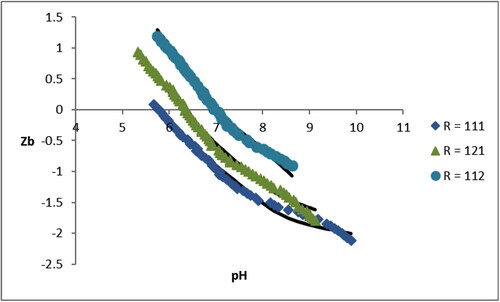
A good fitting between the experimental data and the proposed models can be observed. The models were built considering the species and stability constants given in and . The ZB vs pH of Cu(II)-L-His system is shown in . Supporting material includes data for other studied systems.
From it can be observed that the ternary species [Cu(L)(HA)]+, [Cu(L)(A)], [Cu(L)(A)(OH)]–, [Cu(L)2(A)]–, and [Cu(L)(A)2]- are formed in systems where the amino acid has a HA structure (Ser, Thr, Met, and Phe) exhibiting similar formation constants values. These observations indicate that amino acids Ser, Thr, Met, and Phe are all coordinated to the metal center in a bidentate way through the amino (–NH2) and carboxylato groups (–COO-) groups forming a five membered chelate ring. It is expected that the ternary complexes formed by these amino acids in the Cu(II)-L-amino acid systems have the same geometry and the same sphere of internal coordination [Citation23,Citation26] while the Oxa ligand (L–) is always bidentate.
In the case of the ligands aspartic acid and glutamic acid shown in , there was also coincidence on the formation of ternary species [Cu(L)(HA)], [Cu(L)(A)]-and [Cu(L)(A)(OH)]2- for both amino acids and the value of the formation constants were also very similar in both cases indicating that as the systems on , both amino acids are bound in the same way to the Cu(II) ion, and the ternary complexes have the same geometry and the same sphere of internal coordination [Citation23,Citation26]. As the complexes shown in the Oxa ligand (L) is always bidentate. When the ZB versus pH graph of the system Cu(II)-L-Asp is studied in detail, see Figure 1 of supplementary material, a deviation from the fit lines is observed caused by the presence of small amounts of a undetected species despite that the proposed model is the one that best adjusted to the experimental data.
For the Cu(II)-L-His system the speciation was different with respect those systems formed with amino acids with HL type structure as shown in . These differences are caused by substantial structural differences of the His with respect to the Ser, Thr, Met and Phe ligands, which resulted in tridentate coordination mode of these amino acids unlike the other ligands which are bidentate [Citation23,Citation26–29]. For the cationic ternary complex [Cu(L)(HHis)]+ the Oxa ligand (L) occupy two coordination sites and the His ligand is bound in a bidentate way resulting from the protonation of the imidazole group. This way of coordination is the same exhibited by the amino acids Ser, Thr, Met and Phe when they are bound to Cu(II), forming the neutral complex [Cu(L)(A)]. This is confirmed by the similarity observed when the formation constants of [Cu(L)(A)] from are compared with the ones of [Cu(L)(HHis)]+ from . In contrast for the complexes [Cu(L)(His)], [Cu(L)(A)(OH)]–, and [Cu(L)(A)(OH)2]2– the imidazole ring is deprotonated and His becomes tridentate while Oxa acid remains as bidentate ligand.
Using the formation constants given in and the species distribution diagrams were generated for all Cu(II)-L-amino acid systems studied. As a representative example in are shown the distribution diagrams of Cu(II)-L-Ser, Cu(II)-L-Glu and Cu(II)-L-His systems which correspond to the 1:1:1 relationship. The distribution diagrams for the other ratios and the rest of studied systems are available in the additional material. All distribution diagrams indicate that ternary complexes are the most abundant ones over the entire pH range for each studied system.
Figure 3. Species distribution diagram for the systems: (A) Cu(II)-L-His, (B) Cu(II)-L-Ser and (C) Cu(II)-L-Glu[Cu(II)] = 3.0 mM, R = 1:1:1.
![Figure 3. Species distribution diagram for the systems: (A) Cu(II)-L-His, (B) Cu(II)-L-Ser and (C) Cu(II)-L-Glu[Cu(II)] = 3.0 mM, R = 1:1:1.](/cms/asset/69ccb21c-7885-4307-a3a3-d206372f3f3d/gcoo_a_2249582_f0003_c.jpg)
The distribution diagram of the Cu(II)-L-His system is depicted in where it is seen that the complex [Cu(L)(HHis)]+ predominates at pH ≈ 5 with a relative abundance of 48%. For 5.5 ≤ pH ≤ 6.5, the most important ternary species is [Cu(L)(His)] with a 60% abundance. At more basic pH values, the complex [Cu(L)(His)] is hydrolyzed forming the compound [Cu(L)(His)(OH)]- with an abundance of 70%. Finally, for 8 ≤ pH ≤ 10, the hydrolytic product type [Cu(Phen)(L)(OH)2] is formed in high proportions.
The speciation of the Cu (II)-L-Ser system is shown in where it is observed that the cationic species [Cu(L)(HSer)]+ is formed in the range 4 ≤ pH ≤ 7 with a maximum abundance of 98% at pH = 4. The neutral complex is observed in all studied pH range 4 ≤ pH ≤ 9 and its maximun abundance can be found at 6.9 ≤ pH ≤ 7.5 with a relative abundance of 98%. For pH > 8, the deprotonation of one of the water molecules coordinated to the metallic center occurs, giving rise to the anionic hydroxy complex [Cu(L)(Ser)(OH)]- in the range 6 ≤ pH ≤ 10, with a maximum abundance of 40% at pH = 9.
shows the chemical speciation for the Cu (II)-L-Glu. At pH = 6 the neutral compound [Cu(L)(HGlu)] is formed with a relative abundance of 32%; while the anionic complex [Cu(L)(Glu)]– is the most abundant species in solution at physiological pH. Finally, at pH = 10 there is presence of the anionic hydroxy compound [Cu(L)(Glu)(OH)]2– with a relative abundance of 100%.
In order to compare the stability of the ternary complexes with the binary ones the value of Δlog K [Citation23,Citation26,Citation27,Citation30–32] must be considered. This quantity measures the tendency of the amino acid to coordinate to the Cu(II)-L complexes. The associated equilibrium reaction is represented by EquationEquation (2)(2)
(2) ; The experimental value of Δlog K is determined using Equationequation (3)
(3)
(3) .
(2)
(2)
(3)
(3)
Δlog K was determined for each of the systems studied and are presented in . All values are positive and greater than −0.9 indicating that the ternary complexes are more stable than the binary ones [Citation23,Citation26,Citation27].
Table 7. Δlog K and log χ values calculated for the system studied.
A second way to evaluate the trend toward the formation of ternary species is by calculating logχ values [Citation23,Citation26,Citation27]. The equilibrium constant is calculated by the equilibrium relation (4) and EquationEquation (5)(5)
(5)
(4)
(4)
(5)
(5)
The calculated logχ values are presented in . In all systems studied, the logχ value is greater than the statistical value of 0.6 (χ = 4) indicating that the formation of mixed ligand complexes prevails over the formation of binary complexes [Citation23,Citation26,Citation27].
3.2 Characterization by UV–visible spectroscopy
The UV–vis spectra for all studied systems were obtained at different pH values. From the structural similarity between some of the amino acids used in this work it is expected that the ternary complexes formed by these amino acids in the Cu(II)-L-amino acid systems have an analogous coordination sphere and geometry [Citation23,Citation26,Citation31]. Based on this analysis the Cu(II)-L-Ser, Cu(II)-L-Glu and Cu(II)-L-His systems were selected as representative examples, and their corresponding spectra are shown in . The UV–vis spectra of the rest of systems studied are available in the additional material.
Figure 4. UV–vis spectra at different pH values of the systems: (A) Cu(II)-L-His, (B) Cu(II)-L-Ser and (C) Cu(II)-L-Glu.
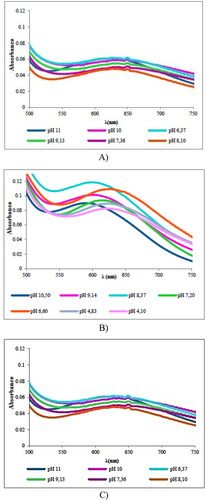
The UV/Vis spectra of all systems studies have a single absorption band corresponding to d-d electronic transitions resulting from having a Cu(II) ion (d9) as metal center. The band appears in the wavelength range 500–800 nm with λmax values between 592 and 650 nm. The experimental results are consistent and indicate that all ternary complexes are pentacoordinated with a square-pyramidal geometry [Citation33–35]. The square-based pyramid complexes, with Cu(II) as metallic nucleus, present in the UV–vis spectrum one absorption band between 580 and 670 nm [Citation36–38]
4. Conclusions
The speciation studies indicate the formation of the species [Cu(L)(HA)]+, [Cu(L)(A)], [Cu(L)(A)(OH)]–, [Cu(L)2(A)]–, and [Cu(L)(A)2]– on the ternary systems with the amino acids Ser, Thr, Met, and Phe which are bound to the metal core in a bidentate way through the amino (–NH2) and carboxylato groups (–COO–) groups forming a five membered chelate ring while the Oxa ligand (L–) is always bidentate. The species [Cu(L)(HA)], [Cu(L)(A)]–, and [Cu(L)(A)(OH)]2– are detected in presence of amino acids Asp and Glu where the value of the formation constants were also very similar in both cases indicating that both amino acids are bound in the same way to the Cu(II) ion, and the ternary complexes have the same geometry and the same sphere of internal coordination. Finally, the compounds [Cu(L)(HA)]+, [Cu(L)(A)], [Cu(L)(A)(OH)]–, and [Cu(L)(A)(OH)2]2– are detected in the Cu(II)-L-His system where the [Cu(L)(HHis)]+ the His ligand is bound in a bidentate way as the L ligand. In contrast for the complexes [Cu(L)(His)], [Cu(L)(A)(OH)]–, and [Cu(L)(A)(OH)2]2– the imidazole ring is deprotonated and His becomes tridentate. The evaluation of Δlog K and logχ confirmed the predominance of ternary complexes which was confirmed when the distribution diagrams of all studied systems were drawn. Finally, the spectroscopic characterization via UV/Vis molecular absorption spectroscopy showed that the ternary complexes are penta-coordinated with a square pyramidal geometry.
Ethical approval
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Authors’ contributions
Neudo Urdaneta: Conceptualization, Supervision, Project administration.
Nubia Grazziani: Methodology, Investigation, Validation.
Vanessa Landaeta: Conceptualization, Supervision, Project administration.
Rafael Rodríguez-Lugo: Conceptualization, Supervision, Project administration.
Mary Lorena Araujo: Conceptualization, Supervision, Writing—review & editing
Lino Hernández: Methodology, Formal analysis, Investigation, Validation, Writing—original draft.
Vito Lubes: Conceptualization, Supervision, Project administration, Formal analysis, Software, Writing—original draft.
gcoo_a_2249582_sm7792.docx
Download MS Word (106.3 KB)Availability of data and materials
Not applicable.
Additional information
Funding
References
- L. Hernández, G. Lubes, M. Rodriguez, V. Lubes. J. Solut. Chem., 41, 840 (2012).
- L. Hernández, M.L. Araujo, W. Madden, E. Del Carpio, G. Lubes, V. Lubes. J. Inorg. Biochem., 229, 111712 (2022).
- M. Geraghty, V. Sheridan, M. McCann, M. Devereux, V. McKee. Polyhedron, 18, 2931 (1999).
- P.R. Reddy, N. Raju, P. Manjula, K.V.G. Redd. Chem. Biodivers., 4, 1565 (2007).
- M. Tabatabaee, M. Bordbar, M. Ghassemzdeh, M. Tahriri, M. Tahriri, M. Tahrir, Z.M. Lighvan, B. Neumüller. Eur. J. Med. Chem., 70, 364 (2013).
- E. Del Carpio, L. Hernández, C. Ciangherotti, V. Villalobos Coa, L. Jiménez, V. Lubes, G. Lubes. Coord. Chem. Rev., 372, 117 (2018).
- C. Marzano, M. Pellei, F. Tisato, C. Santini. Anticancer Agents Med. Chem., 9, 185 (2009).
- T. Storr, K.H. Thompson, C. Orvig. Chem. Soc. Rev., 35, 534 (2006).
- K.G. Daniel, R.H. Harbach, W.C. Guida, Q.P. Dou. Front. Biosci., 9, 2652 (2004).
- S. Chandraleka, K. Ramya, G. Chandramohan, D. Dhanasekaran, A. Priyadharshini, A. Panneerselvam. J. Saudi Chem. Soc., 18, 953 (2014).
- M.S. Mohamed, A.A. Shoukry, A.G. Ali. Spectrochim. Acta A Mol. Biomol. Spectrosc., 86, 562 (2012).
- A. Nobrega, V.R. Landaeta, R. Rodriguez-Lugo, M.L. Araujo, W. Madden, L. Hernández, V. Lubes. Phys. Chem. Liq., 59, 969 (2021).
- M. Geraghty, J.F. Cronin, M. Devereux, M. McCann. Biometals, 13, 1 (2000).
- B.S. Creaven, D.A. Egan, K. Kavanagh, M. McCann, A. Noble, B. Thati, M. Walsh. Inorg. Chim. Acta, 359, 3976 (2006).
- D. Raydan, I.J. Rivas-Lacre, V. Lubes, V. Landaeta, L. Hernández. J. Mol. Liq., 302, 112595 (2020).
- M.J. Bradshaw, A.J. Saviola, E. Fesler, S.P. Mackessy. Cytotechnology, 68, 687 (2016).
- P. Kumar, S. Gorai, M.K. Santra, B. Mondal, D. Manna. Dalton Trans., 41, 7573 (2012).
- D.-Y. Zhang, Y. Nie, H. Sang, J.-J. Suo, Z.-J. Li, W. Gu, J.-L. Tian, X. Liu, S.-P. Yan. Inorg. Chim. Acta, 457, 7 (2017).
- A.I. Matesanz, E. Jimenez-Faraco, M.C. Ruiz, L.M. Balsa, C. Navarro-Ranninger, I.E. León, A.G. Quiroga. Inorg. Chem. Front., 5, 73 (2018).
- N. Urdaneta, W. Madden, V.R. Landaeta, R. Rodríguez-Lugo, L. Hernández, V. Lubes. J. Mol. Liq., 227, 218 (2017).
- F. Brito, M.L. Araujo, V. Lubes, A. D’Ascoli, A. Mederos, P. Gili, S. Domínguez, E. Chinea, R. Hernández-Molina, M.T. Armas, E.J. Baran. E. J. Baran. J. Coord. Chem., 58, 501 (2005).
- L. Hernández, M.L. Araujo, V. Lubes. Speciation: How to Propose Models and Select the Best of Them, Chapter 5, pp. 203–228. Nova Science Publishers, New York (2021).
- L. Hernández, E. Del Carpio, W. Madden, G. Lubes, A. Perez, R.E. Rodríguez-Lugo, V.R. Landaeta, M.L. Araujo, J. Daniel Martínez, V. Lubes. Phys. Chem. Liq., 58, 31 (2020).
- L. Alderighi, P. Gans, A. Ienco, D. Peters, A. Sabatini, A. Vacca. Coord. Chem. Rev., 184, 311 (1999).
- N. Urdaneta, E. Infante, V.R. Landaeta, R. Rodríguez-Lugo, L. Hernández, M.L. Araujo, V. Lubes. Phys. Chem. Liq., 60, 616 (2022).
- L. Hernández, E. Del Carpio, W. Madden, G. Lubes, A. Perez, R.E. Rodríguez-Lugo, V.R. Landaeta, M.L. Araujo, J. Daniel Martínez, V. Lubes. Phys. Chem. Liq., 58, 127 (2020).
- S. Helmut. Angew. Chem. Int. Ed., 14, 394 (1975).
- P. Deschamps, N. Zerrouk, I. Nicolis, T. Martens, E. Curis, M.-F. Charlot, J.J. Girerd, T. Prangé, S. Bénazeth, J.C. Chaumeil, A. Tomas. Inorg. Chim. Acta, 353, 22 (2003).
- P. Deschamps, P.P. Kulkarni, B. Sarkar. Inorg. Chem., 43, 3338 (2004).
- D.P. Martínez, M.L. Araujo, F. Brito, A. Pérez, L. Hernández, V. Lubes. J. Mol. Liq., 220, 681 (2016).
- W. Madden, L. Hernández, A. Perez, E. Del Carpio, V. Lubes. J. Mol. Liq., 221, 88 (2016).
- A. Perez, W. Madden, L. Hernández, E. Del Carpio, V. Lubes. J. Mol. Liq., 233, 288 (2017).
- S. Suzuki, K. Yamaguchi, N. Nakamura, Y. Tagawa, H. Kuma, T. Kawamoto. Inorg. Chim. Acta, 283, 260 (1998).
- F. Zhang, A. Odani, H. Masuda, O. Yamauchi. Inorg. Chem., 35, 7148 (1996).
- O. Yamauchi, A. Odani. J. Am. Chem. Soc., 107, 5938 (1985).
- G.L. Miessler, D.A. Tarr. Inorganic Chemistry, Pearson Prentice Hall, New Jersey (2004).
- A.B.P. Lever. Inorganic Electronic Spectroscopy, Elsevier, Amsterdam (1968)
- J.E. Huheey, E.A. Keiter, R.L. Keiter, O.K. Medhi. Inorganic Chemistry – Principles of Structure and Reactivity, Pearson, London (2006).

![Figure 1. (A) Structure of 2,2′-bipyridine. (B) Structure of 2-(benzo[d]oxazol-2-yl) nicotinic acid.](/cms/asset/cd2c7f47-aa9b-4e3d-8f01-52acb9df398e/gcoo_a_2249582_f0001_b.jpg)