Abstract
A smoke-derived butenolide, 3-methyl-2H-furo[2,3-c]pyran-2-one, has previously been shown to improve seedling vigour. The aim of this study is to examine the effect of hydropriming and butenolide priming treatments on seedling emergence and growth under different sowing depths at 20 and 25°C in two melon (Cucumis melo L.) seed lots of high and low quality. Seeds are subjected to hydropriming (21 h at 25°C) and butenolide priming (10−7 M, 21 h at 25°C) and sown at a depth of 4 or 8 cm in peat moss (field capacity, 64% water by mass). In general, seedlings from butenolide-primed and hydroprimed seeds are superior to those of the control. At 20°C, the effect of butenolide priming is more pronounced than that of hydropriming and the control, particularly for the seeds sown at a depth of 8 cm. Butenolide priming has a ‘repair-inducing’ effect and enhances the low-quality seeds more than those of the high-quality seed lot, an effect which is more obvious at 20°C than 25°C. It can be concluded that butenolide priming may be a useful tool to enhance melon seedling performance under low temperature sowing conditions.
Keywords:
Introduction
Melon (Cucumis melo L.) is a warm-climate crop and the ideal germination temperature is 25–27°C (Bates & Robinson Citation1995). Melon seeds are sown in early spring by direct sowing into the field in some parts of the Mediterranean region in which low temperatures (<20°C) are a common phenomenon (Mavi & Demir Citation2007). One precautionary practice, however, involves sowing seeds slightly deeper (8–10 cm) in order to avoid surface drying and protect the emerging radicle from frost. This results in some additional mechanical stress, along with low temperature stress, during early seedling emergence. Such conditions may result in lower numbers of seedlings and poor stand establishment, particularly when low vigour seed lots are used (Mavi & Demir Citation2007).
Priming is a pre-sowing seed treatment in which seeds are exposed to an external water potential that is low enough to restrict germination by various means (i.e., polyethylene glycol, inorganic salts, matric material, hydration) and yet permit pre-germinative physiological and biochemical activities (Taylor et al. Citation1998). Priming enhances seed performance by increasing the germination rate and uniformity of emergence which results in faster and better seedling development (Bennett et al. 1992). It is a promising technique to improve seed germination resulting in rapid and more synchronous seedling emergence under various adverse field or seed-bed conditions (Khan Citation1992). Various plant growth regulators such as polyamines (Basra et al. Citation1994), jasmonic acid (Korkmaz et al. Citation2004) and salicylic acid (Farooq et al. Citation2008) have been integrated with priming in order to accelerate its effect on germination and seedling growth, particularly under adverse emergence conditions.
Smoke from burning vegetation is an important post-fire germination cue (De Lange & Boucher Citation1990) and a highly active germination promoter, 3-methyl-2H-furo[2,3-c]pyran-2-one (a butenolide compound), was isolated from plant-derived smoke (Flematti et al. Citation2004, van Staden et al. Citation2004). Although smoke was initially investigated for its dormancy-releasing effect, reports investigating the effect of butenolide on rice (Kulkarni et al. Citation2006), maize (van Staden et al. Citation2006), tomato (Jain & van Staden Citation2007), okra (Kulkarni et al. Citation2007) and aubergine (Demir et al. Citation2009) indicate that it can promote seedling vigour and could potentially be used as a seed priming agent. Butenolide priming was also found to be effective for the alleviation of adverse effects of high temperature and low osmotic potential on germination (Ghebrehiwot et al. Citation2008). The effect of priming with butenolide, however, when the seeds are sown deep is not known.
There are a number of physiological events involved in seed germination and seedling development that are affected by butenolide, such as an increase in replicated DNA (Jain & van Staden Citation2007) and induction of the cell division cycle and storage mobilization (Soos et al. Citation2009). The faster germination obtained by butenolide could be associated with enzymatic activity i.e., catalase, through its role in storage compound degradation (Bailly et al. Citation2001). Thus, this study aimed to investigate the effect of priming with water (hydropriming) or butenolide on seedling emergence of two melon seed lots (of low and high vigour) sown at two depths and grown under low or optimal temperature conditions.
Materials and methods
Two melon seed lots (Cucumis melo L. cv. Kırkagac) were obtained from Beta Seed Company (Mithatpasa Street, Ankara, Turkey). Lot 1 had 99% (high vigour) and lot 2 had 94% (low vigour) of initial standard laboratory germination percentages (ISTA Citation2003). A controlled deterioration vigour test (72 h at 45°C, 20% seed moisture content) showed 91% and 69% germination for lot 1 and lot 2, respectively.
Hydropriming was performed by placing 25 seeds on two sheets of Whatman filter paper in 9 cm Petri dishes to which 20 mL of deionized water had been added. The seeds were allowed to imbibe water for 21 h at 25°C in the dark. The butenolide used in the experiment was isolated (99% purity) from smoke-saturated water as described by van Staden et al. (Citation2004) and provided by the Research Centre for Plant Growth and Development, University of KwaZulu-Natal, South Africa.
In the case of butenolide priming, 25 seeds were placed on two sheets of Whatman filter paper in 9 cm Petri dishes containing 20 mL of a 10−7 M butenolide solution and kept in the dark for 21 h at 25°C. Following both priming treatments, seeds were rinsed with distilled water and surface dried with filter paper. Untreated seeds were taken as the control. All procedures were carried out under very dim light (about 2 µmol m−2s−1).
To determine the effect of priming treatments on emergence at various sowing depths (mechanical stress), seeds were sown at depths of 4 cm (shallow) and 8 cm (deep) in peat moss (Klasman, Germany). The peat moss was air dried at room temperature for a week. Water was added (by weight) to give an approximate field capacity. Four replicates of 25 seeds per treatment were sown in the peat moss in plastic containers (2 kg peat moss per container, 21×14×10 cm) at 4 or 8 cm sowing depth. Containers were placed in controlled climatic rooms at 20 and 25°C, with relative humidity of 70±5% throughout the experiment. Cool fluorescent lamps provided photosynthetic photon flux density of 72 µmol m−2s−1 for 12 hd−1 at seedling level. During the first 3 days, containers were covered with plastic bags to maintain constant field capacity by avoiding evaporation. Thereafter, the containers were kept open and distilled water was added daily to each container as necessary.
Seedling emergence (the appearance of a hypocotyl at the surface) was monitored by counting twice daily (for the first 10 days), then once daily thereafter. After 24 days, the seedlings were counted and the fresh and dry mass of the aerial parts was measured. Drying was performed at 80°C for 24 h. Catalase activity was measured by monitoring the disappearance of H2O2 according to the method of Cakmak & Marschner (Citation1992). Catalase activity was measured on melon seeds of unimbibed (control) and imbibed in water (hydroprimed) and butenolide 10−7 M (butenolide treatment). The measurement was conducted on two replicates of 10 whole seeds in each treatment.
One-way analysis of variance (ANOVA) was conducted on all data and means were separated with Duncan's multiple range tests at 5% level. Percentage values were arcsine transformed prior to analysis, and SPPS (9.05, The Predictive Analytics Company) was used for all statistical procedures.
Results
At 25°C, the final seedling emergence percentages of both seed lots did not differ significantly (P≥0.05) between treated and control seeds, with the exception of seeds from lot 1 sown at a depth of 4 cm. At 20°C, however, primed seeds of both seed lots had significantly (P≤0.05) higher emergence than those of the control, regardless of the sowing depth ().
Fig. 1 Cumulative germination of high-quality (A, B: lot 1) and low-quality (C, D: lot 2) melon seeds grown at 20°C. Seeds were primed for 21 h at 25°C with butenolide (10−7 M, •) or water (hydroprimed, ▪) and sown at a depth of 4 cm (A, C: shallow) or 8 cm (B, D: deep). Untreated seeds were used as a control (▴). Mean values with different letters are significantly different (P≤0.05). An asterisk denotes a significant difference (P≤0.05) from the control, but not between the two treatments, and ‘ns’ indicate no significant difference (P≥0.05).
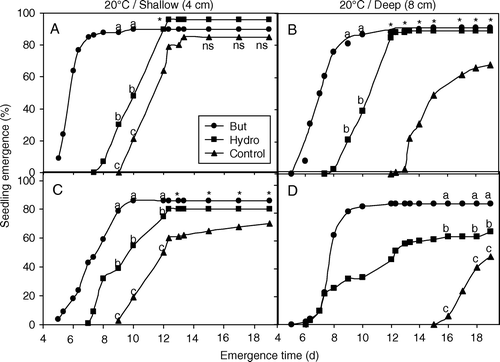
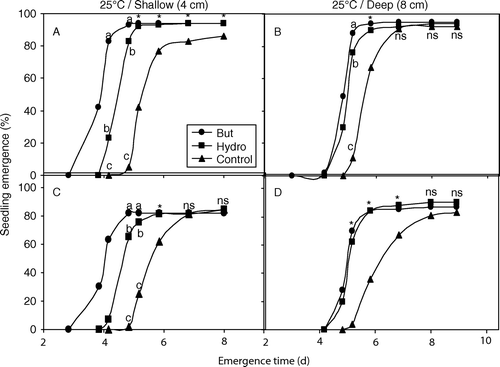
At both temperatures, butenolide-primed seeds of both seed lots started to emerge earlier than hydroprimed and control seeds ( and ). The difference in emergence between treated and control seeds, however, was less evident after approximately 7 days at 25°C. For seeds grown at 20°C, however, the difference in emergence was maintained for more than 18 days (B, C and D). The effect of butenolide-priming was more evident in the lower-quality seeds, under lower temperature and deeper sowing conditions (). For seeds of lot 2 (20°C, 8 cm sowing depth), butenolide-primed seeds reached 83% after 8 days, while hydroprimed seed had 42% germination at this time, and control seeds just started to emerge after 15 days and reached 41% by 20 days (D). Butenolide-primed seeds of lot 2 had significantly (P=0.001) higher emergence than both hydroprimed and control seeds when they were sown at 8 cm at 20°C (). For lot 2 seeds sown at 4 cm under 20°C, the difference between the treatments was significant (P=0.042) until 12 days, after which time the difference between the treatments diminished. Thereafter, there was little difference between both priming treatments, but a significant difference (P=0.031) between the primed and control seeds was observed (). A similar trend was observed for seeds of lot 1 sown at 8 cm (B), although for lot 2 seeds sown at 8 cm, butenolide priming was superior throughout the duration of the experiment (D).
Fig. 2 Cumulative germination of high-quality (A, B: lot 1) and low-quality (C, D: lot 2) melon seeds grown at 25°C. Seeds were primed for 21 h at 25°C with butenolide (10−7 M, •) or water (hydroprimed, ▪) and sown at a depth of 4 cm (A, C: shallow) or 8 cm (B, D: deep). Untreated seeds were used as a control (▴). Mean values with different letters are significantly different (P≤0.05). An asterisk denotes a significant difference (P≤0.05) from the control, but not between the two treatments, and ‘ns’ indicate no significant difference (P≥0.05).
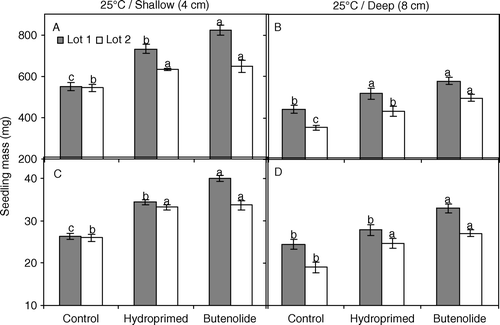
Earlier emergence in butenolide-primed seeds resulted in larger seedling size (fresh and dry mass) than those of hydroprimed and control seeds in lot 2 at 20°C (A–D). While primed seeds of lot 1 gave similar size seedlings at 20°C (except fresh seedling mass at deep sowing [B]), butenolide-priming and hydropriming produced similar size seedlings at 25°C, but both resulted in significantly (P=0.001) larger seedlings than control seeds () except in one case, dry mass at deep sowing in lot 1 (B).
Fig. 3 Fresh (A, B) and dry (C, D) mass of seedlings grown at 20°C from high-quality (solid bars, lot 1) and low-quality (open bars, lot 2) melon seeds following priming treatments. Seeds were primed for 21 h at 25°C with butenolide (10−7 M) or water (hydroprimed) and sown at a depth of 4 cm (A, C: shallow) or 8 cm (B, D: deep). Untreated seeds were used as a control. Mean±s.e. values within each seed lot and sowing depth with different letters are significantly different (P≤0.05).
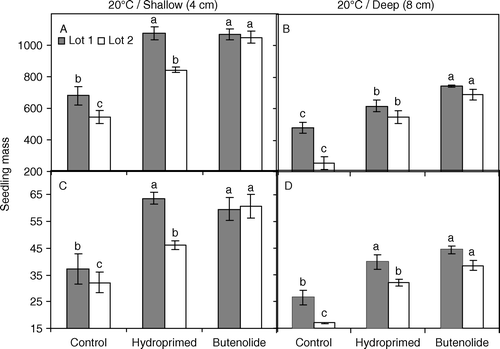
Fig. 4 Fresh (A, B) and dry (C, D) mass of seedlings grown at 25°C from high-quality (solid bars, lot 1) and low-quality (open bars, lot 2) melon seeds following priming treatments. Seeds were primed for 21 h at 25°C with butenolide (10−7 M) or water (hydroprimed) and sown at a depth of 4 cm (A, C: shallow) or 8 cm (B, D: deep). Untreated seeds were used as a control. Mean±s.e. values within each seed lot and sowing depth with different letters are significantly different (P≤0.05).
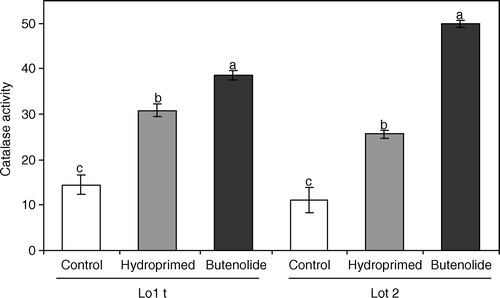
Butenolide treatment increased catalase activity, in both seed lots, compared with both hydroprimed and control seeds. Although catalase activity of hydroprimed seeds was higher than control seeds, activity of control and hydroprimed seeds in both lots was significantly (P=0.004) lower than that of butenolide-primed seeds ().
Fig. 5 Catalase activity (µmol min−1 g−1, fresh mass) of high-quality (lot 1) and low-quality (lot 2) melon seed lots in response to priming treatments. Seeds were primed for 21 h at 25°C with butenolide (10−7 M) or water (hydropriming). Mean±s.e. values of the treatments with different letters in the same lot are significantly different.
Discussion
The present study indicates that butenolide priming enhanced seedling emergence of deep-sown melon seeds grown under suboptimal temperature conditions. The treatment was more effective on seeds from a low-quality seed lot. The finding that priming promoted earlier emergence and increased fresh and dry mass of melon seeds is in agreement with previous findings for other crops (Khan Citation1992; Taylor et al. Citation1998).
Ellis (Citation1992) has defined ‘seed vigour’ as relating to germination (uptake of water and radicle emergence) and ‘seedling vigour’ as relating to the elongation of the embryonic axis and subsequent seedling growth (post-germinative events). Halmer & Bewley (Citation1984) found that most crop emergence losses are due to the failure of seedlings to grow beneath the soil surface, rather than as a result of failure of seeds to germinate. Previous research on watermelon indicated that although seeds were able to germinate in the soil, many were not strong enough to emerge above the surface. However, priming enhanced post-germination seedling growth in watermelon (Demir & van de Venter Citation1999). Similarly, in this study, priming of melon seeds with butenolide improved post-emergence seedling growth, and fresh and dry mass ( and ). Although hydroprimed and butenolide-primed low-quality melon seeds that were deep-sown started to emerge after 6 days, butenolide-primed seeds continued to grow and reached 82% emergence after 8 days, whereas hydroprimed seeds had only 33% emergence by that time. Thus, butenolide-primed seedlings showed more vigorous growth, even when sown at a depth of 8 cm. The stimulatory effect of priming at 25°C, however, was not as great as observed at 20°C. This demonstrates that priming is more advantageous when seeds are sown under suboptimal conditions (Khan Citation1992; Demir & Oztokat Citation2003).
The hydration of seeds during priming enables seeds to undergo a partial germination process, up to a stage before radicle emergence (Taylor et al. Citation1998). Untreated seeds sown under the same conditions as primed seeds, however, would first need to undergo similar germination processes prior to radicle emergence. Moreover, increases in the osmotic gradient between treated seeds and the growth medium results in greater cellular turgor potential at the time of rehydration, leading to more rapid radicle protrusion. Priming seeds for deep sowing may also play a role in alleviating oxygen stress by stimulating oxygen uptake. This is a valuable outcome of seed priming, especially in the case of melon seedling growth, where deep sowing is an important practical technique in order to prevent dehydration and for frost protection in early spring. In the Mediterranean region, cucurbit seeds are sown in early spring in unheated plastic tunnels to develop an early crop. At the end of February and beginning of March the weather is still very cool and often delays emergence, resulting in poor stand establishment. Results from this study indicate that butenolide has the potential to be used in seed technology as a seed pre-sowing treatment, since it enhanced emergence of melon seeds at 20°C, which is below the optimum (). This is in agreement with previous research findings in various agronomical and horticultural crop seeds (van Staden et al. Citation2006; Jain & van Staden Citation2007; Ghebrehiwot et al. Citation2008).
The promotory effect of butenolide on seedling growth has been reported in tomato (Jain & van Staden Citation2007), okra (Kulkarni et al. Citation2007) and tef (Ghebrehiwot et al. Citation2008), although the quality of the seeds used in these studies was not taken into account. In the present study, butenolide was more effective on the lower-quality seed lot tested, indicating that butenolide may have an effect on accentuating repair mechanisms and induction of seed ageing rejuvenation. The rejuvenation effect of priming treatments on aged seed lots has been reported in various crop seeds (Khan Citation1992). Likewise, in our previous study we found that butenolide restored the germination and seedling growth in artificially aged aubergine seeds (Demir et al. Citation2009).
Several different physiological mechanisms may be involved in the repair process effected by priming treatments, and may include enhanced protein and RNA synthesis (Khan Citation1992), embryo enlargement (Hegarthy Citation1970) and DNA replication (Jain & van Staden Citation2007). Smith & Cobb (Citation1992) indicated that enzymes also activate repair in seeds and suggested that ageing induces the generation of reactive oxygen species which may lead to peroxidative stress, resulting in seed deterioration. Several free radical scavenging enzymes such as catalase endow a protective mechanism and keep deleterious compounds to a minimum. Thus, the higher level of catalase activity observed in butenolide-treated melon seeds could be due to the faster hydrolysing ability of endospermic storage reserves, which would result in faster emergence (). Moreover, the butenolide increased catalase activity in seeds of the low-quality seed lot more than in seeds of the high-quality seed lot (). Lower levels of catalase activity in the deteriorated seed lot may be attributable to ageing-enhanced protein degradation (Smith & Cobb Citation1992). The more vigorous seedling growth observed in the butenolide-treated seeds may be due to a protective role of butenolide treatment on protein degradation (Khan Citation1992). The enhancing effect of priming on aged seeds was previously reported in various crops (Taylor et al. Citation1998). The possible use of butenolide in seed priming methodology for deteriorated ‘left-over’ seed from a previous planting season may well be advantageous.
An extended period of the seedling in the seed bed, as is the case when deep sowing, may increase the risk of infection by ‘damping-off’ pathogens such as Fusarium and Pythium. The enhanced and more rapid seedling emergence provided by butenolide treatment reduces the time that seedlings are covered in the seed bed, thereby alleviating the possibility of pathogenic attack. It has also been reported that the treatment of seeds with smoke extract may play a role in protecting seed and seedlings against pathogens in the seed bed.
Plant stand establishment is always an important consideration in commercial field production (Bennett et al. Citation1992). In melon, irregular stand establishment and delayed emergence are common phenomena, particularly in early sowing at low temperature and deep sowing. The findings of this study are important, not only because of the improvement of seedling emergence under suboptimal conditions as a result of butenolide priming, but also due to its rejuvenating influence on deteriorated seed. Although butenolide is currently unavailable commercially, it has the potential to be used as a priming agent for improving seedling emergence and growth of melon seed lots.
Acknowledgements
We thank TUBITAK (The Scientific and Technological Research Council of Turkey), the Scientific Research Project Office of Ankara University (BAPRO) and the National Research Foundation of South Africa for financial support.
References
- Bailly , C , Auidigier , C , Fabienne , L , Wagner , MH , Coste , F , Corbineau , F and Come , D . 2001 . Changes in oligosaccharides content and antioxidant enzyme activities in developing bean seeds as related to acquisition of drying tolerance and seed quality . Journal of Experimental Botany , 52 : 701 – 708 .
- Basra , AS , Singh , B and Malik , CP . 1994 . Priming-induced changes in polyamine levels in relation to vigor of aged onion seeds . Botanical Bulletin of Academia Sinica , 35 : 19 – 23 .
- Bates , MD and Robinson , RW . 1995 . “ Cucumbers, melons and watermelons ” . In Evolution of crop plants , 2nd edn , Edited by: Smartt , J and Simmonds , NW . 89 – 97 . Essex, , UK : Longman Scientific and Technical .
- Bennett , MA , Fritz , VA and Callan , NW . 1992 . Impact of seed treatments on crop stand establishment . HortTechnology , 2 : 345 – 349 .
- Cakmak , I and Marschner , M . 1992 . Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves . Plant Physiology , 98 : 1222 – 1227 .
- De Lange , JH and Boucher , C . 1990 . Autecological studies on Audouinia capitata (Bruniaceae). I. Plant-derived smoke as a seed germination cue . South African Journal of Botany , 56 : 700 – 703 .
- Demir , I and Oztokat , C . 2003 . Effect of salt priming on germination and seedling growth at low temperatures in watermelon seeds during development . Seed Science and Technology , 31 : 765 – 770 .
- Demir , I and van de Venter , HA . 1999 . The effects of priming treatments on the performance of watermelon (Citrullus lanatIus (Thunb.) Matsum ( Nakai) seeds under temperature and osmotic stress . Seed Science and Technology , 27 : 871 – 875 .
- Demir , I , Light , ME , van Staden , J , Kenanoglu , BB and Celikkol , T . 2009 . Improving seedling growth of unaged and aged aubergine seeds with smoke-derived butenolide . Seed Science and Technology , 37 : 255 – 260 .
- Ellis , RH . 1992 . Seed and seedling vigor in relation to crop growth and yield . Plant Growth Regulation , 11 : 249 – 255 .
- Farooq , M , Basra , SMA , Rehman , H and Hussain , M . 2008 . Seed priming with polyamines improves the germination and early seedling growth in fine rice . Journal of New Seeds , 9 : 145 – 155 .
- Flematti , GR , Ghisalberti , EL , Dixon , KW and Trengove , RD . 2004 . A compound from smoke that promotes seed germination . Science , 305 : 977
- Ghebrehiwot , HM , Kulkarni , MG , Kirkman , KP and van Staden , J . 2008 . Smoke-water and a smoke-isolated butenolide improve germination and seedling vigor of Eragrostis tef (Zucc.) Trotter under high temperature and low osmotic potential . Journal of Agronomy and Crop Science , 194 : 270 – 277 .
- Halmer , P and Bewley , JD . 1984 . A physiological perspective on seed vigor testing . Seed Science and Technology , 12 : 561 – 575 .
- Hegarthy , TW . 1970 . The possibility of increasing field establishment by seed hardening . Horticultural Research , 10 : 59 – 64 .
- ISTA (International Seed Testing Association) 2003 . International rules for seed testing . Zurich, , Switzerland , International Seed Testing Association .
- Jain , N and van Staden , J . 2007 . The potential of the smoke-derived compound 3-methyl-2H-furo[2,3-c]pyran-2-one as a priming agent for tomato seeds . Seed Science Research , 17 : 175 – 181 .
- Khan , AA . 1992 . Preplant physiological seed conditioning . Horticultural Reviews , 14 : 131 – 181 .
- Korkmaz , A , Tiryaki , I , Nas , MN and Ozbay , N . 2004 . Inclusion of plant growth regulators into priming solution improves low-temperature germination and emergence of watermelon seeds . Canadian Journal of Plant Science , 84 : 1161 – 1165 .
- Kulkarni , MG , Sparg , SG , Light , ME and van Staden , J . 2006 . Stimulation of rice (Oryza sativa L.) seedling vigor by smoke-water and butenolide . Journal of Agronomy of Crop Science , 192 : 395 – 398 .
- Kulkarni , MG , Ascough , GD and van Staden , J . 2007 . Effects of foliar applications of smoke-water and smoke isolated butenolide on seedling growth of okra and tomato . HortScience , 42 : 179 – 182 .
- Mavi , K and Demir , I . 2007 . Controlled deterioration and accelerated aging tests predict relative seedling emergence potential of melon seed lots . HortScience , 42 : 1431 – 1435 .
- Smith , PT and Cobb , BG . 1992 . Physiological and enzymatic characteristics of primed redried and germinated pepper seeds (Capsicum annuum L.) . Seed Science and Technology , 20 : 503 – 513 .
- Soos , V , Juhasz , A , Light , ME , van Staden , J and Balazs , E . 2009 . Smoke-water-induced changes of expression pattern in Grand Rapids lettuce achenes . Seed Science Research , 19 : 37 – 49 .
- Taylor , AG , Allen , PS , Bennett , MA , Bradford , KJ , Burris , JS and Misra , MK . 1998 . Seed enhancements . Seed Science Research , 8 : 245 – 256 .
- van Staden , J , Jäger , AK , Light , ME and Burger , BV . 2004 . Isolation of the major germination cue from plant-derived smoke . South African Journal of Botany , 70 : 654 – 659 .
- van Staden , J , Sparg , SG , Kulkarni , MG and Light , ME . 2006 . Post-germination effects of the smoke-derived compound 3-methyl-2H-furo [2,3-c]pyran-2-one and its potential as a preconditioning agent . Field Crops Research , 98 : 98 – 105 .