Abstract
Plantlets were regenerated from leaf explants of Actinidia eriantha Benth. cv White (kiwifruit). The best medium for adventitious shoot formation from leaf blade strips was Murashige-Skoog (MS) medium with 0.1 mg L−1 NAA and 2.0 mg L−1 BA. The frequency of shoot regeneration (%) was dependent on the size and the part of the leaf from which the explant was taken. The highest frequency of shoot regeneration was 7.30±2.61 shoots per explant, for explants taken from within 0–3 cm of the leaf blade base, near a vein and not on the leaf edge. When using petiole-derived explants, a maximum frequency of adventitious shoot regeneration of 79.17% with 6.54±2.31 shoots per explant was obtained using 0.1 mg L−1 NAA and 2.0 mg L−1 ZT in MS medium. The highest rate of rooting obtained was 96.67% when using 1/2 MS medium containing 0.6 mg L−1 IBA and 93.75% when using wet moss without IBA. This is the first report to study the effect of explants taken from different parts of the leaf blade on shoot regeneration in A. eriantha. All plantlets were transferred to pots of peat/perlite and grown on before being planted in the field. Plantlets were successfully acclimatized to field conditions and produced healthy plants.
Introduction
Kiwifruit is an increasingly important crop in China and other countries. Although the genus Actinidia contains more than 70 species, at present only three species are commonly cultivated—A. deliciosa (green-fleshed kiwifruit), A. chinensis (gold-fleshed kiwifruit) and A. arguta (‘baby’ kiwifruit), with only A. deliciosa and A. chinensis being grown commercially on a large scale. A. deliciosa and A. chinensis have fruit with non-peelable skins, while fruit of A. arguta have a thin edible skin.
A. eriantha produces the third largest fruit of species in the Actinidia genus (Liang Citation1980) but until 2007 no selections were considered suitable for commercialization. Recently, the species has attracted increasing attention because its fruit contain very high levels of vitamin C (Huang et al. Citation1983) and some genotypes have peelable skins (Seal Citation2003). New A. eriantha cultivars have been selected but some have an average fruit weight of only 25 g (Jo et al. Citation2007). The cultivar ‘White’ has recently been selected in China, and has large fruit and a good flavour (Wu et al. Citation2009).
Regeneration of plants of different Actinidia species through organogenesis and embryogenesis has been the subject of numerous studies (Mezzetti et al. Citation1991a, Citation1991b; Infante et al. Citation1994; Centeno et al. Citation1996; Moncaleán et al. Citation2001; Takahashi et al. Citation2004; Konieczna et al. Citation2008). Regeneration results have varied according to species, cultivar, sex, explant type and culture conditions. Unfortunately, because it is not widely grown commercially, little information is available about the culture of explants from different genotypes of A. eriantha, although initial studies have been made on protoplast culture (Zhang et al. Citation1998) and genetic transformation (Wang et al. Citation2006).
A. eriantha has a number of advantages that mean it could be useful for functional genomics studies in kiwifruit (Wang et al. Citation2006). It is diploid (kiwifruit species range from diploid to octoploid, with the main commercial species, A. deliciosa, being hexaploid), it has a high level of flower formation even on water shoots, and is highly prolific. This includes ‘White’, which has been confirmed by DNA flow cytometry to be diploid (Wu et al. unpublished) and bears fruit on all kinds of canes including watershoots (Wu et al. Citation2009). If ‘White’ is to be a useful species for genetic transformation or for functional genomics studies then an efficient regeneration system is required. In this article, we report the effects of different combinations of plant growth regulator (PGR) and of the size and part of the leaf from which explants are taken, on organogenesis and plant regeneration from leaf tissue. We also report on the regeneration of plants from petiole tissue and adventitious shoots rooting in wet moss. The data should be useful for subsequent micropropagation, genetic transformation and other studies.
Materials and methods
Plant material
Branches from a 3-year-old vine of Actinidia eriantha Benth cv ‘White’ were used for initiation culture and were obtained from the orchard of the Institute of Horticulture, Zhejiang Academy of Agricultural Sciences, Hangzhou, Zhejiang, China.
Explant sterilization, preparation and culture
The branches were flushed in tap water for 1 h then, in a laminar flow cabinet, the leaf blades were immersed in 70% ethanol for 30–45 s, and then transferred to 0.1% HgCl2 for 5 min. They were rinsed five times with sterile water and kept on the sterile filter paper, prior to being cut and placed into culture.
The sterile leaf blades (less than 3 cm long) were cut into 0.5×0.6 cm strips and placed on different media, M1–M8 (), to test the effect on regeneration efficiency. The sterile leaf blades (0–9 cm) were dissected out according to and placed in dishes with medium M3. The leaf blade strips were gently pressed against the surface of the medium, with their adaxial surfaces facing up.
Figure 1 Leaf blade strips dissected from different parts of the leaf blades. Note: Leaves up to 3 cm long (left) were dissected into 12 parts: W = whole leaf blade without petiole; A = apex of leaf blade; Ae = edge of A; Av = veined part of A; M = middle part of leaf blade; Me = edge of M; Mv = veined part of M; T = base of leaf blade; Te = edge of T; Tv = veined part of T; e = edge of leaf blade; v = veined part of leaf blade. Leaves between 3 and 9 cm long (right) were dissected into 16 parts, including some corresponding to parts shown on the smaller leaf blade. Other parts were as follows: b=part between edge and veined; Ab = part between edge and veined part of A; Mb = part between edge and veined part of M; Tb = part between edge and veined part of T. The whole leaf blade was used only when the leaf blade was less than 6 cm. P = petiole.
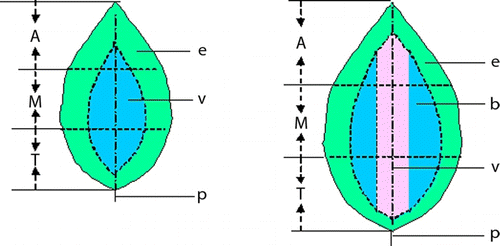
Table 1 Effect of PGRs on callus formation and shoot regeneration from leaves of A. eriantha cv ‘White’.
Petiole segments about 0.5 cm long were cut from sterilized leaf blades and placed flat on different media, P1–P6 (), for the induction of callus formation. After 3 weeks, calli were transplanted to different media, P7–P12 (), for the induction of adventitious shoots.
Table 2 Effect of PGRs on callus formation from petioles of A. eriantha cv ‘White’.
Table 3 Effect of PGRs on shoot regeneration from petioles of A. eriantha cv ‘White’.
The basal medium was Murashige-Skoog (MS) for callus formation and induction of adventitious shoots. All media were supplemented with 2% sucrose (w/v) and solidified with 7.5 g L−1 agar. The pH of the medium was adjusted to 5.8 before autoclaving at 121 oC for 20 min. The cultures were incubated in a growth room at 26±1 oC, under a 16 h light with 2000–3000 lx provided by cool-white fluorescent tubes.
Rooting and acclimation
Two different methods were used for inducing rooting from adventitious shoots. In the first method (termed Ra) the adventitious shoots were transferred to 1/2 MS medium containing 0.1, 0.3, 0.5, 0.6, 0.7 or 0.9 mg L−1 indole-3-butyric acid (IBA), then incubated at 26±1 oC, under a 16 light with 2000–3000 lx provided by cool-white fluorescent tubes. In the second method (termed Rb) the adventitious shoots were dipped in 100 mg L−1 IBA for 1, 5 or 10 s (or dipped in tap water as a control) and then transferred into wet moss and placed in the shade with intermittent mist.
Rooted plantlets were transplanted into 2:1 (v/v) peat:perlite mix under intermittent mist in the shade for 1–2 months until field planting. Ra rooted plantlets required acclimatization by opening the dishes for 3–8 days before transplantation.
Data collection and analysis
All tests were replicated three times with 30 explants per replicate. Means±SEM were calculated and their differences tested for significance using an LSD test at the 5% level of significance.
Results
Shoot regeneration from leaf blade strips
The leaf began to extend after 1 week in culture, and showed four-fold extension after 2 weeks. Many undulations appeared in the leaf blade strips surface during incubation (: L1). After 3 weeks, the leaf blades strips were scored for the number of green and dense calli produced in the vein and edge areas (: L2). After 30 days’ culture and then 5 days after transfer to fresh medium, the first shoot-like structures appeared from the calli on the cut leaf edges and leaf veins (: L3). After about another 2 weeks, these shoot-like structures developed into adventitious shoots (: L4).
Figure 2 In vitro organogenesis and plant regeneration from leaves of Actinidia eriantha Benth. cv ‘White’. L = leaf. L 1 , undulations in the leaf blade strip's surface. L 2 , green and dense calli produced on the veined and edge areas of leaf blade strips. L 3 , shoot-like structures developing into adventitious shoots from leaf blade strip explants. L 4 , adventitious shoots induced from leaf blade strip explants. P = petiole. P 1 , white-yellow and loose calli with nodules on the surface, induced from petioles. P 2 , white-yellow calli growing fast across the whole surface of the medium. P 3 , green and dense calli with smooth surfaces, induced from petioles. P 4 , green shoot-like structures induced from the green calli of petioles. P 5 , adventitious shoots induced from petioles. R = root. PL = plantlet. Ra 1 and Ra 2 , Rooting in medium containing IBA. Rb 1 and Rb 2 , Rooting in wet moss. PL 1 and PL 2 , plantlets potted and maintained in the shade.

The frequency of callus formation varied among MS basal medium supplemented with various types and concentrations of PGR (). Calli were induced in all of the media in the test. The percentages of callus production in a medium with zeatin (ZT) were higher than those in a medium with 6-benzyladenine (BA) for cultures to 20 days. The rates of callus production in a medium with a low ZT concentration were higher than those in a medium with a low BA concentration, and were the same as those in a medium containing higher BA concentrations for culture to 30 days.
The frequency of explants initiating shoots varied depending on the type and concentration of PGR in the medium. Shoot initiation increased as the BA concentration increased from 0.5 to 2.0 mg L−1, but decreased at 3.0 mg L−1 (53.33±2.31%), with a-naphthaleneacetic acid (NAA) maintained at 0.1 mg L−1. Compared with BA, low ZT concentrations induced much higher shoot initiation, but the frequency of shoot initiation declined at ZT concentrations above 1.0 mg L−1. Maximum shoot formation rates of 85.33±2.31% were obtained on MS medium containing a combination of 0.1 mg L−1 NAA and 2.0 mg L−1 BA. Therefore, this medium was used as the regeneration medium to study the effect of leaf length and leaf part on shoot initiation.
The results showed that the shorter the leaf blade, the higher the resulting callus formation and shoot initiation and the lower the rate of browning (). The highest shoot initiation was obtained from leaf blades 0–3 cm long. If the leaf blades were >6 cm, no shoots were obtained. Less shoot initiation occurred when the leaf blades were shorter than 6 cm. No callus formation occurred in the 6–9 cm and >9 cm leaf blades strips except for some (37.33%) in the vein areas in the middle parts (Mv) of the leaf blades.
Table 4 Effect of length and leaf part on the callus and adventitious shoot regeneration from leaf blade strips of A. eriantha cv ‘White’.
Some explants showed browning and de-greening from the outside to the inside of the tissue (). The browning rates varied with the explant length. 40% of explants from leaf blades of >6 cm in length browned and died within 7 days. Even the most tender explants from leaf blades of <3 cm in length did not show browning and explants from leaf blades of 3–6 cm in length showed less browning than those from larger ones.
There was a clear difference between the response of whole leaf blades and the cut strips. The whole leaves grew fast in the medium but most parts of the leaf blades became separated from the medium, and few adventitious shoots formed near the petiole end (31.11%) of 0–3 cm leaf blades. Whole leaf blades>6 cm were too large to culture.
Table 5 Effect of Ra and Rb methods on rooting of A. eriantha cv ‘White’.
The first de-differentiation occurred in Me and e parts of 0–3 cm long leaf blades after culture for 10 days. The highest callus formation (100%), shoot formation (100%) and mean number of shoots per explants (8.75) were all found in explants from leaf blades strips that were 0–3 cm long (). The highest frequency of shoot initiation was found in the Tv part of 0–3 cm long leaf blades.
Shoot regeneration from petiole explants
The first calli were induced from the two ends of the petiole segments after 7 days’ incubation in the medium containing 1.5 mg L−1 ZT and 0.1 mg L−1 NAA (Table 2). After 3 weeks of culture, the only clear variation was in the colour and texture of the calli. Those incubated in medium containing 2,4-D were white-yellow and loose with nodules on their surfaces (: P1), whereas those in medium containing NAA were green and dense with a smooth surface (: P3).
After transfer to shoot initiation medium, the green and dense calli produced green shoot-like structures (: P4) that grew into adventitious shoots after 2 weeks (: P5). However, the white-yellow calli grew quickly across the whole surface of the medium, did not change colour and produced no shoots (: P2). The highest shoot induction of 79.17% with 6.54 shoots/explants, was obtained in the medium containing MS, supplemented with 2.0 mg L−1 ZT and 0.1 mg L−1 NAA (Table 3).
Rooting
With the Ra method (Table 5), roots were induced within 7 days after the adventitious shoots were transferred to the rooting media. After 12 days, 70% of shoots had rooted. When using media containing different concentration of IBA, the highest root initiation percentage (: Ra1 and Ra2) of 96.67% was found in the 1/2 MS medium containing 0.6 mg L−1 IBA. The root initiation percentage was increased when the IBA concentration was less than 0.6 mg L−1.
With the Rb method (Table 5), there was no difference between the rooting obtained in control media compared with treatments with 100 mg L−1 IBA for 1 or 5 s, but the rooting rate declined after treatment with 100 mg L−1 IBA for 10 s (: Rb1 and Rb2).
The survival percentages from both the Ra and Rb methods were higher than 92% (: PL1 and PL2), with the survival rate for Rb (95.56±1.92%) being slightly higher than that for Ra (92.38±1.65%). There was no marked difference in vegetative growth between Ra and Rb, and the plants produced from both methods had grown to 20 cm tall by 6 weeks after planting.
Discussion and conclusions
Many PGRs have been used in tissue culture of Actinidia species, and their effects have varied depending on the species and explant type used (Mezzetti et al. 1991a, Citation1991b; Infante et al. Citation1994; Akbas et al. Citation2009; Moncalean et al. 2009). Most studies have used female kiwifruit, but there is one report for male kiwifruit ‘Tomuri’ (Prado et al. Citation2007). That research showed an organogenic callus induction rate of >80%, with an average of 14 new shoots in the second subculture from leaf explants cultured in media supplement with zeatin and NAA. In an earlier study, we showed that the best medium for A. chinensis cv ‘Hongyang’ was MS supplemented with 1.0 mg L−1 ZT and 0.1 mg L−1 NAA (Long et al. Citation2010). However, the different results obtained here for ‘White’ and for A. deliciosa cv ‘Hayward’ (Wu et al. unpublished) demonstrate that the regeneration characters differ according to species, explant type and PGRs used, even when using the same growth-room facilities. There is little published information on tissue culture of A. eriantha Benth. Wang et al. (Citation2006) reported a synergistic response to a mixture of zeatin and BAP in shoot regeneration of A. eriantha. Zhang et al. (Citation1998) reported that calli induced from protoplasts of A. eriantha regenerated shoots on MS with zeatin and IAA but they did not differentiate on a medium with BAP and 2.4-D. The shoot regeneration in this study showed that low ZT concentrations were clearly superior to low BA concentrations when used for inducing callus and adventitious bud formation in media with 0.1 mg L−1 NAA.
The results showed that the smaller the leaf, the higher the level of callus formation and shoot initiation. This is consistent with results from other species that show that differentiation is more readily achieved using explants of young tissue (Pan Citation2003). Dissected leaf pieces were tested in order to develop a system suitable for genetic transformation because leaf discs are the most common explant type used for genetic transformation. A significant difference was found between the whole leaves and the cut strips: callus formation and shoot formation were much higher from the cut strips. The highest level of shoot initiation was achieved using leaf pieces 0–3 cm long. The high regeneration frequency of these explants suggests they may be suitable for genetic transformation experiments. In particular, based on shoot formation, and considering manipulation required, the basal part of leaf blades strips from 0–3 cm long leaf blades may be the most appropriate explants, and provide a very good basis for a genetic transformation system.
We tested two methods for shoot rooting, including a new method (Rb) for kiwifruit tissue culture that does not require sterile media. ‘White’ adventitious shoots were easy to be rooted using either method. The good rooting performance in culture may reflect an inherent character of A. eriantha, which has been used as a root-stock for many years in South China due to its strong rooting ability. The rate of root initiation, mean number and length of roots per shoot were higher from Ra than from Rb. However, the rate of survival of plants in the field from Rb was higher than those from Ra, and shoots grown in Rb needed no acclimation, thus shortening their establishment time and simplifying the procedure. Therefore, Rb may be a new useful method for tissue culture of Actinidia.
Acknowledgements
This research was funded by the general programme of the Science and Technology Department of Zhejiang Province (2008C22007), the general programme of the National Natural Science Foundation of Zhejiang Province (Y307030, Y3110405) and by a grant from the Zhejiang Academy of Agricultural Sciences (2010CX65). We would like to thank Alan Seal of Plant and Food Research, New Zealand, for editing this paper.
References
- Akbas , F , Isikalan , C and Namli , S . 2009 . Callus induction and plant regeneration from different explants of Actinidia deliciosa . Applied Biochemisty and Biotechnology , 158 ( 2 ) : 470 – 475 .
- Centeno , ML , Rodriguez , A , Feito , I and Fermindez , B . 1996 . Relationship between endogenous auxin and cytokinin levels and morphogenic responses in Actinidia deliciosa tissue cultures . Plant Cell Reports , 16 : 58 – 62 .
- Huang , ZF , Liang , MY , Huang , CG and Li , RG . 1983 . A preliminary study on the character and nutritive composition of Actinidia fruits . Guihaia , 3 : 53 – 56 .
- Infante , R , Rotondi , A , Marino , G and Fasolo , F . 1994 . Solar light effects on growth, net photosynthesis, and leaf morphology of in vitro kiwifruit (Actinidia deliciosa) cv Hayward . In Vitro Cellular and Developmental Biology , 30 : 160 – 163 .
- Jo , YS , Cho , HS , Park , MY and Bang , GP . 2007 . Selection of a sweet A. eriantha, ‘Bidan’ . Acta Horticulture , 753 : 253 – 257 .
- Konieczna , MP , Kiszkurno , MK , Świerczyńska , J , Góralski , G , Lesak , HS and Bohdanowicz , J . 2008 . Ultrastructure and histochemical analysis of extracellular matrix surface network in kiwifruit endosperm-derived callus culture . Plant Cell Reports , 27 : 1137 – 1145 .
- Liang , CF . 1980 . Outline of taxonomy on Actinidia in China . Guangxi Plants , 1 : 30 – 45 .
- Long , QJ , Wu , YJ and Xie , M . 2010 . Studies on tissue culture and rapid micro-propagation from leaves and stems of kiwifruit (Actinidia chinensis ‘Hongyang’) . Acta Agriculturae Zhejiangensis , 22 ( 4 ) : 429 – 432 .
- Mezzetti , B , Rosati , P , Casalicchio , G and Liang , CF . 1991a . Actinidia deliciosa in vitro I. Growth and mineral uptake by explants. Plant Cell . Tissue and Organ Culture , 25 : 91 – 98 .
- Mezzetti , B , Conte , LS and Rosati , P . 1991b . Actinidia deliciosa in vitro II. Growth and exogenous carbohydrates utilization by explants. Plant Cell . Tissue and Organ Culture , 26 : 153 – 160 .
- Moncaleán , P , Rodríguez , A and Fernández , B . 2001 . In vitro response of Actinidia deliciosa explants to different BA incubation periods . Plant Cell, Tissue and Organ Culture , 67 : 257 – 266 .
- Moncaleán , P , Fal , MA , Castanon , S and Rodríguez , A . 2009 . Relative water content, in vitro proliferation, and growth of Actinidia deliciosa plantlets are affected by benzyladenine . New Zealand Journal of Crop and Horticultural Science , 37 ( 4 ) : 351 – 359 .
- Prado , MJ , Gonzalez , MV , Romo , S and Herrera , MT . 2007 . Adventitious plant regeneration on leaf explants from adult male kiwifruit and AFLP analysis of genetic variation . Plant Cell, Tissue and Organ Culture , 88 : 1 – 10 .
- Pan RZ 2003 . Plant tissue culture . GuangDong higher education press Guangzhou, Guangdong, , China 170
- Seal , AG . 2003 . The plant breeding challenges to making kiwifruit a worldwide mainstream fresh fruit . Acta Horticulture , 610 : 75 – 80 .
- Sotiropoulos , TE and Dimassi , KN . 2004 . Response to increasing rates of boron and NaCl on shoot proliferation and chemical composition of in vitro kiwifruit shoot cultures . Plant Cell, Tissue and Organ Culture , 79 : 285 – 289 .
- Takahashi , W , Sugawara , F , Yamamoto , N , Bando , E , Matsushita , J and Tanaka , O . 2004 . Plant regeneration in Actinidia polygama Miq. by leaf, stem, and petiole culture with zeatin, and from stem-derived calli on low-sucrose medium . Journal of Forest Research , 9 : 85 – 88 .
- Wang , TC , Ran , YD , Atkinson , RG , Gleave , AP and Cohen , D . 2006 . Transformation of Actinidia eriantha: A potential species for functional genomics studies in Actinidia . Plant Cell Reports , 25 : 425 – 431 .
- Wu , YJ , Xie , M , Zhang , QC , Jiang , GH , Zhang , HQ , Long , QJ , Han , WJ , Chen , JW and Shong , GH . 2009 . Characteristics of ‘White’: a new easy-peel cultivar of Actinidia eriantha . New Zealand Journal of Crop and Horticultural Science , 37 : 369 – 373 .
- Zhang , YJ , Qian , YQ , Mu , XJ , Cai , QG , Zhou , YL and Wei , XP . 1998 . Plant regeneration from in vitro-cultured seedling leaf protoplasts of Actinidia eriantha Benth . Plant Cell Reports , 17 : 819 – 821 .