Abstract
A spectroscopic method was developed to measure the nitrogen status of winter wheat (Triticum aestivum L.) canopies. Two years of field experiments, including a range of cultivars grown with differing levels of nitrogen fertilization, were conducted and ground-based hyperspectral data were collected to develop and validate an empirical model for early detection of low canopy chlorophyll content. Canopy reflectance was measured with a spectrometer, fitted with a 25° field of view fibre-optic adaptor. Canopy chlorophyll density (CCD), representing the total amount of chlorophyll present in the canopy per unit ground area, was combined according to the contribution of winter wheat leaves in different layers of the canopy and related to canopy reflectance. Combined canopy chlorophyll density (CCCD) calculated with both layers 1 and 2 and with layers 1, 2 and 3 were better related to difference vegetation index (DVI=RNIR−RRED, where RNIR and RRED were reflectance at 890 nm and 670 nm, respectively) than CCD in any individual layer. Statistical prediction models of canopy chlorophyll status in winter wheat were developed. The CCCD1+2 model demonstrated lower root mean square errors and higher modelling efficiencies than those of the CCCD1 and CCCD1+2+3 models. Chlorophyll status in the two uppermost layers of the wheat canopy could be quantified using DVI. Therefore, early detection of canopy nitrogen deficiency in winter wheat was achieved.
Introduction
Chlorophyll largely determines the amount of photosynthetically active radiation (PAR) absorbed by the leaf, photosynthetic rate and primary production. High chlorophyll content of plant communities is related to a large leaf area index (LAI), increasing radiant energy absorption, and total dry matter production (Anderson Citation1967). A precise estimation of plant chlorophyll status is important for nitrogen (N) fertilization recommendations because much of leaf N is incorporated in chlorophyll (Filella et al. Citation1995). The determination of chlorophyll status with traditional laboratory methods can be labour intensive, time consuming and expensive, especially when a large number of samples are involved.
Rapid and non-destructive chlorophyll status detection can be made using a portable leaf chlorophyll meter (e.g. SPAD-502, Soil Plant Analysis Development, Minolta Camera Co Ltd, Japan) through measurements of leaf chlorophyll based on the spectral transmittance properties of leaves in wheat (Reeves et al. Citation1993), sweet pepper (Madeira et al. Citation2003), peace lily (Wang et al. Citation2004), butterhead lettuce (León et al. Citation2007) and grapevine (Steele et al. Citation2008). Previous research has shown that canopy reflectance at wavebands around 550 nm (green region) and 670 nm (red region) was strongly related to leaf chlorophyll concentration (Thomas & Gausman Citation1977). Schlemmer et al. (Citation2005) reported that reflectance of corn leaves in the 630 nm region increased (the leaf turned from green to a more yellowish green colour) as chlorophyll content decreased. Gao et al. (Citation2008) reported that the optimal band for estimation of chlorophyll content was in the 698–715 nm range. Vegetation indices involving green and red wavebands, such as Normalized Pigment Chlorophyll-a Index (=(R680−R430)/(R680+R430)) (Peñuelas et al. Citation1993), Green Normalized Difference Vegetation Index (=(R750-R550)/(R750+R550)) (Gitelson et al. Citation1996) and R750/R550 (Gitelson & Merzlyak Citation1996), have been developed and used to estimate chlorophyll status on leaf and canopy levels of various plant species. Recently, Xue & Yang (Citation2009) stated that the normalized derivative difference ratio [=(D722–D700)/(D722+D700), (Le Maire et al. Citation2004)] gave a good estimation of chlorophyll content in vegetable leaves.
In general, estimation of chlorophyll status has been based on the relationships between chlorophyll content of leaves in the upper layer or whole canopy and spectral parameters (Serrano et al. Citation2000; Wright et al. Citation2004; Vuolo et al. Citation2010), whereas contributions of chlorophyll and/or spectral reflectance from specific vertical layers in the canopy has not been considered. As the most mobile element of the plant and a very important constituent of chlorophyll, N content declines with leaf aging (Monks & Efford Citation2006) and is always transferred from aged leaves to new ones (Charles-Edwards et al. Citation1987). Yamasaki et al. (Citation1996) reported that the content of chlorophyll per leaf area was highest in the uppermost young leaves and decreased with decreasing height level, indicating that there was a vertical gradient of chlorophyll abundance per leaf area through the plant canopy. Poorter et al. (Citation1995) stated that chlorophyll content per unit leaf weight changed with canopy layer. Zhou et al. (Citation2010) studied the relationship between the vegetation index, as a modified simple ratio (mSR705), and the chlorophyll content of the uppermost leaf and the third uppermost leaf of rice plants and concluded that use of the difference of the modified simple ratio between layer 1 and layer 3, i.e. mSR705L1–mSR705L3, greatly reduced the influence of the growth stage and genotype in assessing the N status using reflectance data. If leaf chlorophyll status in the middle and bottom canopy layers could be detected, corresponding N treatments may be applied in a timely manner due to the strong relationship between chlorophyll and N.
The objectives of this study were, first, to develop a spectral reflectance model for early detection of declining chlorophyll content in winter wheat canopies and, second, to validate the precision and accuracy of the resulting model.
Materials and methods
Experimental design
Modelling experiment
The modelling experiment was conducted during the 2002–03 growing season at the China National Experimental Station for Precision Agriculture (40°11′N, 116°27′′E), located in Changping district, Beijing, China. The soil was a silt clay loam. Pre-sowing soil (0–0.2 m) tests indicated organic matter at 14.2–14.8 g kg−1, total N at 0.8–1.0 g kg−1, available phosphorus (P) at 20.1–55.4 mg kg−1 and available potassium (K) at 117.6–129.1 mg kg−1. Soil was tested and fertilized to ensure no nutrient limitation.
The investigated wheat cultivars included eight erectophile plant types—4P3, 9128, 95128, 98-100, CA16, Chaoyou66 (CY66 for short), Jing411 (J411) and Lunxuan201 (LX201)—and seven planophile plant types—9428, 9507, Lunai17 (LA17), Linkang2 (LK2), Linkang502(LK502), Nongda3214 (ND3214) and Zhongmai9 (ZM9). All the cultivars are popularly planted in northern China. The model developed with these cultivars is anticipated to be suitable for early wheat N deficiency detection in northern China. A randomized complete block design with three replicates of each cultivar was employed. Each plot (3×5 m) received 180 kg ha−1 urea, 225 kg ha−1 (NH4)2HPO4 and 150 kg ha−1 K2SO4 before sowing. Topdressing N with an amount of 280 kg ha−1 urea was applied with two splits, 50% at Feekes 5.0 and 50% at Feekes 7.0. Sowing was on 21 October 2003 at a rate of 225 seeds m−2. The between-row space was 0.23 m. At elongation, the average plant density was 476.6 stems m−2 for the erectphile type and 434.4 stems m−2 for the planophile type, and the corresponding figures at anthesis were 409.8 stems m−2 and 381.9 stems m−2, respectively, because weaker stems died after elongation.
Validation experiment
The validation experiment was carried out at a site near the modelling experiment during the 2001–02 growing season at the same research station and on the same soil type as the modelling experiment. The nutrient contents of soil from 0–0.2 m depth were: organic matter 12.1–13.2 g kg−1, total N 0.9–1.2 g kg−1, available P 25.2–48.3 mg kg−1 and available K 96.6–128.8 mg kg−1.
An erectophile plant type, Jingdong8, and a planophile plant type, 9507, were investigated in this experiment. A randomized complete block design with four N fertilizer treatments (N0, N1, N2 and N3) and three replicates was used. Each treatment received 300 kg ha−1 (NH4)2HPO4 before sowing. No additional N was applied to N0. N1, N2 and N3 were supplied with an additional 150, 300 and 450 kg N ha−1, respectively. The N source was urea, which was applied with three splits: at pre-sowing (50% of the total amount), Feekes 5.0 (25% of the total amount) and Feekes 7.0 (25% of the total amount). Sowing was on 16 October 2002 at a rate of 225 seeds m−2. The between-row space was 0.23 m. At elongation, the average plant density was 554.9 stems m−2 for Jingdong8 and 596.1 stems m−2 for 9507, and the corresponding figures at anthesis were 426.3 stems m−2 and 453.4 stems m−2, respectively, with more weaker stems dying after elongation compared with the modelling experiment.
Field and laboratory measurements
In each plot, an area (1 m2) of the wheat canopy was selected for canopy spectral reflectance measurements, physiological and biochemical analyses. Measurements were performed at elongation and anthesis in both modelling and validation experiments.
Wheat canopy radiance measurements were taken from a height of 1.3 m above ground (the height of the wheat was 0.9±0.05 m at maturity), under clear sky conditions between 10:00 and 14:00, using an ASD FieldSpec Pro spectrometer (Analytical Spectral Devices, Boulder, CO, US) fitted with 25o field of view (FOV) fibre optics, operating in the 350–2500 nm spectral region with a sampling interval of 1.4 nm between 350 and 1050 nm, and 2 nm between 1050 and 2500 nm, and with spectral resolution of 3 nm at 700 nm, 10 nm at 1400 nm. The 1.3 m measurement height coupled with 25o FOV equated to 0.26 m2 area on the ground, which gives the appropriate ground sample area with less soil background influence under our experimental design (the between-row space was 0.23 m). A 0.4×0.4 m BaSO4 calibration panel was used as a reference (Herrmann et al. Citation2010). Twenty scans were recorded for each plot, and the canopy radiance measurements were taken by averaging these at an interval of 1 sec, with a dark current correction with every spectral measurement. Reference panel radiance measurements were taken before and after the canopy radiance measurement. The wheat canopy radiance was calculated using the equation below:
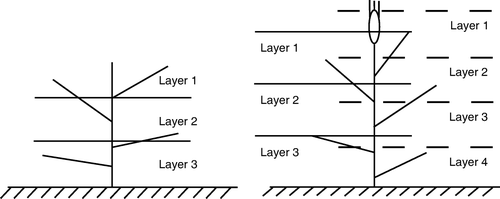
Following canopy spectral measurements, the PAR at the top of every layer and at the bottom of the whole canopy was measured with a line quantum sensor (LI-191SA, LI-Cor Inc, Lincoln, NE, US), which measures PAR under spatially non-uniform canopies. Plants in the 1 m2 area were divided into three to four layers of equal vertical thickness (three in the modelling experiment, four in the validation experiment, ). The height was standardized as the actual height of the plant at elongation, and the average height from the top of the flag leaf to the ground at anthesis. The line quantum sensor was held with both ends between two rows and parallel with the row direction.
Figure 1 Sketch map of canopy layers at elongation (left) and anthesis (right) stages. At anthesis, the unbroken line is for the modelling experiment, and the broken line for the validation experiment.
Leaf area index (LAI) and chlorophyll analysis were performed at elongation and anthesis for both modelling and validation experiments. Whole-plant samples were taken immediately after the spectral and PAR measurements, placed in a cooled black plastic bag and transported to the laboratory for subsequent analysis. The plants in the 1 m2 area were cut into three or four layers of equal vertical thickness according to the standard used for PAR determination. Leaves in each layer were collected together for LAI, leaf mass per unit area (LMA, g m−2), and chlorophyll content (mg g−1 fresh leaf weight) measurements. The LAI was determined by a dry weight method (Zhu et al. Citation1998). Approximately 60 cm2 reference leaf segment was cut from the central part of 20–30 leaves in each individual layer. The reference leaf segments and the rest of the sample leaves were oven-dried at 70 oC to constant weight and weighed, respectively. Leaf area index was calculated as:
Chlorophyll was extracted with 80% acetone immediately after the samples were transported to the laboratory. The absorption of the extracts at wavelengths of 645 nm and 663 nm were measured with a Helios spectrophotometer (Thermo Electron Company, Cambridge, UK). The chlorophyll content was calculated using the formula of Arnon (Citation1949) as total chlorophyll (mg g−1)=20.3×D645+8.04×D663, where D645 and D663 are chlorophyll absorbance at 645 and 663 nm, respectively.
Data analysis
Hinzman et al. (Citation1986) reported that canopy chlorophyll density (CCD)—the total amount of chlorophyll present in the canopy per unit of ground area—was a sensitive indicator of N deficiencies in wheat. Referring to their concept, we calculated CCD (g m−2) as in equation (Equation3) below:
Because leaves in layers 2 and 3 (or 2–4 for the validation experiment) were not fully exposed to sunlight (Woolley Citation1971), we calculated the combined canopy chlorophyll density (CCCD) from each individual layer using the attenuation degree (AD) of PAR in each layer as a weighting factor. A series of calculations were necessary, beginning with the calculation of actual radiation (RAD) received by a given layer as in equation (Equation4):
Difference vegetation index (DVI) (Jordan Citation1969), calculated from the reflectance in red (670 nm) and NIR (890 nm) wavelength regions, was used to estimate CCD. Filella et al. (Citation1995) and Serrano et al. (Citation2000) pointed out that increases in red reflectance were related to the decreases in chlorophyll content resulting from lower N supply. Soudani et al. (Citation2006) reported that reflectance in the NIR region correlated significantly with LAI.
The leaf chlorophyll content and CCD in each layer across all the 15 cultivars, and the averages of both leaf chlorophyll content and CCD in each layer across erectophile and planophile plant types in the modelling experiment were subjected to analysis of variance (ANOVA). The least significant difference test was performed using the GLM procedure in SAS 9.1 (SAS Institute 2003). The observed and estimated CCCDn were subjected to linear regression analysis to evaluate the value of the model. We calculated both root mean squared error (RMSE), as in equation (Equation8), and modelling efficiency (MEF) (Loague & Green Citation1991), as in equation (Equation9), to quantify the degree of fit between observations and simulations.
Results and discussion
Leaf chlorophyll content and canopy chlorophyll density in different layers in the modelling experiment
Leaf chlorophyll content decreased with canopy layer at elongation and anthesis in both erectophile and planophile plant types (), confirming the results of previous studies (Yamasaki et al. Citation1996; Wang et al. Citation2005). ANOVA showed that there was no significant difference in leaf chlorophyll content among cultivars in each layer (data not shown). Compared with canopy layer 1, layers 2 and 3 had 9–21% less chlorophyll at elongation. However, at anthesis, layers 2 and 3 had as much as 16–20% less chlorophyll. Differences among layers in both erectophile and planophile plant types were significant at both elongation (P<0.01) and anthesis (P<0.0001) ().
Figure 2 Leaf chlorophyll content (mg g−1) in different canopy layers at elongation and anthesis of both erectophile and planophile plant types. Data for erectophile and planophile plant types are averaged from eight erectophile plant type cultivars and seven planophile plant type cultivars, respectively. Letters A, B and C following lines at elongation indicates significance at P < 0.01, and letters A, B and C following lines at anthesis indicates significance at P < 0.0001.
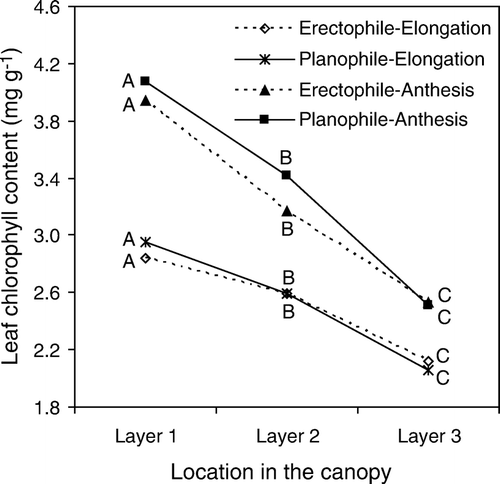
Table 1 Canopy chlorophyll density (CCD, g m−2) in different layers at elongation and anthesis.
Filella et al. (Citation1995) noted that LAI values are related to the amount of leaf tissue in the field of view of the reflectance sensor. Stanhill et al. (Citation1972) stated that biomass was the main factor in the reflectance response of N-deficient wheat canopies. Hinzman et al. (Citation1986) suggested that chlorophyll density in the canopy was related to both leaf chlorophyll content and LAI, which correlated with spectral reflectance at red and NIR wavelength regions (Gitelson et al. Citation1996; Soudani et al. Citation2006), respectively. Therefore, canopy chlorophyll status in this study was expressed as an integrated measure, i.e. CCD, of leaf chlorophyll content, LAI and LMA. In our study, the CCD in layer 2 was generally greater than that in layer 1 at both elongation and anthesis stages, while the CCD value in layer 3 was the lowest among the three layers ( and ). At elongation, the percentage CCD in layers 1 and 2 accounts for 77.0% of the CCD of the whole canopy for the erectophile plant type and 81.3% for the planophile plant type. At anthesis, the corresponding figures were 75.1% and 80.6% (). For the erectophile plant type, highly significant differences in CCD were observed among the three layers at elongation (P<0.0001), and between layers 1 and 2, on one hand, and layer 3, on the other hand, at anthesis (P<0.01). In the planophile plant type, there was no significant difference in CCD between layer 1 and layer 2, while CCD in layer 3 was significantly different from those in both layer 1 and layer 2 (P<0.0001 at elongation, and P<0.01 at anthesis) (). The results indicated that the planophile plant type had higher CCD in the upper two layers, and lower CCD in the bottom layer in comparison with the erectophile plant type. No significant difference in the CCD among all the 15 investigated cultivars was observed within each layer (data not shown).
Figure 3 Canopy chlorophyll density (CCD, g m−2) in different layers at elongation and anthesis of both erectophile plant type and planophile plant type. Data for erectophile and planophile plant types are averaged from eight erectophile plant type cultivars and seven planophile plant type cultivars, respectively. Letters A, B and C following lines at elongation indicates significance at P < 0.0001, and letters A and B following lines at anthesis indicates significance at P < 0.01. The same letters between layers show no significant difference.
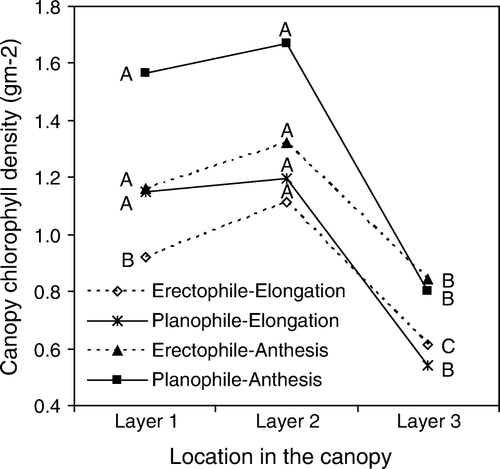
Table 2 The percentage of CCD (canopy chlorophyll density, g m−2) in each layer of the whole canopy of erectophile and planophile plant types at elongation and anthesis.
Model development
The coefficients of determination (R2) between CCD in layers 1, 2 and 3 and DVI were 0.59, 0.71 and 0.30 at elongation and 0.55, 0.68 and 0.52 at anthesis, respectively, in the modelling experiment (). Since each layer had a different contribution to the canopy reflectance, the degree of contribution of each individual layer in the canopy was calculated as a combined weight (w). The CCCD1, CCCD1+2 and CCCD1+2+3, which included CCD from layer 1, layers 1 and 2, and all three layers, respectively, were calculated with the w value. The R2 between CCCD1 + 2 and CCCD1 + 2+3 and DVI were 0.73 and 0.80 at elongation, and 0.70 and 0.74 at anthesis, respectively (). The relationship between chlorophyll status and DVI was strengthened by CCCD1 + 2 and CCCD1 + 2+3 when compared with layer 1 alone. Therefore, the information in layers 1 and 2 or layers 1, 2 and 3, instead of the information in layer 1 alone, should be taken into consideration when assessing canopy chlorophyll status. The layers involved should be combined according to their contribution to the canopy reflectance. The models developed with the regression method based on the relationship between DVI and CCCD_n are presented in .
Table 3 The coefficient of determination (R2) between CCD (canopy chlorophyll density, g m−2) and combined CCD (CCCD, g m−2) and DVI (difference vegetation index) in the modelling experiment.
Model validation
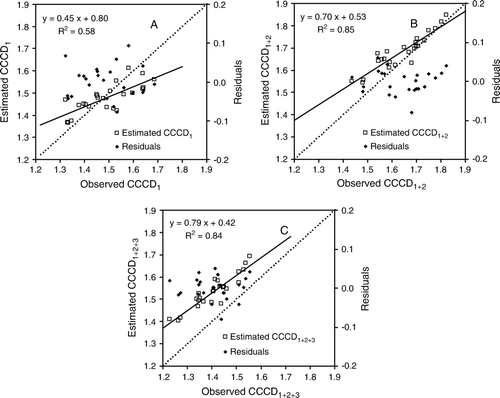
Data collected during the elongation phase of the validation experiment were used to validate the three developed models (). Regression residual plots showed that all three models were well supported (). The RMSE of estimated vs. observed CCD of models CCCD1, CCCD1 + 2 and CCCD1 + 2+3 were 0.07, 0.06 and 0.13, respectively, and the MEF of CCCD1, CCCD1+ 2 and CCCD1 + 2+3 were 0.54, 0.66 and −1.39, respectively. Model CCCD1 + 2 had relatively higher MEF and lower RMSE. However, the MEF of model CCCD1 + 2+3 was negative with an absolute value larger than 1, indicating that chlorophyll status was being overestimated (all the points produced by estimations and observations in C were above the y=x line). Overestimation using the CCCD1+2+3 may have been due to an inflated value for w. From , the R2 and slope of estimated vs. observed CCCD for the CCCD1 + 2 and CCCD1 + 2+3 models were higher than that of CCCD1, and their intercepts were lower.
Figure 4 Comparison of: A, observed CCCD1 and estimated CCCD1; B, observed CCCD1 + 2 and estimated CCCD1 + 2; and C, observed CCCD1 + 2+3 and estimated CCCD1 + 2+3 at elongation of the validation experiment. CCCD is combined canopy chlorophyll density. F0.0(1,22)=0.515.
Unfortunately, the models developed based on the data at anthesis could not be validated. Data collected at anthesis were not used for model validation because layers at anthesis in the validation experiment were not equal to those in the modelling experiment. Because radiation in the NIR region had strong transmittance (Woolley Citation1971) and CCCD1 + 2+3contained the chlorophyll information in the whole canopy, the CCCD1 + 2+3 model was supposed to yield higher accuracy and precision. However, this hypothesis was not supported in this study. This could also be attributed to the w value used in the CCCD1 + 2+3 model. An alternative method to define the contribution of layer 3 to whole canopy reflectance should be developed in a future study.
Table 4 Models established based on the relationship between DVI and CCCDn. DVI is difference vegetation index; CCCD (g m−2) is combined canopy chlorophyll density. The n in CCCD is 1, 1 + 2 and 1 + 2+3, respectively. Data are from the modelling experiment, and data from both planophile and erectophile are used together to develop the model. Chl, chlorophyll; N, nitrogen. The units of leaf N content, leaf chlorophyll concentration are mg g−1 dry leaf weight and mg g−1 fresh leaf weight, respectively.
Conclusion
Remotely-sensed chlorophyll status offers the possibility of rapidly estimating crop N status for improved N management strategies. A method based on the relationship between canopy vertical chlorophyll distribution and the characteristics of canopy reflectance was developed to detect chlorophyll status in lower layers in winter wheat canopy. Both CCCD1 + 2 (CCD combined with both layers 1 and 2) and CCCD1 + 2+3 (CCD combined with layers 1, 2 and 3) were better related to DVI than CCD in any individual layer. A model based on CCCD1 + 2 explained the majority of the variability in chlorophyll status (R2=0.73 and 0.70 at elongation and anthesis, respectively) and showed lowest RMSE and highest MEF when predicting winter wheat canopy chlorophyll status. Therefore, chlorophyll status in the two uppermost layers of the wheat canopy could be quantified using the DVI, and early detection of chlorophyll deficiency was achieved.
Acknowledgements
This work was supported by National Natural Science Foundation of China (40571118, 40701119, 40701120) and the National High Tech R&D Program of China (2006AA10A302, 2006AA12Z138). Thanks to Dr Zhihong Ma, Ms Hong Chang, Mr Beihong Wang, Mr Weiguo Li and Mr Yanbo Wang for their contributions in sample analysis and hyperspectral data obtaining, and to Dr Akira Hirano (JIRCAS, Japan International Research Center for Agricultural Sciences) for his suggestions in writing.
References
- Anderson , MC . 1967 . Photon flux, chlorophyll content, and photosynthesis under natural conditions . Ecology , 48 : 1050 – 1053 .
- Arnon , DI . 1949 . Copper enzymes in isolated chloroplasts Polyphenoloxidae in Beta vulgaris . Plant Physiology , 24 : 1 – 15 .
- Charles-Edwards , DA , Stutzel , H , Ferraris , R and Beech , DF . 1987 . An analysis of spatial variation in the nitrogen content of leaves from different horizons within a canopy . Annals of Botany (London) , 60 : 421 – 426 .
- Filella , I , Serrano , L , Serra , J and Peñuelas , J . 1995 . Evaluating wheat nitrogen status with canopy reflectance indices and discriminant analysis . Crop Science , 35 : 1400 – 1405 .
- Gao Y , Chen L , Zhou X , Li L , Liu Q , Tian G 2008 . Analysis on optimal bands for retrieval of mixed canopy chlorophyll content based on remote sensing The International Archives of the Photogrammetry, Remote Sensing and Spatial Information Sciences. XXXVII, Part B7 : 1391 – 1395 .
- Gitelson , AA , Kaufman , YJ and Merzlyak , MN . 1996 . Use of a green channel in remote sensing of global vegetation from EOS-MODIS . Remote Sensing and Environment , 58 : 289 – 298 .
- Gitelson , AA and Merzlyak , MN . 1996 . Signature analysis of leaf reflectance spectra: algorithm development for remote sensing of chlorophyll . Journal of Plant Physiology , 148 : 494 – 500 .
- Herrmann I , Pimstein A , Karnieli A , Cohen Y , Alchanatis V , Benfil JD 2010 . Utilizing the VENµS red-edge bands for assessing LAI in crop fields. ISPRS Archive Vol. XXXVIII, Part 4-8-2-w9, Core Spatial database—Updating, maintenance and services—From Theory to Practice . Haifa, Israel . Pp. 34 – 39 .
- Hinzman , LD , Bauer , ME and Daughtry , CST . 1986 . Effects of nitrogen fertilization on growth and reflectance characteristics of winter wheat . Remote Sensing and Environment , 19 : 47 – 61 .
- Jordan , CF . 1969 . Derivation of leaf area index from quality of light on the forest floor . Ecology , 50 : 663 – 666 .
- Le Maire , G , Francois , C and Dufrene , E . 2004 . Towards universal broad leaf chlorophyll indices using PROSPECT simulated database and hyperspectral reflectance measurements . Remote Sensing of Environment , 89 : 1 – 28 .
- León , AP , Viña , SZ , Frezza , D , Chaves , A and Chiesa , A . 2007 . Estimation of chlorophyll contents by correlations between SPAD-502 meter and chroma meter in butterhead lettuce . Communications in Soil Science and Plant Analysis , 38 : 2877 – 2885 .
- Loague , K and Green , RE . 1991 . Statistical and graphical methods for evaluating solute transport models: Overview and application . Journal of Contaminant Hydrology , 7 : 51 – 73 .
- Madeira , A , Ferreira , A , De Varennes , A and Vieira , M . 2003 . SPAD meter versus tristimulus colorimeter to estimate chlorophyll content and leaf color in sweet pepper . Communications in Soil Science and Plant Analysis , 34 : 2461 – 2470 .
- Monks , A and Efford , MG . 2006 . Selective herbivory by brushtail possums: Determining the age of ingested leaves using n-alkanes . Austral Ecology , 31 : 849 – 858 .
- Peñuelas , J , Gamon , JA , Griffin , KL and Field , CB . 1993 . Assessing community type, plant biomass, pigment composition, and photosynthetic efficiency of aquatic vegetation from spectral reflectance . Remote Sensing of Environment , 46 : 110 – 118 .
- Poorter , L , Oberbauer , SF and Clark , DB . 1995 . Leaf optical properties along a vertical gradient in a tropical rain forest canopy in Costa Rica . American Journal of Botany , 82 : 1257 – 1263 .
- Reeves , D , Mask , P , Wood , C and Delano , D. 1993 . Determination of wheat nitrogen status with hand held chlorophyll meter: Influence of management practices . Journal of Plant Nutrition , 16 : 781 – 796 .
- SAS Institute . 2003 . SAS for Windows. v. 9.1 . SAS Inst., Cary, NC.
- Schlemmer , MR , Francis , DD , Shanahan , JF and Schepers , JS . 2005 . Remotely measuring chlorophyll content in corn leaves with differing nitrogen levels and relative water content . Agronomy Journal , 97 : 106 – 112 .
- Serrano , L , Filella , I and Peñuelas , J . 2000 . Remote sensing of biomass and yield of winter wheat under different nitrogen supplies . Crop Science , 40 : 723 – 731 .
- Soudani , K , François , C , Le Maire , G , Le Dantec , V and Dufrêne , E . 2006 . Comparative analysis of IKONOS, SPOT, and ETM+ data for leaf area index estimation in temperate coniferous and deciduous forest stands . Remote Sensing of Environment , 102 : 161 – 175 .
- Stanhill , G , Kafkaf , V , Fuchs , M and Kagan , Y . 1972 . The effect of fertilizer application on solar reflectance from a wheat crop . Israel Journal of Agricultural Research , 22 : 109 – 118 .
- Steele , MR , Gitelson , AA and Rundquist , DC . 2008 . A comparison of two techniques for nondestructive measurement of chlorophyll content in grapevine leaves . Agronomy Journal , 100 : 779 – 782 .
- Thomas , JR and Gausman , HW . 1977 . Leaf reflectance vs. leaf chlorophyll and carotenoid concentrations for eight crops . Agronomy Journal , 69 : 799 – 802 .
- Vuolo F , Atzberger C , Richter K , D'Urso G , Dash J 2010 . Retrieval of biophysical vegetation products from Rapideye imagery . In : Wagner W , Székely B ISPRS TC VII Symposium—100 Years ISPRS, Vienna, Austria, 5–7 July 2010, IAPRS, XXXVIII, Part 7A : 281 – 286 .
- Wang , Q , Chen , J and Li , Y . 2004 . Nondestructive and rapid estimation of leaf chlorophyll and nitrogen status of peace lily using a chlorophyll meter . Journal of Plant Nutrition , 27 : 557 – 569 .
- Wang J , Zhao C , Huang W , Liu L , Wang Z 2005 . Extracting canopy vertical distribution information by canopy-reflected spectrum in winter wheat . In : Larar AM , Suzuki M , Tong Q Multispectral and Hyperspectral Remote Sensing Instruments and Applications II. Proceedings of the SPIE 5655 : 388 – 397 .
- Wright DL , RasmussenVP , Ramsey RD , Baker DJ 2004 . Canopy reflectance estimation of wheat nitrogen content for grain protein management . GIScience and Remote Sensing 41 : 287 – 300 .
- Woolley , JT . 1971 . Reflectance and transmittance of light by leaves . Plant Physiology , 47 : 656 – 662 .
- Xue , L and Yang , L . 2009 . Deriving leaf chlorophyll content of green-leafy vegetables from hyperspectral reflectance . Journal of Photogrammetry and Remote Sensing , 64 : 97 – 106 .
- Yamasaki , T , Kudoh , T , Kamimura , Y and Katoh , S . 1996 . A vertical gradient of the chloroplast abundance among leaves of Chenopodium album . Plant and Cell Physiology , 37 : 43 – 48 .
- Zhou , Q , Liu , Z and Huang , J . 2010 . Detection of nitrogen-overfertilized rice plants with leaf positional difference in hyperspectral vegetation index . Journal of Zhejiang University-Science B (Biomedicine & Biotechnology) , 11 : 465 – 470 .
- Zhu Y , Cui J , Guo T , Wang C , Zhang S , Li J , Wang Y 1998 . Study on the growth and development regularity of Wenmai 6 and the crucial cultivated technique for super-high-yielding. Acta Agronomica 24 : 947 – 951 . ( in Chinese with English abstract )