Abstract
Despite being rated as one of the world's worst weeds, Equisetum hyemale was until recently imported into New Zealand for use by the floristry industry, subject to devitalization. It was subsequently found that imported stems were not being successfully devitalized and importation was banned. This study, in two trials, used different dilutions of six herbicides, including Roundup, the one listed in the MAF Biosecurity NZ standard, to investigate if it were possible to render stems non-propagable without destroying their commercial use. This research found that a Sharpshooter (oryzalin) formulation at 1× and 1:2 dilutions of the field application strength was the only herbicide that completely devitalized the stem material with minimal impact on stem quality. Roundup at the concentration required by MAF Biosecurity NZ destroyed the stems, while 1:5 and 1:10 dilutions reduced the ability of the stems to be propagated, but still caused stem damage. AGPRO activated amitrole, Duplosan, Agritone and Kamba were not successful as devitalization agents.
Introduction
New Zealand's horticulture and agriculture industries rely on strong biosecurity systems to prevent the arrival of unwanted organisms that could become pests. A range of cut flowers and foliages are imported into New Zealand for the floristry trade from a number of countries, including Australia, the US, India, Colombia, Singapore, Malaysia, Thailand and several Pacific islands. Importation of these products is controlled in part by the New Zealand Ministry of Agriculture and Forestry Biosecurity Standard 152.09.05. Some of the plants listed in this standard could be considered biosecurity risks: some are known to be invasive alien species elsewhere; some are in the same genus as species that have become invasive in other countries; and others may harbour pests as yet unknown in New Zealand. Ministry of Agriculture and Forestry (CitationMAF) Standard 152.09.05 requires that listed imported material must be subjected to an approved devitalization treatment to render it non-propagable, to prevent invasion by these potential weeds or other pests they may carry.
Horsetails (Equisetum spp.) are rated as being among the world's worst weeds (Holm et al. Citation1977), yet until recently, E. hyemale L. was imported from the US or Australia into New Zealand for use by the floristry industry (Large et al. Citation2006). The base of stems were required to be devitalized by immersion for 20 min in a solution of 0.5% Roundup® (360 g/L glyphosate), as per Ministry of Agriculture and Forestry Standard 152.09.05.
Large et al. (2006) found that imported stems of E. hyemale were not in fact being successfully devitalized and could be grown and propagated easily. They suggested that research into alternative treatments would be desirable. MAF subsequently modified the standard to prohibit the importation of E. hyemale into New Zealand, which while reducing the threat for further incursions of this pest species, does cause some inconvenience to the floristry industry.
Successful devitalization of floristry products has two main components:
| 1. | to render the plant material unpropagable by spore, shoot or rhizome production | ||||
| 2. | to do this without significantly reducing its commercial use i.e. retaining floral quality and a dark green stem colour. | ||||
There is little information in the literature on the use of herbicides for devitalization of floristry materials. Non-residual translocated herbicides such as glyphosate are directed at the foliage of a plant, which then absorbs the molecules of the herbicide active ingredient(s). Herbicide molecules are then translocated in the phloem. While devitalization treatments may not contact the foliage of the plant material, stems can also absorb herbicides, in some cases as successfully as through the leaves, and many herbicides are also capable of being transported in the xylem (Rao Citation1999). The Ministry of Agriculture and Forestry Standard 152.09.05 relies on uptake of the herbicide through the cut stems and transport to the leaves and flowers.
Simple inexpensive treatments are the most practical as devitalization is currently carried out in exporting countries under local conditions. For this study it was decided to trial a range of commercially available herbicides with known ability to control species of Equisetum in the field. A number of experiments have used different post-emergence herbicides to try to control the related E. arvense in the field. Some trials have shown glyphosate to be effective (Uchida et al. Citation1998), ineffective (Banaszkiewicz & Kopytowski Citation2003) or effective depending on which line of E. arvense was tested (Uchida et al. Citation2000). Several authors reported achieving good control with MCPA (Weide & Slabbekoorne Citation2001), MCPA and duplosan (Hallgren Citation1996), MCPA and dicamba (Rapparini et al. Citation2002) and amitrole (Vezina Citation1990). Kuriyama et al. (Citation1994) used the pre-emergence herbicide oryzalin (the active ingredient of Sharpshooter) to inhibit the growth of Equisetum in culture. It was decided to investigate whether commercially available versions of these products at field dilutions, and further dilutions of these, would successfully devitalize E. hyemale.
Materials and methods
Six herbicides (AGPRO activated amitrole [hereafter referred to as amitrole], Agritone 720, Duplosan® KV, Kamba 500, Roundup Transorb® and Sharpshooter) were trialled. Details of common names, chemical names, strength of concentrates and suppliers are given in . Trade names of the herbicide treatments are used throughout this paper to indicate the formulation of the active ingredient. Stock solutions of all herbicides were made by diluting concentrates with tap water. The Roundup stock solution was equivalent to the MAF Standard 152.09.05 (0.5% Roundup® [the newer 540 g/L formulation was converted to 360 g/L glyphosate rate, as per MAF instructions]). For the other herbicides where there are no recommendations, dilutions were made to the approximate broadscale field application dilutions on the commercial container labels i.e. an average hectare product rate was divided by an average hectare water rate to give the dilution used in the trial. Details of the stock dilutions (1×) and further concentrations are given in . These stock solutions were labelled as 1× concentration and each was further diluted. Controls of tap water were also used. Two separate trials were conducted. Both trials used 1×, 1:10 and 1:100 concentrations for each herbicide. Trial 2 included additional 1:5 treatments for Agritone 720, Duplosan® KV, amitrole and Roundup Transorb®, and 1:2 for Sharpshooter, based on the results of the first trial. Treatment concentrations used in this paper are shown in .
Table 1 Herbicide details.
Stems of E. hyemale were cut from live plants to the same length (30 cm) and the bases of a set of five stems were, within minutes of cutting, immersed in each treatment to 20 cm depth for 20 min, air dried for 30 min at room temperature, then placed in beakers of fresh tap water. Stems were placed in a culture room with cool white fluorescent lights (16 h light/8 h dark) at 24 °C in November 2008 for 25 days to simulate a commercially accepted vase life.
For each stem, dieback, colour changes, root growth and shoot growth were recorded 25 days after treatment. These recordings were used to grade stems for floristry quality at both 14 and 25 days (1 = dead or dying; 2 = some discoloration, significant tip dieback; 3 = stems green with minimal discoloration) (). The degree of devitalization was then rated on a scale of 1–3 (1 = minimal devitalization, growth of suckers, shoots and roots; 2 = some devitalization, no suckers but growth of shoots and/or roots; 3 = complete devitalization, material dead) (). Means and standard errors are given in and .
Figure 1 Floristry ratings for stem colour after treatment. Left, Dead stems, score 1.0. Right, A–D, Acceptable quality, score 3.0. E, Discoloured, score 2.0. Bar = 20 mm.
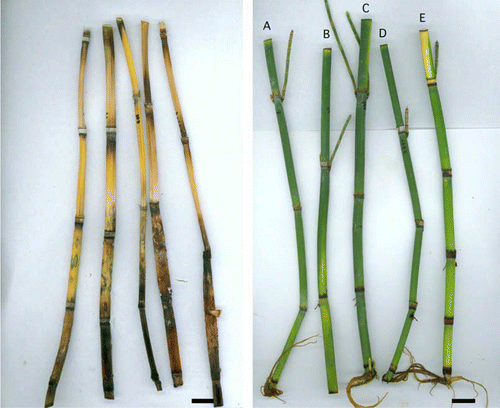
Figure 2 Biosecurity ratings (suckers, shoots, roots, dead). Left, A, C, Minimal devitalisation, score 1.0. B (shoots), D (roots), Partial devitalisation, score 2.0. Right, A–D, Complete devitalisation, score 3.0. E, Minimal devitalisation, score 1.0. Bar = 20 mm.

Table 2 Means and standard errors of all treatment results for Trial 1.
For Trial 2, after making the final observations of stem quality, all living stems were planted in standard potting mix and placed in a greenhouse under shade cloth with irrigation for a further 140 days to determine their ability to grow. The degree of devitalization was again rated using the above scoring system. Means and standard errors are given in .
Table 3 Means and standard errors of all treatment results for Trial 2.
Means and standard errors were generated using Excel version 2003. The experimental design was a completely randomized design, using five replicates of a single stem.
Results and discussion
Potentially successful treatments
Treatments were considered to be successful if devitalization was achieved as well as a commercially viable vase life. Treatment means for devitalization with wider variability raise the possibility of shoot or root growth in some samples and so these treatments were considered unsuccessful devitalizing treatments ( and ). After 25 days in treatment solutions none of the treatments in either trial seemed to consistently devitalize stems of E. hyemale without causing discoloration or death ( and and ). Potentially successful treatments were identified 140 days after planting the stems in commercial potting mix ().
Figure 3 Devitalisation vs floristry quality at 25 days, Trial 1. Devitalisation (1 = minimal devitalisation—growth of suckers, shoots and roots; 2 = some devitalisation, no suckers but growth of shoots and/or roots; 3 = complete devitalisation, material dead). Floristry quality (1= dead or dying; 2 = some discoloration, significant tip dieback; 3 = stems green with minimal discoloration).
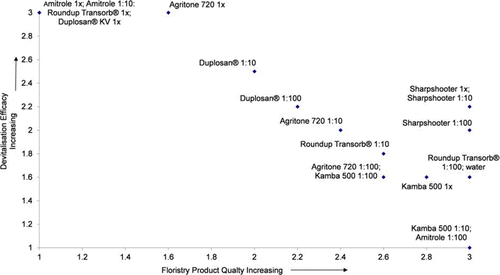
In Trial 1, at 25 days, stems treated with Sharpshooter at 1×, 1:10 and 1:100 maintained stem quality and produced no roots. Shoots were produced in these treatments which reduced their devitalization score. A similar result was seen in Trial 2 for Sharpshooter at 1×, 1:2 and 1:100. After being planted out in potting mix for 140 days, stems treated with the two stronger treatments of Sharpshooter (at 1× and 1:2) did not grow and the adventitious shoots died (). Some shoot growth continued on stems treated with Sharpshooter at 1:100.
Figure 4 Devitalisation vs floristry quality at 25 days, Trial 2. Devitalisation (1 = minimal devitalisation—growth of suckers, shoots and roots; 2 = some devitalisation, no suckers but growth of shoots and/or roots; 3 = complete devitalisation, material dead). Floristry quality (1 = dead or dying; 2 = some discoloration, significant tip dieback; 3 = stems green with minimal discoloration).
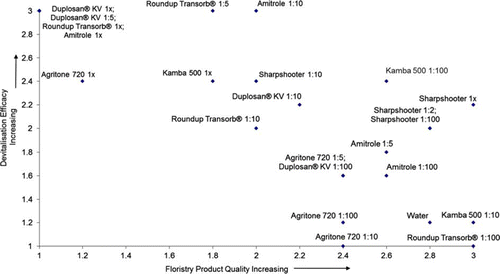
Unsuccessful treatments
Treatments that did not devitalize E. hymale after 25 days in both trials were: water; Roundup Transorb®1:100; Kamba 500 1:10; Agritone 720 1:100; and amitrole 1:100. Agritone 720 1:10 caused partial devitalization in Trial 1 but did not in Trial 2. After 140 days in potting mix, the Trial 2 treatments with the least effect were: water; Roundup Transorb®1:100; Duplosan® KV 1:100; and Agritone 720 1:10.
Figure 5 Devitalisation after 140 days in greenhouse vs floristry quality at 25 days, Trial 2. Devitalisation (1 = minimal devitalisation—growth of suckers, shoots and roots; 2 = some devitalisation, no suckers but growth of shoots and/or roots; 3 = complete devitalisation, material dead). Floristry quality (1 = dead or dying; 2 = some discoloration, significant tip dieback; 3 = stems green with minimal discoloration).
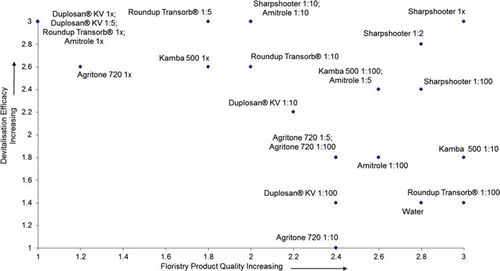
Stem quality for commercial floristry use was reduced at 25 days for both trials by the following treatments: Duplosan® KV 1×; Roundup Transorb® 1×; amitrole 1×; and Agritone 720 1×. In Trial 1, amitrole 1:10, and in Trial 2 Kamba 500 1×, Duplosan® KV 1:5 and Roundup Transorb® 1:5 also caused deterioration in stem quality ().
Variability in treatment results was high for Kamba across all concentrations which may indicate issues with variability in stem absorption characteristics across E. hymale stems.
Individual herbicides
Roundup Transorb® at the concentration required by MAF Biosecurity NZ for devitalization (1× concentration in this study) caused complete mortality and destruction of the stem material in both trials making stems unusable for commercial floristry (–). Roundup Transorb® at 1:5 and 1:10 dilutions reduced the ability of the stems to propagate, but caused deterioration in quality at 25 days in Trial 2. These treatments could potentially reduce the possibility of shoot growth, but not eliminate it, and compromised extended commercial use of the plant material. Only the 1:100 dilution left the stems in an optimum condition as a floristry product, but provided minimal devitalization. These results indicate that glyphosate is not suitable for the devitalization of horsetails produced for use in floristry.
Glyphosate, the active ingredient of Roundup, disrupts the shikimic acid pathway that is responsible for producing the amino acids involved in protein synthesis and cell division in the meristems of plants (Amrhein et al. 1980; Ross & Childs Citation1996). Higher concentrations of Roundup inhibited both roots and shoot growth and caused yellowing, tip death and complete death of material. The 1:10 treatment caused blackening of the lower internodes. This activity of glyphosate on meristems may be the cause of the blackened lowest internodes of the glyphosate treated E. hyemale stems.
The reasons behind the original use of glyphosate for devitalization of horsetails are not completely clear. Apart from Large et al. (Citation2006), there does not seem to be any literature on the devitalization of horsetails using glyphosate or any other product. The literature on the use of glyphosate for devitalization appears to be limited to one study where Ahmed & Zaharah (Citation1998) investigated the effects of glyphosate on roses and carnations. They found that glyphosate accumulated in rose buds and carnation nodes, inhibiting propagation from treated rose buds, but killing the carnations (Ahmed & Zaharah Citation1998).
Amitrole treatments did not both devitalize stems and maintain stem quality for floristry in this experiment. Treatment with amitrole at 1× and the 1:10 dilution effectively devitalized E. hyemale stems, treatment at a dilution of 1:5 produced partial devitalization and the dilution of 1:100 did not effectively devitalize E. hyemale stems (–). Amitrole caused rapid yellowing and death of stems (1× and 1:10), but allowed stems to remain green at 1:5 and 1:100 concentrations. However, both of these concentrations also allowed some root and shoot growth. It is not clear why the 1:5 concentration allowed some root and shoot growth in the second trial in some stems. Amitrole is a chlorophyll/carotenoid pigment inhibitor and travels symplastically, generally causing the depigmentation of new growth (Ross & Childs Citation1996) which is consistent with the rapid yellowing observed.
Duplosan® KV (Mecoprop/MCPP) and Agritone 720 (MCPA) are phenoxyaliphatic acids and Kamba 500 (dicamba) is a benzoic acid. High concentrations of all three herbicides caused mortality, lower concentrations caused discolouring, inhibition and deformation of shoots and roots, but allowed the formation of suckers when stems were planted in soil. All three herbicides are auxin growth inhibitors and are downwardly mobile, travelling symplastically from the leaves to sites of metabolic activity such as meristems. Symptoms tend to appear in new growth first, with pigment loss, stoppage of growth and distorted new growth when used as a herbicide (Ross & Childs Citation1996). These symptoms are similar to those observed in this study in E. hyemale.
The control treatment (water) did not devitalize stems and stems remained acceptable as a florist product. Stems remained dark green and produced normal shoots and roots.
Sharpshooter at the higher concentrations of 1× and 1:2 effectively devitalized E. hyemale stems. There was some tip dieback, but stem colour remained green. This herbicide allowed some shoot growth, but severely inhibited root growth. After stems were planted out in potting mix, these shoots and the original stems died. At 1:100 the Sharpshooter treatment did not effectively render all of the treated stems unpropagable. These results show that effective elimination of propagability while maintaining stem quality is only likely with Sharpshooter (oryzalin) at standard field application rates, while reduction of propagability can be achieved with slightly lower concentrations.
The active ingredient of Sharpshooter, oryzalin, is a dinitroaniline. As herbicides, dinitroanilines are pre-emergent herbicides causing distorted growth in higher plants (Kuriyama et al. Citation1994). In roots, cell division and mitosis are disrupted (Bayer al. Citation1967; Ross & Childs Citation1996). Kuriyama et al. (Citation1994) found that oryzalin inhibited sporophytic shoot initiation in cultures of E. arvense particularly at temperatures of 20–40 °C. Shoot development was also inhibited, apparently due to the meristem becoming inactive (Kuriyama et al. Citation1994). The root initials seen in this experiment on E. hyemale stems treated with Sharpshooter at all except the 1:100 treatment did not grow any further, suggesting disruption of cell division may have occurred.
Dinitroanilines such as oryzalin (Sharpshooter) do not have much foliar activity and do not tend to translocate well. Most of the group are not very soluble, although oryzalin is more soluble than most, and the safety data sheet for Sharpshooter (Imtrade Australia 2005) states that it is soluble in water. This could have implications for use of this product by growers or exporters. However, from the results of these trials, it would seem that enough oryzalin is entering the stems through the xylem of the cut stem or the epidermis to reduce propagability. Observations during treatment were that the material did not completely dissolve, although active suspension was maintained through stirring. Actual dilution rates may have varied for this reason, which may explain why the 1:2 dilution was not as effective as the 1:10 dilution in this experiment.
It is important to note that this research used commercial formulations where we were not provided with the full information on commercially sensitive additives/adjuvants which could have significant effects on the performance of the product.
Further research may be required to confirm that any treatment consistently eliminates shoot growth on a commercial scale. It would also be useful to trial Sharpshooter with other imported floristry products to determine if this chemical formulation is more effective than the currently used glyphosate at 0.5% (MAF Standard 152.09.05).
Acknowledgements
We thank Nufarm, AGPRO and Key Industries for supplying the herbicides, the Unitec Research Fund for funding the project, Felicity Bowden for technical assistance and the two anonymous reviewers for critically reviewing this manuscript.
References
- Ahmed SH , Zaharah AR 1998 . Postharvest treatment with glyphosate to devitalize rose and carnation cut flowers . Acta Horticulturae 464 : 541 .
- Amrhein N , Deus B , Gehrke P , Steinrucken HC 1980 . The site of the inhibition of the shikimate pathway by glyphosate . II. Interference of glyphosate with chorismate formation in vivo and in vitro . Plant Physiology 66 : 830 – 834 .
- Banaszkiewicz , T and Kopytowski , J . 2003 . Some aspects of glyphosate use in apple orchards . Sodininkyste ir Darzininkyste , 22 : 41 – 46 .
- Bayer , DE , Foy , CL , Mallory , TE and Cutter , EG . 1967 . Morphological and histological effects of trifluran on root development . American Journal of Botany , 54 : 945 – 952 .
- Hallgren E 1996 . Control of common horse-tail (Equisetum arvense) in fallow . Agriculture—pests, diseases and weeds. 37th Swedish Crop Protection Conference, Uppsala, Sweden, 26–27 January 1996. Uppsala, Swedish University of Agricultural Sciences . Pp. 325 – 329 .
- Holm LG , Plucknett DL , Plancho JV , Herberger JP 1977 . The world's worst weeds: distribution and biology . Honolulu , The University Press of Hawaii . 609 p.
- Kuriyama , A , Kawai , F and Kanamori , M . 1994 . Influence of the herbicide oryzalin on the sporophytic morphogenesis of Equisetum arvense . Journal of Plant Physiology , 143 : 353 – 358 .
- Large , MF , Blanchon , DJ and Angus , ML . 2006 . Devitalisation of imported horsetail (Equisetum hyemale) . New Zealand Journal of Crop and Horticultural Science , 34 : 151 – 153 .
- MAF Biosecurity Authority (Plants) Standard 152.09.05 . Clearance of fresh cut flowers and foliage. Wellington, Ministry of Agriculture and Forestry . 25 p.
- Rao VSP 1999 . Principles of weed science. 2nd edition . Enfield, USA, Science Publishers Inc USA .
- Rapparini , G , Paci , F , Pradolesi , G , Cioni , F and Tugnoli , V . 2002 . Horsetail control in maize as a precursor to beet . Informatore Agrario , 58 : 67 – 73 .
- Ross MA , Childs DJ 1996 . Herbicide mode-of-action summary, WS-23-W . Cooperative Extension Service, Purdue University, West Lafayette, Indiana .
- Uchida , S , Araki , J , Aoyama , R and Nishi , S . 1998 . Glyphosate phytotoxicity to Equisetum arvense as affected by growth stage and water stress . Journal of Weed Science and Technology , 43 : 300 – 306 .
- Uchida , S , Aoyama , R , Iwai , J and Fujiwara , S . 2000 . Differential susceptibility of Equisetum arvense L. lines to glyphosate and development of an assay method . Journal of Weed Science and Technology , 45 : 244 – 249 .
- Vezina , L . 1990 . Efficacy of herbicide programmes used in barley and maize on Equisetum arvense L. populations in Quebec, Canada . Weed Research (Oxford) , 30 : 71 – 79 .
- Weide RY van der , Slabberkoorne JJ 2001 . Control of horsetail: old pesticides are still needed . PPO-Bulletin Akkerbouw 1 : 20 – 21 .