Abstract
Dasineura mali Kieffer (Diptera: Cecidomyiidae) is an important pest of apple in Europe, North America and New Zealand and contaminates fresh fruit. Platygaster demades Walker (Hymenoptera: Platygasteridae) is the most important parasitoid of D. mali. A high percentage of P. demades individuals enter aestivation in the field which lasts more than 20 weeks, resulting in outbreaks of the pest in the summer. Therefore, the ability to induce and terminate aestivation in the laboratory is important for the improvement of biological control of D. mali using P. demades. Our results show that the highest proportion of P. demades individuals entered aestivation at 14:10 h (L:D) and 25 °C with the critical day-length and temperature for aestivation induction being 13.8 h light and 22.8 °C, respectively. The best aestivation termination condition was 12 h light + 15 °C. It is suggested that: (1) the aestivation peak in the field that occurs in mid-summer may be caused by environmental conditions P. demades have experienced in the spring; (2) laboratory colonies of P. demades should be maintained between 15 and 20 °C with a photoperiod of 12:12 h (L:D) to avoid the occurrence of aestivation; (3) treating P. demades at 14 h light + 25 °C for 15 days is sufficient to induce aestivation; and (4) aestivation can be terminated in 10–13 weeks at 12 h light + 15 °C.
Introduction
Platygaster demades Walker is an egg endoparasitoid of apple leaf-curling midge, Dasineura mali Kieffer, and pear leaf-curling midge, D. pyri (Bouché). It is a Palaearctic species (Walker, Citation1836), which was introduced from France (Paris) to New Zealand to control D. pyri in 1925 (Miller Citation1926) and found to attack D. mali in 1955 (Todd Citation1956). Dasineura mali, probably also native to the Palaearctic region (Marrison Citation1953), is a monophagous herbivore that attacks young apple leaves of growing shoots in Europe, North America and New Zealand (Marrison Citation1953; Gagné Citation1989) and contaminates fresh fruit (Tomkins et al. Citation1994). Platygaster demades is the only parasitic wasp that attacks and causes high mortality of D. mali populations in New Zealand (He & Wang Citation2007).
Dasineura mali eggs are laid only on young apple leaves of growing shoots and larvae feed and develop on the young leaves, making them rolled (Todd Citation1956). Platygaster demades eggs are laid into D. mali eggs and start hatching after the mature D. mali larvae (third instar) leave the curled leaves, penetrate into the soil and commence spinning their cocoons; the parasitoid larvae feed inside D. mali larvae and complete their development in the cocooned larvae of D. mali (Todd Citation1956). Parasitised D. mali larvae fail to pupate. Both D. mali and P. demades have four generations a year in Palmerston North, New Zealand (He et al. Citation2010).
Parasitoids have developed a number of strategies to synchronise their life cycles with those of their hosts (Tauber et al. Citation1986; Boivin Citation1994; Goehring & Oberhauser Citation2002; Jervis et al. Citation2005). Diapause is a primary adaptation of insects to cope with adverse environmental conditions and for synchronisation of their life cycles (Tauber et al. Citation1986; Danks Citation1987; Saunders et al. Citation2002). Our previous work (He et al. Citation2010) shows that in New Zealand, P. demades undergoes aestival–hibernal diapause, i.e. after aestivation at the embryonic stage during the summer, they terminate aestivation in late autumn and enter overwintering at the larval and pupal stages. It produces both aestivating and non-aestivating phenotypes between late spring and mid-autumn, and regulates the frequency and duration of aestivation over the season (He et al. Citation2010). In New Zealand the D. mali population sharply declines in the third and fourth generations between mid-summer and early autumn (He et al. Citation2010) in accordance with the seasonal dynamics of its host plant: the number of new apple shoots reaches the peak in mid- to late spring, then sharply declines between early and mid-summer, and ends in early autumn (Shaw et al. Citation2005). Therefore, the plastic life history displayed by P. demades is thought to be a temporal risk-spreading strategy for maximal survival (He et al. Citation2010).
Aestivation in P. demades is an adaptive strategy to survive under adverse environmental conditions and substantially reduces the potential of this parasitoid for biological control of D. mali, resulting in outbreaks of the pest in the summer (He et al. Citation2010). Various studies suggest that the day-length and temperature are the major factors causing diapause in parasitoids (Tauber et al. Citation1986; Milonas & Savopoulou-Soultani Citation2000; Jervis et al. Citation2005; Larios et al. Citation2007) although other factors, such as host and host-plant effects may also play a role (Tauber et al. Citation1986; Boivin Citation1994; Polgár & Hardie Citation2000). The ability to induce and terminate diapause in the laboratory has important implications for the use of parasitoids in biological control programmes (Laing & Corrigan Citation1995; Milonas & Savopoulou-Soultani Citation2000; Mehrnejad and Copland Citation2005). However, the optimal conditions that trigger and terminate aestivation in P. demades are still poorly understood, making effective biological control of D. mali using P. demades difficult.
In the present paper we examined the roles that day-length and temperature played in the occurrence of aestivation in P. demades with two objectives: (1) to determine the critical day-length and temperature that induced aestivation; and (2) to identify the environmental conditions that cued the termination of aestivation. To achieve the first objective we carried out a series of experiments in the laboratory including four day-length and three temperature treatments where we continuously examined P. demades by daily observation and weekly dissecting. In the second objective we allowed P. demades to remain in the aestivation induction condition for four different periods and then transferred them to the non-aestivation condition. We then determined the aestivation termination rate and the time required to terminate aestivation.
Materials and methods
Insects
A breeding colony of P. demades in the laboratory was established from the field-collected mature D. mali larvae (third instar) in a mature organic apple orchard in Plant Growth Unit, Massey University, Palmerston North, New Zealand. Larvae were maintained on the rearing medium (vermiculite) in Petri dishes (5.5 cm in diameter×1.3 cm in height) for pupation at 20±1 °C, 65±5% relative humidity and 15 h day-length. Twenty dishes were established with 50 larvae per dish. Newly emerged adults of both P. demades and D. mali were released into an aluminium-framed experimental cage (43×42×40 cm) in which 10 potted apple seedlings (~20 cm height, bred from rootstock MM 106 FSV) were maintained for oviposition. The cage had a fine metal mesh (aperture size = 0.25 mm) on the back and both sides and Perspex® on the top and front and aluminium alloy on the bottom. Parasitoids were reared in the laboratory for three generations before being used for experiments.
Effect of day-length and temperature on aestivation induction
To investigate the effect of day-length on development and aestivation of P. demades, we set up four treatments (day-lengths): 12, 13, 14 and 15 h light at 20 °C in the laboratory. To obtain parasitised D. mali individuals at each test day-length condition, we released eight mated parasitoid females collected from the breeding colony into an aforementioned experimental cage with eight apple seedlings infested by D. mali eggs (<24 h old) under the environmental conditions for the breeding colony. Seedlings were removed after 12 h oviposition during the photophase and individually caged by transplant permeable plastic bags (42 cm in length×23 cm in width with aperture = 0.25 mm). Two seedlings were used for each treatment. Mature D. mali larvae in each treatment were collected from the rolled leaves, and transferred to the rearing medium in Petri dishes, which continued to be maintained under the same condition for each treatment. We established a total of 20 dishes with 30 mature D. mali larvae per dish for each treatment.
We dissected all D. mali larvae of one dish per treatment per week under a stereomicroscope (Olympus, Japan) to determine the development state of P. demades. Petri dishes were also observed daily to record the emergence of P. demades adults. According to our dissecting, all P. demades eggs had developed to embryos when D. mali larvae became mature (He et al. Citation2010). Platygaster demades individuals were considered having entered aestivation if they still remained at the embryonic stage after all other individuals from the same treatment had developed to adults and emerged (He et al. Citation2010). At this time we dissected all D. mali cocoons in remaining dishes for each treatment (five to seven dishes per treatment depending on treatments) to determine the aestivation rate of P. demades.
To investigate the effect of temperature on P. demades aestivation, we set up three temperature treatments: 15, 20 and 25 °C with a day-length of 15 h light in the laboratory. The experiment was performed and data recorded as in the day-length experiment. We established 20, 15 and 15 dishes for treatments 15, 20 and 25 °C, respectively. At the time when aestivation was detected for each treatment, we dissected all D. mali cocoons in remaining dishes for that treatment (five to seven dishes per treatment depending on treatments) to determine the aestivation rate of P. demades.
In both the previous experiments, we mixed D. mali larvae collected from the same treatment before transferring them to Petri dishes to reduce the variation of parasitism rate between dishes in each treatment.
Effect of day-length and temperature on aestivation termination
We obtained and maintained parasitised D. mali as in the previous aestivation induction experiments.
The previous experiments showed that the highest and lowest proportion of P. demades individuals entered aestivation at 14 h light+25 °C and 12 h light + 15 °C, respectively (see Results). Thus, the former was considered the aestivation-inducing condition, and the latter the aestivation-terminating condition in this experiment.
In this experiment we maintained the parasitised D. mali at 14 h light + 25 °C for 15, 25, 35 and 45 days after parasitisation (four treatments) and then transferred them to 12 h light + 15 °C. Parasitoids that remained at 14 h light + 25 °C permanently were used as a control. We set up 35 Petri dishes, each with 30 mature D. mali larvae, for each treatment and the control. Twenty-five dishes per treatment or control were used for weekly dissecting to determine the development state of P. demades and for daily observation of P. demades adult emergence, using the methods in the previous experiments. For each treatment the period between the time when P. demades entered aestivation and that when P. demades embryos started developing to larvae was recorded as the time required for aestivation termination by P. demades. At the end of 25 weeks’ weekly dissecting and daily observation, we dissected all remaining 10 dishes for the control and each treatment to determine the aestivation termination rate in P. demades.
Statistical analysis
Data on aestivation induction and termination were analysed using ANOVA followed by a multiple Tukey's honestly significant difference (HSD) test. Data were arcsine transformed to achieve normal distribution prior to analysis. Data on the time required by parasitoids to terminate aestivation were not normally distributed, even after transformation, and thus analysed using the non-parametric Kruskal–Wallis test (KWT) followed by Dunn's procedure for multiple comparisons.
Results
Effect of day-length and temperature on aestivation induction
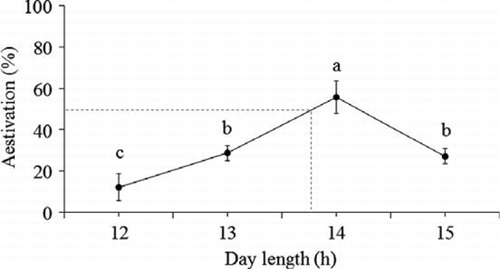
Results indicated that no D. mali entered aestivation. Aestivation in P. demades was detected under all tested day-lengths () and temperatures (). The proportion of parasitoid individuals that entered aestivation significantly increased when day-length increased from 12 to 14 h but decreased at the day-length of 15 h (ANOVA: F3,18=10.19, P < 0.001) (). The critical day-length that induced 50% of P. demades to enter aestivation at 20 °C was estimated to be 13.8 h ().
Figure 2 Aestivation rate in Platygaster demades under different temperatures at the day-length of 15 h.
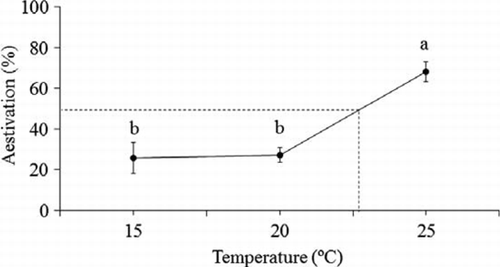
A significantly higher proportion of parasitoids entered aestivation at 25 °C than at 15 and 20 °C (ANOVA: F2,12=22.21, P < 0.001) (). The critical temperature that induced 50% parasitoids to enter aestivation under the day-length of 15 h was estimated to be 22.8 °C ().
Effect of day-length and temperature on aestivation termination
No parasitoids that had been held at 14 h light + 25 °C (control) terminated aestivation within 175 days (25 weeks) of observation, while about 80% of those that were transferred to 12 h light + 15 °C terminated aestivation in 66–90 days (10–13 weeks) (ANOVA: F4,45=88.78, P < 0.001) (A and B).
Figure 3 Aestivation in Platygaster demades. A, Aestivation termination rate. B, Time required for aestivation termination.
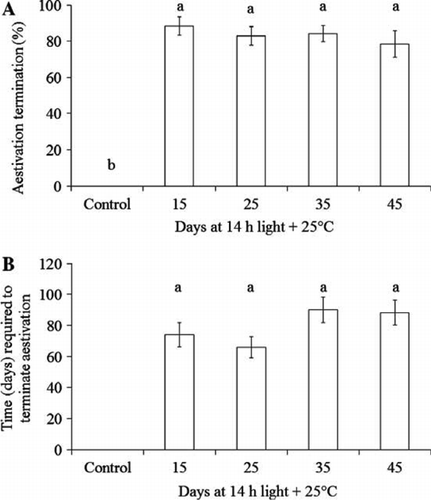
However, treatments did not significantly affect aestivation termination rate (ANOVA: F3,36=0.85, P > 0.05; A) or the time required to terminate aestivation (KWT: χ2=5.51, df = 3, P > 0.05; B).
Discussion
Insect aestivation is usually induced before the start of the summer and terminated in the autumn or winter (Tauber et al. Citation1986; Saunders et al. Citation2002). Our previous study on P. demades in the field in Palmerston North, New Zealand (He et al. Citation2010) shows that the embryonic aestivation starts to occur in mid-November (late spring, when day-length = 14.1 h and mean T = 13.9 °C) and peaks in mid-January (mid-summer, when day-length = 15.1 h and mean T = 17.5 °C) (climate data from New Zealand Meteorological Service [Citation2001–2007]).
The present study indicates that the highest proportion of P. demades individuals entered aestivation at the day-length of 14 h with the critical day-length for aestivation induction being 13.8 h (). This photoperiod condition occurs in mid- to late spring in Palmerston North (New Zealand Meteorological Service, Citation2001–2007). Although the aestivation of the field population reaches the peak at the day-length of 15 h (He et al. Citation2010), the aestivation rate at this day-length was significantly lower than at 14 h light (). It is thus suggested that: (1) the day-length in the spring is one of the main triggers of the aestivation process in this species; and (2) the aestivation peak in the field that occurs in mid-summer may be caused by environmental conditions P. demades have experienced in the spring. Similar phenomena are also reported in the cabbage butterfly Pieris melete Ménétriés (Xiao et al. Citation2008).
He et al. (Citation2010) show that in the field no P. demades individuals enter aestivation after early March (early autumn or later, when day-length ≤ 12.9 h and mean T ≤ 16.8 °C [New Zealand Meteorological Service, Citation2001–2007]). However, in the present study about 10% of individuals entered aestivation under the day-length of 12 h (). This could be explained by maternal influence (Milonas & Savopoulou-Soultani Citation2000; Mehrnejad & Copland Citation2005) because the breeding colony of P. demades was maintained under the day-length of 15 h before being transferred to the day-length of 12 h.
More than 25% of P. demades individuals in all temperature treatments entered aestivation (), with the highest aestivation rate occurring at 25 °C. This could be attributed to the fact that both the breeding colony and temperature treatments were maintained at the day-length of 15 h, under which condition about 30% individuals entered aestivation (). The laboratory data show that the critical temperature for aestivation induction was 22.8 °C at the day-length of 15 h (). Unlike the photoperiod effect, the mean temperature in the field and consistent temperature in the laboratory may not be comparable (Tauber et al. Citation1986; Goehring & Oberhauser Citation2002). Therefore, the present study on the effect of temperature on aestivation induction may not be used to predict what happens in the field. However, our laboratory results suggest that a rearing temperature higher than 20 °C could trigger some individuals to enter aestivation.
These studies reveal that laboratory colonies of P. demades should be maintained between 15 and 20 °C with the photoperiodic cycle of 12:12 h (L:D) to avoid the occurrence of aestivation.
In the field P. demades start to terminate aestivation between mid-April and mid-May (day-length = 10–11 h and mean T = 12.1–13.4 °C [New Zealand Meteorological Service, Citation2001–2007]) depending on generations, and by the end of May (day-length = 9.5 h and mean T = 11.9 °C [New Zealand Meteorological Service, Citation2001–2007]) almost all aestivated individuals from all generations have terminated aestivation (He et al. Citation2010). However, the present study shows that only about 80% of aestivated P. demades terminated aestivation at 12 h light + 15 °C (A). It is suggested that 10 h light + 12 °C could be a better condition for aestivation termination. Larios et al. (Citation2007) also report that in a pupal parasitoid of leafminers, Chrysocharis pubicornis (Zetterstedt), shorter day-length and lower temperature can increase aestivation termination rate.
Our results show that the duration of treatment under aestivation-induction conditions (14 h light + 25 °C) did not significantly affect the aestivation termination rate (A) and the time required for aestivation termination (B). It is thus indicated that: (1) treating P. demades at 14 h light + 25 °C for maximum 15 days is sufficient to induce aestivation; and (2) once aestivation occurs it will take 10–13 weeks to terminate regardless of treatment duration. In the natural environment the aestivation can last as long as 23 weeks (He et al. Citation2010). These results suggest that we can substantially shorten the aestivation duration in the laboratory.
In conclusion, the induction and termination of aestivation in P. demades are mainly regulated by day-length and probably also by temperature. The nature of this physiological phenomenon is to synchronise life cycles of P. demades with seasonal changes in their host availability and climatic conditions suitable for development (He et al. Citation2010). These findings are important for the improvement of biological control programmes of D. mali using P. demades. For example, in the development of introduction and augmentation strategies for the parasitoid, we can consider maintaining the parasitoid in aestivation induction conditions before mass-rearing and subsequence release. In programmes for mass production of the parasitoid, we should rear the insect under aestivation termination conditions. Finally, using the knowledge generated in the present study we can speed up the aestivation-termination process of field-collected parasitoids and then release them to the field to achieve better control of apple leaf-curling midge during the summer. Because the aestivation peak in the field that occurs in mid-summer is caused by environmental conditions P. demades have experienced in the spring, it is unlikely that the aestivation-free wasps that are released during the summer would re-enter aestivation.
Acknowledgements
The authors thank Dr M. Butcher and two anonymous referees for constructive comments on the manuscript, Pipfruit NZ and MURF for funding, and the Plant Growth Unit in Massey University, New Zealand for allowing us to carry out experiments in its orchard.
References
- Boivin , G . 1994 . “ Overwintering strategies of egg parasitoids ” . In Biological control with egg parasitoids , Edited by: Wajnberg , E and Hassan , S . 219 – 244 . Wallingford , , UK : CAB International .
- Danks , HV . 1987 . Insect dormancy: an ecological perspective , 439 Ottawa , , Canada : Biological Survey of Canada .
- Gagné , RJ . 1989 . The plant feeding gall midges of North America , 356 New York : Cornell University Press, Ithaca .
- Goehring , L and Oberhauser , KS . 2002 . Effects of photoperiod, temperature, and host plant age on induction of reproductive diapause and development time in Danaus plexippus . Ecological Entomology , 27 : 674 – 685 .
- He , XZ and Wang , Q . 2007 . Aestivation in apple leaf-curling midge (Diptera: Cecidomyiidae) parasitoid, Platygaster demades Walker (Hymenoptera: Platygastridae) . New Zealand Plant Protection , 60 : 21 – 25 .
- He , XZ , Wang , Q , Walker , JTS , Rogers , DJ and Lo , PL . 2010 . A sophisticated life history strategy in a parasitoid wasp: producing univoltine and multivoltine phenotypes in a local population . Biology Control , 54 : 276 – 284 .
- Jervis , MA , Copland , MJW and Harvey , JA . 2005 . “ The life cycle ” . In Insects as natural enemies: a practical perspective , Edited by: Jervis , MA . 219 – 260 . Dordrecht and New York : Springer .
- Laing , JE and Corrigan , JE . 1995 . Diapause induction and post-diapause emergence in Trichogramma minutum Riley (Hymenoptera: Trichogrammatidae): the role of host species, temperature, and photoperiod . Canadian Entomologist , 127 : 103 – 110 .
- Larios , GLB , Ohno , K and Fukuhara , F . 2007 . Effects of photoperiod and temperature on preimaginal development and summer diapause of Chrysocharis pubicornis (Zetterstedt) (Hymenoptera: Eulophidae), a pupal parasitoid of leafminers (Diptera: Agromyzidae) . Applied Entomology and Zoology , 42 : 189 – 197 .
- Marrison , LG . 1953 . Apple leaf-curling midge in New Zealand . New Zealand Journal of Agriculture , 86 : 565 – 566 .
- Mehrnejad , M and Copland , MJW . 2005 . Diapause strategy in the parasitoid Psyllaephagus pistaciae . Entomologia Experimentalis et Applicata , 116 : 109 – 114 .
- Miller , D . 1926 . Parasites of the pear midge (Peyysia pyri): first attempt at the establishment in New Zealand . New Zealand Journal of Agriculture , 31 : 379 – 393 .
- Milonas , PG and Savopoulou-Soultani , M . 2000 . Diapause induction and termination in the parasitoid Colpoclypeus florus (Hymenoptera: Eulophidae): role of photoperiod and temperature . Annals of the Entomological Society of America , 93 : 512 – 518 .
- New Zealand Meteorological Service 2001 –2007 Daily climatological observations: monthly reports . New Zealand , Massey University .
- Polgár , LA and Hardie , J . 2000 . Diapause induction in aphid parasitoids . Entomologia Experimentalis et Applicata , 97 : 21 – 27 .
- Saunders , DS , Steel , CGH , Vafopoulou , X and Lewis , RD . 2002 . Insect clocks , 3rd edition , 560 p Amsterdam : Elsevier .
- Shaw , PW , Wallis , DR , Alspach , PA and Sandanayaka , WRM . 2005 . Phenology of apple leafcurling midge (Dasineura mali) in relation to parasitism by Platygaster demades . New Zealand Plant Protection , 58 : 306 – 310 .
- Tauber , MJ , Tauber , CA and Masaki , S . 1986 . Seasonal adaptations of insects , 411 New York : Oxford University Press .
- Todd , DH . 1956 . A preliminary account of Dasyneura mali Kieffer (Cecidomyiidae: Dipt.) and an associated hymenopterous parasite in New Zealand . New Zealand Journal of Science and Technology , A37 : 462 – 464 .
- Tomkins AR , Wilson DJ , Hutchings SO , June S 1994 . A survey of apple leafcurling midge (Dasyneura mali) management in Waikato orchards . Proceedings of the 47th New Zealand Plant Protection Conference , Waitangi , New Zealand , 9–11 , August 1994 . Pp. 346 – 349
- Walker , F . 1836 . On the species of Platygaster . Entomological Magazine , 3 : 217 – 274 .
- Xiao , H , Li , F , Wei , XT and Xue , FS . 2008 . A comparison of photoperiodic control of diapause between aestivation and hibernation in the cabbage butterfly Pieris melete . Journal of Insect Physiology , 54 : 755 – 764 .