Abstract
Citrus trees growing in the Mediterranean region suffer from iron (Fe) deficiency because of the presence of high levels of carbonate ions in calcareous soils. In this context we have evaluated the effects of different citrus rootstocks on photosynthetic capacity and plant development of young ‘Navelina’ orange trees under Fe deprived conditions. Leaf number, shoot length, plant dry mass, leaf chlorophyll (Chl) concentration, net photosynthetic rate (P N), stomatal conductance (gS), leaf transpiration rate (E), intercellular CO2 concentration (Ci) and photosynthetic water use efficiency (WUE) of ‘Navelina’ orange scion budded on to eight citrus rootstocks with different agronomic characteristics were investigated. Significant decreases in leaf number, shoot length and plant dry mass under high pH conditions were observed. Plants on Tuzcu 31 31 and Gou Tou sour orange rootstocks were the least affected in terms of plant growth. The highest reduction in the leaf Chl content was observed in (-)Fe plants of Navelina on local trifoliate and C-35 citrange. These rootstocks also had significantly reduced PN, gS and E rates in the (-)Fe treatment. Shoots budded on Tuzcu 31 31 and Gou Tou sour oranges were the least affected by the induced Fe deficiency.
Introduction
Citrus production is increasing throughout the Mediterranean and Turkey and more and more citrus orchards are being planted on marginal soils. Mediterranean countries have a suitable climate for citrus, but it is estimated that 20%–50% of fruit trees grown in the Mediterranean basin suffer from iron (Fe) deficiency. The most prevalent cause of Fe deficiency in this region is the presence of high levels of carbonate ions in calcareous soils, characterized by a high pH (Mengel Citation1994). These soils often have more than 20% of calcium and magnesium carbonates and are strongly buffered, with a pH between 7.5 and 8.5 (Pestana et al. Citation2005). Fe uptake is highly dependent on soil pH, and Fe activity in solution decreases 1000-fold for each pH unit rise to reach a minimum within the range of 7.4 to 8.5 (Byrne et al. Citation1995). Leaf Fe chlorosis in plants is a persistent problem occurring in areas of calcareous and/or alkaline soils. Yield reductions from Fe induced leaf chlorosis have been found in tomato, raspberry, kiwifruit, pineapple, vines and citrus (Alvarez-Fernández et al. Citation2006). Moreover, the severity of leaf chlorosis and the differential behaviour of genotypes can be determined by the chlorophyll (Chl) concentration in the leaves (Byrne et al. Citation1995; Pestana et al. Citation2001, Citation2005, Citation2011; Castle & Nunnallee Citation2009).
The high level of bicarbonate ions in the soil affects metabolic processes in roots and leaves, decreasing soil and plant Fe availability, leading to the condition known as lime induced iron chlorosis. The most evident effect of Fe chlorosis is a decrease in photosynthetic pigments, resulting in a relative enrichment of carotenoids over chlorophylls, and production of yellow, chlorotic leaves. The loss of pigmentation is caused by decreased chlorophyll content in chloroplasts. This negatively affects the rate of photosynthesis and, therefore, the development of biomass. Fe deficiency affects the physiology and biochemistry of the whole plant, as Fe is an important cofactor of many enzymes, including those involved in the biosynthetic pathway of chlorophylls (Marschner Citation1995; Pestana et al. Citation2005).
The use of rootstocks in fruit production includes not only stronger resistance against pathogens, but also a higher tolerance to abiotic stress conditions such as salinity, heavy metals, nutrient stress, water stress and alkalinity (Rouphael et al. Citation2012). Recent studies showed that different citrus rootstocks had different tolerance levels to iron deficiency (Castle & Nunnallee Citation2009; Pestana et al. Citation2011). Studies emphasized that high pH conditions reduced iron uptake in citrus rootstocks (Hamze et al. Citation1986; Ferguson et al. Citation1990; Campbell Citation1991; Sudahono et al. Citation1994; Castle & Nunnallee Citation2009; Pestana et al. Citation2011). Also, different rootstocks variously affect tree growth, fruit quality and yield (Castle Citation1987; Forner-Giner et al. Citation2003; Castle & Nunnallee Citation2009). Moreover, scion behaviour depends in part on the rootstock induced effects on leaf gas exchange (González-Mas et al. Citation2009). González-Mas et al. (Citation2009) indicated that in calcareous soils, citrus production depends on availability of suitable rootstocks that are tolerant of low Fe soil conditions. Studies have found that Volkameriana and sour orange plants were tolerant; Carrizo and Troyer citranges were intermediate, whereas the Poncirus trifoliata rootstock was more sensitive to iron chlorosis (Hamze et al. Citation1986; Sudahono et al. Citation1994; Byrne et al. Citation1995; Castle & Nunnallee Citation2009). However, there are no studies that have evaluated plant growth and photosynthetic rates of these rootstocks together under Fe deprived conditions.
Among physiological processes, photosynthesis is the basic determinant of plant growth and productivity, and the ability to maintain the rate of carbon assimilation under environmental stress is of fundamental importance to plant production (Lawlor Citation1995). Since Fe catalyses chlorophyll biosynthesis (Bollivar & Beale Citation1996), it would be expected to promote the photosynthetic rate (Pn), whereas Fe deficiency would be expected to reduce it (Terry Citation1984; Davis et al. Citation1986). Most of the knowledge about the effect of Fe deficiency on photosynthetic parameters has been obtained using annual plants. Relatively few studies have focused on the consequences of induced Fe deficiency on photosynthesis in evergreen fruit trees and especially in citrus. Moreover, little information is available on assessing the response of citrus rootstocks and gas exchange measurements under Fe deficient conditions. Chouliaras et al. (Citation2004) indicated that there are no data available on the effect of Fe deficiency on stomatal conductance (g S) and leaf water relations.
The present study evaluated eight commonly used rootstocks in citriculture on young ‘Navelina’ orange trees grown under both Fe sufficient and deprived conditions. The tolerance levels were assessed by plant growth, chlorophyll concentration and gas exchange measurements.
Materials and methods
Plants, treatments and growth conditions
Navelina (Citrus sinensis L.) scions budded on Tuzcu 31 31 sour orange (Citrus aurantium L.), Gou Tou sour orange (Citrus aurantium L. var. Gou Tou), Volkameriana (Citrus volkameriana V. Ten. & Pasq), Cleopatra mandarin (Citrus reshni Tan.), Carrizo citrange (Citrus sinensis [L.] Osb. × Poncirus trifoliata [L.] Raf.), Troyer citrange (Citrus sinensis [L.] Osb. × Poncirus trifoliata [L.] Raf.), C-35 citrange (Citrus sinensis. Osb. ‘Ruby’ × Poncirus trifoliata [L.] Raf.) and local trifoliate (Poncirus trifoliata [L.] Raf.) were used as plant material. Seeds were germinated in the dark at 22 °C in plastic trays with sterilized 1:1 peat:soil. After germination the seedlings were grown for 8 months in the greenhouse. Uniform seedlings were selected and transferred into pots for scion budding. After budding, seedlings were grown in a modified Hoagland nutrient solution for citrus for 8 months. Just before starting the treatments, the plants were transferred to 10 L pots that were filled with quartz sand and transferred to a plant growth chamber. Until stress was applied, all plants were irrigated with a solution of the following composition: 1.25 mM KNO3, 0.625 mM KH2PO4, 2.00 mM MgSO4, 2.00 mM Ca(NO3)2, EDTA-Fe (125 µM), 25.0 µM H3BO3, 2.00 µM MnSO4, 2.00 µM ZnSO4, 0.50 µM CuSO4, 0.065 µM (NH4)6Mo7O24 for 2 months in order to ensure plant adaptation to growth chamber conditions. Final solution pH and electrical conductivity (EC) were adjusted to 5.8 and 1.05 mS, respectively. For Fe deprived conditions, the solution was adjusted to 7.8 ± 0.1 and Fe was added to the solution as 10−2 mM Fe EDTA together with 19.98 mM CaCO3 + 3 mM NaHCO3, EC = 0.967 ([-]Fe treatment). Although EDTA activity is reduced under high pH conditions, it provided some available Fe for rootstock growth and can mimic the natural conditions in a calcareous soil (Pestana et al. Citation2005). For control plants, 10−4 M Fe EDTA was added to the solution and the pH of the control nutrient was adjusted to 6.0 ± 0.1 in order to provide optimal conditions for Fe uptake in citrus. These treatments were imposed for 5 months. Plants in the growth chamber experienced 65% relative humidity, 450 ± 25 µmol m−2s−1 photosynthetic photon flux density (PPFD) at the top of the plant canopy, and a photoperiod of 16 h day/8 h night with a 26 °C day and 20 °C night air temperature.
Plant growth measurement
At the beginning of the experiment, the shoot length and number of leaves for each plant were recorded. After 5 months, the shoot length and number of leaves produced for each treatment were recorded again. The differences between the latter and initial values were calculated to assess plant growth during the experimental period. After leaf chlorophyll concentration and leaf gas exchange measurements, the plants were harvested and separated into shoots (all leaves and stem) and roots for dry mass (DM) measurements and dried at 72 °C for 48 h using a thermo ventilated oven.
Gas exchange measurements
Transpiration rate (E) (mmol m−2s−1), stomatal conductance (g S) (mmol m−2s−1), net photosynthetic rate (P N) (µmol[CO2] m−2s−1), intercellular CO2 concentration (C i) (µmol [CO2] mol−1 [air]) were measured at the end of the experiment using a portable photosynthesis system (model LCA-4, ADC Bioscientific, Hoddesdon, UK) on five young, fully expanded young Navelina orange leaves (4th–5th leaf from the shoot apex) per treatment and rootstock. The instantaneous photosynthetic leaf water use efficiency (WUE) was calculated as WUE = P N/E, according to Ribeiro et al. (Citation2009). All gas exchange measurements were taken on attached mature leaves that were about 6 months old. The leaf temperature range was between 26 and 28 °C and the relative humidity was 65% during the experimental period. PPFD was 250–300 µmol m−2 s−1.
Leaf chlorophyll concentration
After gas exchange measurements, the same leaves were used to estimate leaf Chl concentrations by a SPAD-502 meter (Minolta, Osaka, Japan). SPAD readings were used to estimate leaf Chl concentration because there is a strong relationship between SPAD readings and chlorophyll levels in citrus leaves (Jifon et al. Citation2005).
Statistical analysis
The experiment was arranged as 2 × 8 × 8 (two treatments, eight rootstocks, eight replicates) in a complete randomized design. Data were subjected to two-way analysis of variance (ANOVA). The means and calculated standard deviations are reported. Significant differences between means were evaluated using Tukey's multiple range test at P ≤ 0.05 and P ≤ 0.01. Control and (-)Fe treatments for each rootstock were compared using ‘Student's t test’ at P ≤ 0.05. Also the correlation coefficients between all measured parameters were calculated. All statistical analyses were performed by using SAS v9.00 statistics software and SigmaPlot® version 11.00 (Systat Software, San Jose, CA, USA) was used for data presentation.
Results
Growth
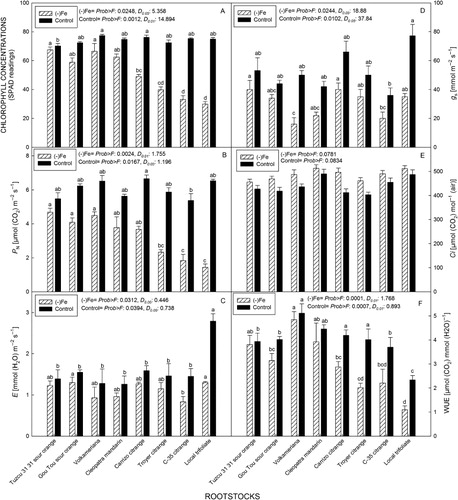
Rootstocks significantly affected plant DM grown under Fe sufficient and deficient treatments (). A two-way ANOVA indicated a significant main effect of rootstock and Fe treatment and also their interaction (P ≤ 0.05) on the shoot DM (). Average shoot DM of all rootstock grown under control conditions (26.36 g) was significantly reduced compared with the (-)Fe treatment (22.13 g). The highest shoot DM was obtained in plants on Volkameriana and Troyer citrange, whereas the lowest was on the local trifoliate grown under (-)Fe conditions. No significant differences were obtained in plants budded on Volkameriana, Cleopatra mandarin, Tuzcu 31 31 and Gou Tou sour oranges between the control and (-)Fe treatments (). The (-)Fe treatments, however, significantly decreased shoot DM on plants budded on other rootstocks. The C-35 citrange and local trifoliate rootstocks experienced the highest DM loss (25.06 and 31.66%, respectively), whereas losses were minimal with the Tuzcu 31 31 and Gou Tou rootstocks.
Table 1 Results of two-way analysis of variance (ANOVA) of rootstock (R) and Fe treatment (F) effects and their interaction (R×F) for the dependent variables considered.
Table 2 Shoot dry mass (g) of ‘Navelina’ orange budded on different rootstocks under Fe sufficient and deprived conditions.
Significant main effects of rootstock and Fe treatment on the root DM and leaf number were also found (). Similar to the shoot DM, no significant decreases were obtained in root DM from Volkameriana, Cleopatra mandarin, Tuzcu 31 31 and Gou Tou sour oranges according to t tests (). In addition, Tuzcu 31 31 and Gou Tou sour oranges had 6.29% and 9.65% less root DM under Fe deprived vs. sufficient conditions, respectively. The largest decrease in root DM (33.89%) was found in plants budded on local trifoliate (). The (-)Fe treatments also affected leaf number resulting in marked decreases with induced Fe deficiency. In the (-)Fe treatment, the Cleopatra mandarin rootstocks produced the highest leaf number, whereas the C-35 citrange yielded the lowest (). Fe deprived conditions did not affect leaf number in plants budded on Tuzcu 31 31 and Gou Tou sour orange. ‘Navelina’ scions on all rootstocks, except the sour orange rootstocks, had lower leaf numbers under bicarbonate induced Fe deficiency, with the greatest decline occurring on local trifoliate and C-35 citrange rootstocks (). Thus, (-)Fe conditions reduced the photosynthetic capacity of all rootstocks except sour orange in this study.
Table 3 Root dry mass (g) of ‘Navelina’ orange budded on different rootstocks under Fe sufficient and deprived conditions.
Table 4 Leaf number of ‘Navelina’ orange budded on different rootstocks under Fe sufficient and deprived conditions.
In terms of shoot length, significant main effects and interaction effect were observed (). Similar to the other measurements, young ‘Navelina’ trees budded on Tuzcu 31 31 and Gou Tou sour oranges were the least affected and the plants on local trifoliate were the most affected by the high pH in the nutrient solution in plant growth parameters ().
Table 5 Shoot length (cm) of ‘Navelina’ orange budded on different rootstocks under Fe sufficient and deprived conditions.
Chlorophyll concentration
The SPAD measurements were used as an estimate of leaf chlorophyll concentration, because there is a positive linear relationship between these two parameters (r2 > 0.8, Jifon et al. Citation2005). In the present study, there were significant rootstock and Fe treatment effects on leaf Chl concentrations. Also, a two-way ANOVA indicated a significant (P ≤ 0.05) interaction effect on leaf Chl (). Leaves of shoots grafted on to C-35 citrange and local trifoliate had the lowest Chl concentration, wheres leaves of shoots budded on to Tuzcu 31 31 and Volkameriana had the highest Chl concentration. Furthermore, slight decreases were determined in plants budded on to those rootstocks between (-)Fe and control treatments. In contrast, marked decreases in Chl concentration were observed from leaves on C-35 citrange and local trifoliate ().
Gas exchange measurements
In the Fe deprived leaves of ‘Navelina’, the net photosynthetic rate was inhibited on all rootstocks. A two-way ANOVA indicated a significant (P ≤ 0.05) rootstock × treatment interaction effect on P N (). ‘Navelina’ budded on Tuzcu 31 31 sour orange, Gou Tou and Volkameriana had higher P N than those on other rootstocks under (-)Fe conditions. The lowest P N values recorded in plants on C-35 citrange and local trifoliate were 1.84 and 1.43 µmol m−2s−1, respectively. Under Fe deprived conditions, the greatest loss in P N was found in local trifoliate in comparison with control plants. With the exception of Tuzcu 31 31, plants on all of the other rootstocks had lower gas exchange measurements under (-)Fe conditions (). There were also significant differences in both control and (-)Fe treatments in terms of E and g S of ‘Navelina’ trees on different rootstocks. Leaves of shoots on local trifoliate had the highest E values in the control but the lowest under Fe deprived conditions. Therefore, the highest E decrease was recorded in leaves on local trifoliate (). Although Fe deprived leaves on Volkameriana had the lowest g S, the greatest decrease was recorded in local trifoliate in comparison with the control (). Induced Fe deficiency resulted in slight decreases in C i values of ‘Navelina’ leaves without significant differences (). Significant interaction effects were determined in WUE. Scions on Volkameriana rootstock had the greatest WUE in both control and (-)Fe treatments, whereas the lowest WUE was recorded in local trifoliate grown under (-)Fe conditions ().
Correlation coefficients analysis
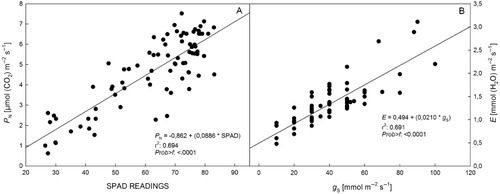
Significant correlations between investigated parameters were determined with the exception of C i (). The correlations between Chl content and P N (0.83) and between E and g S (0.83) were very significant. Therefore, regression analyses were performed between these correlations and strong relationships were determined concerning high r2 values (). The regression analysis confirmed that P N rate was increased by the high Chl content in leaves.
Table 6 Correlation coefficient analysis between investigated parameters.
Discussion
In this article, we described how rootstock affected the growth and the photosynthetic performance of leaves from ‘Navelina’ trees grown under Fe deprived conditions. Fe deprivation caused a significant decline in plant growth compared with the control. In addition, rootstock × Fe treatment interactions were significant in terms of all estimated growth parameters except root dry weight according to a two-way ANOVA. The number of leaves, plant height and plant DM were negatively affected by the induced Fe deficiency. Previous studies also claim that bicarbonate ions hamper plant growth and development (Mengel Citation1994; Sabir et al. Citation2010). High soil pH levels also limit plant growth and development under alkaline conditions (Yang et al. Citation2007, Citation2008a,Citationb, Citation2009). Iron uptake is highly dependent on soil pH and iron activity in solution decreases 1000-fold for each pH unit rise to reach a minimum within the range of 7.4 to 8.5 (Byrne et al. Citation1995). In our study, rootstocks significantly affected scion growth under Fe deprived conditions. Shoots on Tuzcu 31 31 and Gou Tou sour oranges were the least affected, whereas shoots on local trifoliate were the most affected. The inhibition of plant growth by Fe deficiency has been found in similar rootstock studies in citrus (Pestana et al. Citation2001, Citation2005, Citation2011; Castle & Nunnallee Citation2009).
Forty five days after (-)Fe treatments, leaf iron chlorosis symptoms were visible. Fe deficiency caused severe ‘Navelina’ leaf chlorosis budded on to C-35 citrange and local trifoliate. Moderate chlorosis was found on Troyer and Carrizo citranges leaves. The rootstocks that produced the least leaf Fe chlorosis symptoms included Antalya Cleopatra mandarin, Volkameriana, Tuzcu 31 31 and Gou Tou sour orange. Tuzcu 31 31 and Gou Tou sour orange rootstocks, in particular, expressed very little leaf chlorosis under (-)Fe conditions. Similar to leaf Fe chlorosis symptoms on these rootstocks, the highest leaf active Fe concentrations were found on ‘Navelina’ leaves on Gou Tou sour orange (37.90 ppm) and Tuzcu 31 31 (36.68 ppm) whereas the lowest were on C-35 citrange (22.52 ppm) and local trifoliate (22.60 ppm) (Cimen et al. Citationin press). Several authors have classified Fe tolerance of citrus rootstocks based on chlorosis parameters of shoots (Hamze et al. Citation1986; Sudahono et al. Citation1994; Byrne et al. Citation1995; Castle & Nunnallee Citation2009). Similar to our study, they found that trifoliate rootstocks were sensitive and Carrizo, Troyer and sour orange rootstocks were more tolerant to Fe depravity, based on leaf chlorosis levels.
However, plant growth parameters may not be sufficient to evaluate tolerance to Fe chlorosis of citrus rootstocks (Pestana et al. Citation2005) because of the iron paradox (i.e. Fe chlorotic leaves often have high Fe concentrations). Therefore, physiological parameters such as Chl and CO2 gas exchange measurements can substantiate the tolerance of plants to Fe deficiency or high pH conditions (Morales et al. Citation2000; Chouliaras et al. Citation2004; Baveresco et al. Citation2006; Larbi et al. Citation2006; Molassiotis et al. Citation2006). In comparison with all rootstocks used in this experiment, (-)Fe treatment led to a significant decrease in leaf Chl content of ‘Navelina’ shoots budded on local trifoliate.
In addition, the highest leaf chlorophyll contents were found in the leaves on Tuzcu 31 31 sour orange in the (-)Fe treatment. Iron induced leaf chlorosis results in decreases in growth and photosynthetic parameters possibly because Fe is important in chlorophyll synthesis and thylakoid stabilization. Under Fe deprived conditions, sour oranges maintained significantly greater Fe and chlorophyll contents compared with local trifoliate. The ability of sour orange rootstocks to maintain high leaf Fe and chlorophyll concentrations under Fe deprived conditions have been documented previously (Hamze et al. Citation1986; Sudahono et al. Citation1994; Byrne et al. Citation1995; Castle & Nunnallee Citation2009). In the present study, sour orange rootstocks produced similar root dry matter in control and (-)Fe conditions and significantly more root biomass than some Fe sensitive rootstocks. In addition to improved root growth, the sour orange rootstocks may have more membrane Fe transporter than the other rootstocks. However, the exact mechanism involved in Fe uptake needs to be elucidated in future studies. Iron deficiency causes various physiological changes in leaves, as Fe is an important cofactor of many enzymes, including those involved in the biosynthetic pathway of chlorophylls (Marschner Citation1995). In the present study, many leaf gas exchange parameters were reduced by induced Fe deficiency. Several authors indicated that the lime induced iron deficiency reduced the net photosynthetic rate in the leaves (Morales et al. Citation1994; Abadía et al. Citation1999; Larbi et al. Citation2006; Nenova Citation2009). In our study, the leaves of shoots grafted on Volkameriana, Tuzcu 31 31 and Gou Tou sour oranges had the highest P N, which differed significantly from leaves on the other rootstocks under Fe deprived conditions. Also, Chouliaras et al. (Citation2004) claimed that the reduction in photosynthesis in Valencia leaves grafted on to Swingle citrumelo was higher than those on sour orange. This finding is in agreement with the present study. In addition, González-Mas et al. (Citation2009) indicated that different rootstocks had different effects on the net photosynthetic rate of ‘Navelina’ orange trees grown in calcareous soil. Fe deficiency has a noticeable effect on the stomatal conduction of seedlings because of its importance in many enzyme systems and for energy transfer during photosynthesis. The seedlings that were not affected by the high pH, calcareous soil had higher photosynthetic rates, leaf chlorophyll levels and stomatal conductance. Stomatal movements provide the leaf with the opportunity to change both the partial pressure of CO2 at the sites of carboxylation and the rate of transpiration. In turn, changes in transpiration rate can cause changes in the temperature and water potential of the leaf (Farquhar & Sharkey Citation1982). On the other hand, leaf water potential directly regulates leaf transpiration, gas exchange and stomatal conduction in trees suffering from Fe deficiency (Meinzer Citation2002; Sperry Citation2002; Brodribb & Holbrook Citation2003; Eichert et al. Citation2010). In addition, Chouliaras et al. (Citation2004) indicated that stomatal conduction was significantly reduced in two orange cultivars on Swingle citrumelo under Fe deprived conditions.
Rootstocks significantly affected E and g S of young ‘Navelina’ seedlings both in control and Fe deprived conditions. Although leaves of the trifloliate rootstock had the highest transpiration rates in both control and (-)Fe treatments, this rootstock also had the greatest reduction in transpiration rate under Fe deprived conditions. This decrease in transpiration rate may have resulted in the Fe deficiency symptoms noticeable in the trifoliate rootstocks. The rate of transpiration is related to the degree of stomatal opening and to the evaporative demand of the atmosphere surrounding the leaf. Deficiency of elements can influence stomatal opening, and thus the transpiration rate could be reduced (Fracheboud Citation1999). Despite a decline in g S, the leaves of shoots grafted on to local trifoliate had high C i values in both (-)Fe and control plants. This can be explained by the fact that, as occurs under water stress, only very critically low levels of g S affect P N and C i (Flexas et al. Citation2004). Also, abiotic stresses increase the C i of leaves by impairing the ability of the photosynthesis machinery to utilize internal CO2 efficiently (Ahmadi & Siosemardeh Citation2005).
In our study, there were significant differences in ‘Navelina’ leaves among rootstocks in terms of WUE. The leaves of shoots grafted on to Volkameriana had the highest and those on local trifoliate had the lowest. In general, Fe deprived leaves were less efficient in contrast with healthy leaves in terms of water use efficiency. WUE decreased in chlorotic leaves compared with healthy green leaves. The reasons for the reduced WUE in chlorotic leaves are not yet fully understood, but there is some evidence suggesting that this may at least in part be a result of an increased cuticular E. In addition, Larbi et al. (Citation2006) suggested that Fe deprived leaves of plants such as sugar beet, peach and pear have to transpire more to produce carbohydrates in comparison with healthy leaves, resulting in a decrease in plant WUE. On the other hand, there are studies that suggest that stomata regulation may be reduced in chlorotic leaves, a phenomenon that may also contribute to reducing WUE (Eichert et al. Citation2010).
Conclusions
We investigated the effects of several rootstocks on scion under induced Fe deficiency based on growth, chlorophyll content and gas exchange characteristics. Young ‘Navelina’ orange trees budded on Tuzcu 31 31 and Gou Tou sour oranges performed best under Fe deprived conditions in a plant growth chamber. ‘Navelina’ on Volkameriana and Cleopatra were moderately adapted to induced Fe deficiency, whereas C-35 citrange and local trifoliate were poorly adapted, regarding the parameters considered. However, further studies are needed to evaluate these rootstocks under Fe deficient field conditions.
Acknowledgements
This research was supported by the grant from the Çukurova University, Scientific Research Projects Coordinating Office (project CU-BAP-ZF2010YL30). The authors are grateful to Dr Richard Rosecrance for critical reading and language correction of the manuscript.
References
- Abadía J, Morales F, Abadía A 1999. Photosystem II efficiency in low chlorophyll, iron-deficient leaves. Plant Soil 215: 183–192. 10.1023/A:1004451728237
- Ahmadi A, Siosemardeh A 2005. Investigation on the physiological basis of grain yield and drought resistance in wheat: leaf photosynthetic rate, stomatal conductance and non stomatal limitation. International Journal of Agriculture and Biology 7: 807–811.
- Alvarez-Fernández A, Abadía J, Abadía A 2006. Iron deficiency, fruit yield and fruit quality. In: Barton LL, Abadía J eds. Iron nutrition in plants and Rizospheric microorganisms. Dordrecht, Springer. Pp. 437–448.
- Baveresco L, Bertamini M, Iacono F 2006. Lime-induced clorosis and physiological responses in grapevine (Vitis vinifera L. Cv. Pinot blanc) leaves. Vitis 45: 45–46.
- Bollivar DW, Beale SI 1996. The chlorophyll biosynthetic enzyme-Mgprotoporphyrin IX monomethylester (oxidative) cyclase. Plant Physiology 112: 105–114.
- Brodribb TJ, Holbrook NM 2003. Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiology 132: 2166–2173. 10.1104/pp.103.023879
- Byrne DH, Rouse RE, Sudahono DHB 1995. Tolerance to citrus rootstocks to lime-induced iron chlorosis. Subtropical Plant Science 47: 7–11.
- Campbell CW 1991. Rootstocks for the Tahiti lime. Proceedings of the Florida State Horticultural Society 104: 28–30.
- Castle WS 1987. Citrus Rootstocks. In: Roy C, Robert F eds. Rootstocks for fruit crops. New York, Willey Interscience. Pp. 361–399.
- Castle WS, Nunnallee J 2009. Screening citrus rootstocks and related selections in soil and solution culture for tolerance to low-iron stress. Horticultural Science 44: 638–645.
- Chouliaras V, Therios I, Molassiotis A, Patakas A, Diamantidis G 2004. Effect of iron deficiency on gas exchange and catalase and peroxidase activity in citrus. Journal of Plant Nutrition 27: 2085–2099. 10.1081/PLN-200034638
- Cimen B, Yesiloglu T, Incesu M, Yilmaz B in press. Physiological investigation of tolerance to iron chlorosis of ‘Navelina’ orange budded on different citrus rootstocks. Acta Horticulturae.
- Davis T, Jolley V, Walser R, Brown J, Blaylock A 1986. Net photosynthesis of Fe-efficient and Fe-inefficient soybean cultivars grown under varying iron levels. Journal of Plant Nutrition 9: 671–681. 10.1080/01904168609363473
- Eichert A, Peguero-Pinab T, Gil-Pelegrinb JJ, Herediac A 2010. Effects of iron chlorosis and iron resupply on leaf xylem architecture, water relations, gas exchange and stomatal performance of field-grown peach (Prunus persica). Physiologia Plantarum 138: 48–59. 10.1111/j.1399-3054.2009.01295.x
- Farquhar GD, Sharkey TD 1982. Stomatal conductance and photosynthesis. Annual Reviews of Plant Physiology 33: 317–345. 10.1146/annurev.pp.33.060182.001533
- Ferguson L, Sacovich N, Roose M 1990. California citrus rootstocks. Publication 21477. Oakland, CA, Division of Agriculture and Natural Resources, University of California.
- Flexas J, Bota J, Cifre J, Escalona JM, Galmes J, Gulias JE, Lefi SF, Moreno MT, Ribas-Carbo M, Riera D, Sampol B, Medrano H 2004. Understanding down-regulation of photosynthesis under water stress: future prospects and searching for physiological tools for irrigation management. Annals of Applied Biology 144: 273–283. 10.1111/j.1744-7348.2004.tb00343.x
- Forner-Giner MA, Alcaide A, Primo-Millo E, Forner JB 2003. Performance of Navelina orange on 14 rootstocks in Northern ‘Valencia’ (Spain). Science Horticulture 98: 223–232. 10.1016/S0304-4238%2802%2900227-3
- Fracheboud Y 1999. Cold adaptation of the photosynthetic apparatus of maize by growth at suboptimal temperature. In: Sánchez-Díaz M, Irigoyen J, Aguirreola JJ, Pithan K eds. Crop development for the cool and wet climate of Europe. Brussels, Office for the Official Publications of the European Communities. Pp. 88–98.
- González-Mas MC, Llosa MJ, Quijano A, Forner-Giner A 2009. Rootstock Effects on Leaf Photosynthesis in ‘Navelina’ Trees in Calcareous Soil. Horticulture Science 44: 280–283.
- Hamze M, Ryan J, Zaabout N 1986. Screening of citrus rootstocks for lime-induced chlorosis tolerance. Journal of Plant Nutrition 9: 459–469. 10.1080/01904168609363459
- Jifon JL, Syvertsen JP, Whaley E 2005. Growth environment and leaf anatomy affect nondestructive estimates of chlorophyll and nitrogen in Citrus sp. leaves. Journal of American Society Horticulture Science 130: 152–158.
- Larbi A, Abadía A, Abadía J, Morales F 2006. Down co-regulation of light absorption, photochemistry, and carboxylation in Fe-deficient plants growing in different environments. Photosynthesis Research 89: 113–126. 10.1007/s11120-006-9089-1
- Lawlor DW 1995. The effect of water deficit on photosynthesis. In: Smirnoff N ed. Environment and plant metabolism, flexibility and acclimation. London, BIOS Scientific Publisher. Pp. 129–160.
- Marschner H 1995. Mineral nutrition of higher plants. 2nd edition. London, Academic Press.
- Meinzer FC 2002. Co-ordination of vapour and liquid phase water transport properties in plants. Plant Cell Environment 25: 265–274. 10.1046/j.1365-3040.2002.00781.x
- Mengel K 1994. Iron availability in plant tissues, Iron chlorosis on calcareous soils. Plant Soil 165: 275–283. 10.1007/BF00008070
- Molassiotis A, Tanou G, Diamantidis G, Patakas A, Therios I 2006. Effects of 4- month Fe deficiency exposure on Fe reduction mechanism, photosynthetic gas exchange, chlorophyll fluorescence and antioxidant defense in two peach rootstocks differing in Fe deficiency tolerance. Journal of Plant Physiology 163: 176–185. 10.1016/j.jplph.2004.11.016
- Morales F, Abadía A, Belkhodja R, Abadía J 1994. Iron deficiency-induced changes in the photosynthetic pigment composition of fieid-grown pear (Pyrus communis L.) leaves. Plant Cell and Environment 17: 1153–1160. 10.1111/j.1365-3040.1994.tb02013.x
- Morales F, Belkhodja RL, Abadía A, Abadía J 2000. Photosystem II efficiency and mechanism of energy dissipation in iron-deficient, field grown pear trees (Pyrus communis L.). Photosynthesis Research 63: 9–21. 10.1023/A:1006389915424
- Nenova VR 2009. Growth and photosynthesis of pea plants under different iron supply. Acta Physiologia Plantarum 31: 385–391. 10.1007/s11738-008-0247-2
- Pestana M, Correia PJ, David M, Abadía A, Abadía J, de Varennes A 2011. Response of five citrus rootstocks to iron deficiency. Journal of Plant Nutrition Soil Science 174: 837–846. 10.1002/jpln.201000341
- Pestana M, David M, Varennes A, Abadía J, Faria EA 2001. Responses of ‘Newhall’ orange trees to iron deficiency in hydroponics: effects on leaf chlorophyll, photosynthetic efficiency, and root ferric chelate reductase activitiy. Journal of Plant Nutrition 24: 1609–1620. 10.1081/PLN-100106024
- Pestana M, Varennes A, Abadía J, Faria EA 2005. Differential tolerance to iron deficiency of rootstocks grown in nutrient solution. Scientia 104: 25–36.
- Ribeiro RV, Machado EC, Santos MG, Oliveira RF 2009. Photosynthesis and water relations of well-watered orange plants as affected by winter and summer conditions. Photosynthetica 47: 215–222. 10.1007/s11099-009-0035-2
- Rouphael Y, Cardarelli M, Rea E, Colla G 2012. Improving melon and cucumber photosynthetic activity, mineral composition, and growth performance under salinity stress by grafting onto Cucurbita hybrid rootstocks. Photosynthetica 50: 180–188. 10.1007/s11099-012-0002-1
- Sabir A, Bilir-Ekbiç H, Erdem H, Tangolar S 2010. Response of four grapevine (Vitis spp.) genotypes to direct or bicarbonate-induced iron deficiency. Spanish Journal of Agricultural Research 8: 823–829. 10.5424/sjar/2010083-1284
- Sperry JS 2002. Hydraulic constraints on plant gas exchange. Agricultural and Forest Meteorology 104: 13–23. 10.1016/S0168-1923%2800%2900144-1
- Sudahono DHB, Byrne DH, Rouse RE 1994. Greenhouse screening of citrus rootstocks for tolerance to bicarbonate-induced iron chlorosis. Horticultural science 29: 113–116.
- Terry N 1984. Limiting factors in photosynthesis: iron stress mediated changes in light-harvesting and electron transport capacity and its effects on photosynthesis in vivo. Plant Physiology 71: 855–860. 10.1104/pp.71.4.855
- Yang CW, Chong JN, Li CY, Kim CM, Shi DC, Wang DL 2007. Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversiana during adaptation to salt and alkali conditions. Plant Soil 294: 263–276. 10.1007/s11104-007-9251-3
- Yang CW, Wang P, Li CY, Shi DC, Wang DL 2008a. Comparison of effects of salt and alkali stresses on the growth and photosynthesis of wheat. Photosynthetica 46: 107–114. 10.1007/s11099-008-0018-8
- Yang CW, Shi DC, Wang DL 2008b. Comparative effects of salt and alkali stresses on growth, osmotic adjustment and ionic balance of an alkali-resistant halophyte Suaeda glauca (Bge.). Plant Growth Regulation 56: 179–190. 10.1007/s10725-008-9299-y
- Yang CW, Zhang ML, Liu J, Shi DC, Wang DL 2009. Effects of buffer capacity on growth, photosynthesis, and solute accumulation of a glycophyte (wheat) and a halophyte (Chloris virgata). Photosynthetica 47: 55–60. 10.1007/s11099-009-0010-y