Abstract
The influence of two commercial Prunus rootstocks (‘Myrabolan 29/C’ and apricot ‘Seedling’) on fruit entity and quality of ‘Pisana’ apricot cultivar (Prunus armeniaca L.) was evaluated over two consecutive crop seasons. The effect of rootstock on the total antioxidant capacity, total phenols and physical-chemical traits of fresh apricots was studied at the ready-to-eat stage and after 14 days at 4 °C cold storage. The rootstocks had no significant influence on the flowering and fruiting entity but affected some fruit quality traits. The ‘Myrabolan 29/C’ appeared to induce the highest fruit weight, total antioxidant capacity and total phenols. The results showed an important role of the climatic conditions in determining the fruit antioxidant content. A drought ripening period improved the antioxidant potential of fruit independently of the rootstock. After cold storage, apricot fruits maintained good levels of antioxidants.
Introduction
Apricot (Prunus armeniaca L.) is characterized by non-surplus production, a wide range of possible valorisation and an adaptation to different environmental conditions due to a richness of local genotypes (Audergon et al. Citation2006). Apricot can be grown in a range of different pedo-climatic areas. However, heavy and calcareous soils with high pH cause root asphyxia and iron-induced chlorosis (Viti et al. Citation1989; Massai & Loreti Citation2009). Under these conditions, a commonly used rootstock is apricot ‘Seedling’, which is compatible with all cultivars. However, this rootstock showed some limits with regard to elevated vigour, non-cropping precocity and susceptibility to Phytophthora and Armillaria, particularly under irrigated conditions.
The tendency is to substitute apricot ‘Seedling’ with clonal rootstocks, such as Myrabolan (Prunus cerasifera), because they produce a variety of desirable traits including tree uniformity (Moreno Citation2009). The clonal selection ‘29/C’ has been established at nursery level, showing good adaptability to various types of soil, from drought to limestone (Cinelli & Viti Citation1995), high tree vigour, early entry to production, good yield efficiency and fruit size (Egea et al. Citation2004; Pennone & Abbate Citation2006). Moreover, some clonal rootstocks may influence the fruit quality by improving the pomological and organoleptic characteristics (Caste Citation1995; Milošević et al. Citation2013). New additional qualitative traits, such as antioxidant properties, can develop innovative cultivation and marketing strategies. The healthy benefits of fruits are appreciated by consumers who are becoming more interested in functional foods. In apricot, recent studies showed that the bioactive compounds, such as the antioxidant content, are mainly related to the genotype and the pedo-climatic conditions (Leccese et al. Citation2008). A screening of several international and Italian germplasm genotypes of apricot revealed some interesting cultivars that showed a high antioxidant capacity of fruits: among these cultivars, ‘Pisana’ stood out for excellent fruit properties (Leccese et al. Citation2012a). ‘Pisana’ was patented in Italy by the University of Pisa's breeding programmes and is characterized by late blooming and ripening time, and strong fruit attractiveness for the fresh market (Guerriero & Monteleone Citation1992; Guerriero et al. Citation2006). This cultivar, usually grafted on to the clonal rootstock ‘Myrabolan’, is also well appreciated in non-EU regions such as Latin America (Seibert et al. Citation2010).
The aim of this research was to assess the influence of two commercial Prunus rootstocks (‘Myrabolan 29/C’ and apricot ‘Seedling’) on fruit entity and quality of ‘Pisana’ cultivar, at ready-to-eat stage and after storage. In particular, the effect of rootstock on antioxidant properties, which has been poorly investigated until now, was studied.
Materials and methods
Plant material
Two commercial Prunus rootstocks (‘Myrabolan 29C’ and apricot ‘Seedling’), grafted with the ‘Pisana’ cultivar, were compared over two harvesting seasons (2010–11). The trial was conducted at the research station of the Department of Agriculture, Food and Environment at the University of Pisa, located in a coastal area of Tuscany (Italy, altitude 6 m asl, 43°02′N, 10°36′E). The site is characterized by mild winters and the average annual rainfall is about 600 mm; the soil in the orchard is loam, moderately deep, medium texture, slightly alkaline and non-calcareous.
Trees (10 years old), trained to a free palmette system (4 × 4.5 m) with rows facing east-west, were not irrigated and routine conventional horticultural management (pruning, thinning, fertilization, pest and disease protection) was performed. Two groups of trees, in a randomized experimental design (five single-tree replications for each scion-stock combination), were established: (1) thinned trees subjected to hand thinning of fruit to compare canopies with similar crop load (about 20 kg/canopy, regular yield for apricot adult trees) to determine the physical-chemical and biochemical traits of fruits; (2) unthinned trees were used to observe the natural behaviour for flowering and fruiting processes.
The main climatic data were acquired. Hourly temperatures were registered by automatic data-loggers (Tynitag Plus®, West Sussex, UK, 2003) and rainfall data were provided by the Regional Agro-meteorological Service of Florence (Agenzia Regionale per lo Sviluppo e l'Innovazione nel settore Agricolo Forestale, Tuscany, Italy). The winter cold received by the plants was quantified in terms of chill units (CU), starting from the end of vegetative season. The threshold of 1000 CU was considered a determinant amount for the fulfilment of the chilling requirement in most apricot cultivars (including ‘Pisana’) under Mediterranean conditions (Viti et al. Citation2006; Guerriero et al. Citation2010). The heat requirements, from breaking of endodormancy to ecodormancy release, were calculated as the growing degree hours (GDH). CU and GDH were quantified according to the model proposed by Richardson et al. (Citation1974).
Flowering and fruiting entity
Flowering and fruiting entity was measured on unthinned trees from four branches per tree (20 branches in total per each scion-stock combination). Fruiting shoots were homogeneous with a similar length (60–80 cm) and marked at different canopy positions when flower buds were at phenological stage A (Bartolini et al. Citation2004). The following parameters were recorded: bud drop rate by the monthly count of persisting flower buds; endo- and ecodormancy overcoming, according to Andreini et al. (Citation2012); blooming time expressed as Julian Day (JD 1 = 1 January); rate of bloom determined as initial (10% of opened flowers), full (50% of opened flowers) and end (90% of opened flowers); and fruiting percentage calculated as the number of fruit set per total number of open flowers.
Physical-chemical fruit parameters
At physiological maturity (ready-to-eat stage), samples of 60 fruits per graft combination were randomly collected from thinned trees and the main physical-chemical parameters, total antioxidant capacity and total phenol content determined. Half the fruits were immediately used for analysis, while the other half were cold stored at 4 ± 0.5 °C (90% relative humidity) and analysed after 14 days.
From each fruit, measurements of fresh weight, peel and flesh colour, flesh firmness, total soluble solids (TSS) and titratable acidity (TA) were determined. The skin colour of the unblushed side, ranging from yellow to orange with all shades in between, was evaluated using a colour chart for apricot fruit created by the Centre Technique Interprofessionnel des Fruits et Legumes, France (Lichou et al. Citation2003). This chart provides the development of the unblushed skin colour according to 10 shades of growing intensity from 1 (green) to 10 (red-orange) through different categories (1–4 yellow-green; 5–8 yellow-orange; 9–10 red-orange). The skin colour of the blushed side ranges from pink to red. The area of the blushes was evaluated visually by classifying the red area according to the following categories: <35% (class 1), 35%–65% (class 2), >65% (class 3). Firmness was evaluated with a manual penetrometer (Model 53200SP TR, TR-Turoni & Co Inc, Forlì, Italy) on two peeled opposite sides at the equatorial region of the apricot, using an 8 mm wide plunger. TSS was measured using a refractometer (Model 53015C TR, TR-Turoni & Co Inc, Forlì, Italy) and expressed in °Brix at room temperature. TA was determined in fruit juice by titrating a known volume of juice with 0.1 N sodium hydroxide (NaOH) to an end point of neutral pH (8.1). TA was expressed as milliequivalents per 100 g of fresh weight (meq 100 g−1 FW).
Biochemical fruit parameters
The total antioxidant capacity (TAC) and total phenol (TP) analyses were carried out on the same fruits that had been subjected to the physical and chemical determinations. Samples of 3 g (three replicates) of fresh material were homogenized using an ultra-Turrax T25 (Ika, Staufen, Germany) at 4 °C to avoid oxidation. The extraction was performed in 80% ethanol for 1 h in a shaker in the dark and subsequently centrifuged at 2600 g for 10 min at 2–4 °C. For each rootstock/fruit stage (ready-to-eat and stored), the supernatant of three extractions was used.
TAC assay
Total antioxidant capacity was evaluated using the improved Trolox equivalent antioxidant capacity (TEAC) method (Arts et al. Citation2004). The TEAC value was calculated in relation to the reactivity of Trolox, a water-soluble vitamin E analogue, which was used as an antioxidant standard. In the assay, 40 µL of the diluted samples, blanks, were added to 1960 µL ABTS•+ solution, which resulted in a 20%–80% inhibition of the absorbance. The decrease in absorbance at 734 nm was recorded at 6 min after an initial mixing, and plotted against a dose-response curve calculated for Trolox (0–30 µM). Antioxidant activity was expressed as micromoles of Trolox equivalents per gram of fresh fruit weight (µmol TE g−1 FW). Trolox was purchased from Sigma Chemical Co (St Louis, MO, USA).
TP assay
Total phenolic content was determined according to the improved Folin-Ciocalteu (F-C) method(Waterhouse Citation2001). The assay provides a rapid and useful indication of the antioxidant status of the studied material and has been widely applied to different food samples. Gallic acid (GA) (Sigma Chemical Co, St Louis, MO, USA) was used as a standard compound for the calibration curve. Total phenol content was calculated as milligrams of GA equivalent (GAE) per gram of fresh fruit weight (mg GAE g−1 FW). The absorbance of the blue coloured solutions was read at 765 nm after incubation for 2 h at room temperature.
Statistical analysis
Data were reported as means ± standard errors of the means (SEM). Analysis of variance (ANOVA) was performed using the statistical package Statistica (StatSoft, Tulsa, OK, USA). Differences between means from ANOVA were considered statistically significant at P ≤ 0.05, according to the Newman-Keuls test.
Results and discussion
Climatic conditions
The climatic trend of the years 2010–11 and the relative CU amount () fell within a seasonal mean over several years in the same environmental area (Guerriero et al. Citation2002; Viti et al. Citation2010a). In both years, the ‘Pisana’ cultivar at medium chilling requirement (Guerriero et al. Citation2000) overcame the flower bud endodormancy, even though the 1000 CU accumulation was reached at different times. The winter of 2011 was colder than 2010 allowing early attainment of the 1000 CU (22 January); the warm winter of 2010 caused a late attainment of the 1000 CU which occurred on 11 February. From January to March, a total of six frost events occurred in both years but with different distribution and intensity.
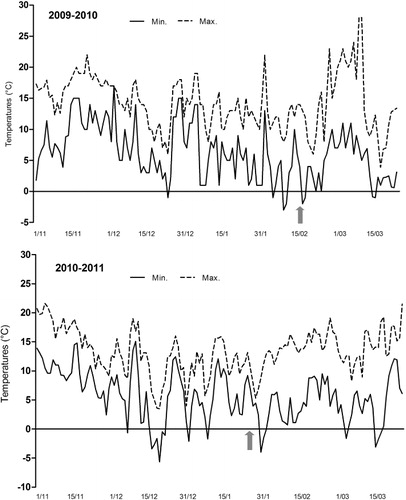
Maximum and minimum temperatures and the amount of rainfall over the last period of fruit growth and ripening are shown in . Daily temperatures over the two harvest periods were similar with averages ranging from 16–21 °C from April to June. Not relevant fluctuations of temperatures were recorded with respect to the pluriennial seasonal averages of the considered climatic area (Guerriero et al. Citation2010). In the same period, strong differences were found in the distribution and cumulative amount of rainfall. The cumulative rainfall was 193 and 47 mm in 2010 and 2011, respectively. In 2010, the wettest conditions were recorded at the time of the final stages of fruit growth, during the month of May, when 105 mm of rainfall was registered against 11.7 mm in 2011.
Table 1 Monthly mean maximum and minimum temperatures (°C), relative average (Avg) and cumulative rainfall (mm) from April to June over two growing seasons (2010–11).
Flowering and fruiting parameters
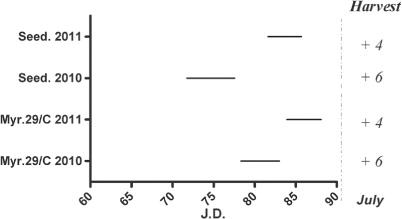
In both years, the start of flowering was influenced by the graft combination (): ‘Pisana’ on to ‘Seedling’ showed an earlier time than ‘Pisana’ grafted on to ‘Myrabolan 29/C’, ranging from a difference of seven days in 2010 to a difference of three days in 2011. Similar results were reported by Egea et al. (Citation2004) who found a slightly later flowering induced by Myrabolan. An early flowering time (second decade of March) was recorded in 2010, which was characterized by a late chilling accumulation, whereas in 2011 the flowering time happened in the third decade of March. The different flowering times may be attributed to the influence of the heat requirement expressed as GDH, from endo- to ecodormancy overcoming, which was higher in 2010 (3300 GDH) than 2011 (2300 GDH). This occurrence suggested a compensation between CU accumulation and GDH requirement, in agreement with previous research (Viti et al. Citation2010b).
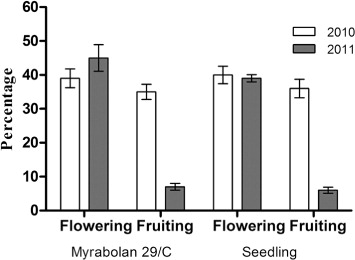
In both years, flowering and fruiting entity was not influenced by the rootstock (), and the winter flower bud drop (about 25%) and the number of unswelled buds were within usual values (Viti et al. 2006) allowing good flowering levels (≥40%). However fruiting percentage ranged from 35% in 2010 to 7% in 2011 for both rootstocks. The lowest fruit percentages, recorded in 2011, could be attributed to the climatic conditions during the flowering and fruit-set period: four consecutive spring frost events took place, reaching a low of −3 °C (). From the pre-flowering stages onwards, the reproductive organs become particularly sensitive to spring frosts as a consequence of the morphological and physiological status of swelling buds, characterized by a fast dehardening against sub-zero temperatures (Szalay et al. Citation2006). In particular, apricot is sensitive to freezing temperatures at the fulfilment of flower bud endodormancy with the beginning of active growth (Rodrigo Citation2000; Bartolini et al. Citation2006; Viti et al. Citation2010a).
No relevant variations were observed with regard to harvest time, which occurred during the first decade of July, in both graft combinations and years ().
Apricot fruit quality
Physical-chemical parameters
The main physical-chemical traits of apricots at ready-to-eat stage are shown in . In both years, the highest fruit weight was significantly induced by ‘Myrabolan 29/C’ (about 73 g): an increase in size of 30% was recorded, in comparison with ‘Pisana’ grafted on to ‘Seedling’. Apricot fruit quality characteristics are affected by rootstock (Hernandez et al. Citation2010); a positive influence of ‘Myrabolan 29/C’ on most fruit physical features, including the fruit volume, was recently proved by Milošević et al. (Citation2013). The smallest fruits from ‘Seedling’ rootstock were characterized by a more extensive skin colour on the blushed side, characterized by a uniform bright red colour. Colour has a significant impact on consumer's perception of apricot quality, especially regarding fruit attractiveness (Ruiz & Egea Citation2008).
Table 2 Main physical-chemical traits in apricot fruits of ‘Pisana’ cultivar grafted on to ‘Myrabolan 29/C’ and apricot ‘Seedling’ recorded at ready-to-eat stage and after 14 days of 4 °C cold storage. Mean of a 2-year period ± SEM.
Apricots from both rootstocks showed similar excellent levels of flesh firmness (about 2 kg 0.5 cm−2) and total soluble solids (13–14 °Brix), while titratable acidity showed values ranging from 11.7 to 14.4 meq 100 g−1 FW in ‘Seedling’ and ‘Myrabolan 29/C’, respectively. The ‘Pisana’ cultivar has high-quality attributes such as a notable TSS which influences the fruit taste. Several authors reported that values higher than 13 °Brix are representative of gustative quality in fresh apricots at harvest maturity (Cemagref Citation1981; Bassi & Selli Citation1990; Badenes et al. Citation1998). The high TSS content was positively related with an increase in the TSS/TA ratio responsible for a balanced flavour, improving the fruit eating quality, which may influence consumers’ acceptance and the commercial value of apricots (Bassi & Audergon Citation2006; Ruiz & Egea Citation2008). These parameters are comparable to previous studies carried out on ‘Pisana’ cultivars under different environmental conditions (Leccese et al. Citation2012b), suggesting the key role of genotype in qualitative fruit performance. The ANOVA results showed a year-by-year variation in flesh firmness and TSS values, in accordance with previous research carried out on several apricot cultivars (Ruiz & Egea Citation2008; Milinovic et al. Citation2012).
In relation to the susceptibility of apricot fruit to handling, storage and transport, pomological and chemical performances were tested after 14 days at 4 °C cold storage (). After storage, fruit weight loss occurred and the highest decrease (about 14%), was recorded for apricots from ‘Myrabolan 29/C’. Fruits from ‘Seedling’ rootstock had a lower weight decrease (about 8%). These fruit weight losses were similar to values previously recorded in studies carried out on a wide number of apricot genotypes after storage (Leccese et al. Citation2012b).
The most storage-susceptible parameter was flesh firmness; fruits from both rootstocks showed a loss of firmness from 23.3% and 32.5% for ‘Seedling’ and ‘Myrabolan 29/C’, respectively. The TSS and TA were not affected by the storage conditions in agreement with previous studies showing a loss of firmness after storage without changes in TSS and TA levels (Aubert et al. Citation2010; Leccese et al. Citation2010).
Total antioxidant capacity and total phenols
TAC and TP levels measured in ‘Pisana’ confirmed its excellent antioxidant powers as previously found when this cultivar was compared with a large number of commercial apricot genotypes (Leccese et al. Citation2012b).
During the trial, differences between rootstocks and years were observed (). Regarding the role of rootstock, ‘Myrabolan 29/C’ induced higher antioxidant levels than ‘Seedling’ in both years. At ready-to-eat stage, as averages of the two crop seasons, values of TAC ranged from 6.74 µmol TE g−1 FW (‘Myrabolan 29/C’) to 5.05 µmol TE g−1 FW (‘Seedling’) and TP ranged from 1.20 mg GAE g−1 FW (‘Myrabolan 29/C’) to 0.84 mg GAE g−1 FW (‘Seedling’). This performance was maintained after 14 days’ cold storage, suggesting that the Myrabolan rootstock induces a higher antioxidant capacity, in agreement with Scalzo et al. (Citation2005). This occurrence could be one of the effects related to the vigour of Myrabolan, which might have an important role in the uptake and translocation of nutrients (Nadernejad et al. Citation2013). Good storability was confirmed in apricots from ‘Seedling’, which maintained good antioxidant levels during storage.
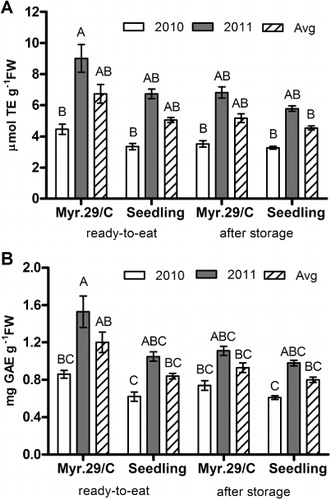
In 2011, at ready-to-eat stage, TAC and TP reached very high levels in both graft combinations: the TAC ranged from 6.74 µmol TE g−1 FW (‘Seedling’) to 9.01 µmol TE g−1 FW (‘Myrabolan 29/C’) and the TP ranged from 1.05 mg GAE g−1 FW (‘Seedling’) to 1.53 mg GAE g−1 FW (‘Myrabolan 29/C’). These values were about two-fold higher than in 2010 when fruits had TAC and TP levels similar to those recorded in previous studies carried out on ‘Pisana’ cultivar grafted on to ‘Myrabolan 29/C’ (Leccese et al Citation2012a). In a classification to order the apricot cultivars according to the antioxidant status (AOX) by a TEAC score, ‘Pisana’ showed the highest AOX placing at the top of the class which included TAC values above 4.53 µmol TE g−1 FW (Leccese et al. Citation2008).
The variability in TAC and TP levels observed between the two crop seasons could be influenced by different rainfall events which occurred during the last fruit growth period and ripening stages. These conditions might be determinant considering that the examined apricot orchard was under dry management. Water availability, together with light intensity and temperature, is an important climate factor affecting the nutritional value of vegetables and fruits (Salunke & Desai Citation1998). Several studies carried out on different fruit species showed a relationship between antioxidant activity and water availability (Navarro et al. Citation2010; Tavarini et al. Citation2011). The lowest cumulative rainfall was recorded in 2011 when only 47 mm of rain fell from April to June () and might have induced the antioxidant increase in ‘Pisana’ apricots. Moreover, several experimental trials on regulated deficit irrigation showed a positive effect of this practice on pomological properties and phenolic composition of fruits at harvest and during cold storage (Dragovic-Uzelac et al. Citation2007; Navarro et al. Citation2010).
The ANOVA results comparing harvest year and rootstock effect showed significant interactions ‘year × rootstock’ for TP properties (). In recent years, in apricot, a positive strong correlation has been established between phenols and the total antioxidant capacity (Leccese et al. Citation2008).
Table 3 Two-way ANOVA results at ready-to-eat. Variables: TAC (µmol TE g−1 FW) and TP (mg GAE g−1 FW).
Conclusions
The ‘Pisana’ cultivar, regardless of the interaction between rootstocks and climatic conditions, showed high qualitative fruit traits confirming previous studies on the key role played by the genetic background in shaping the pomological traits and the antioxidant potential of fruits. Data suggest an effect of the rootstock in determining, under the same growing conditions, variations in some traits of fruits, allowing an improvement of the qualitative profile. Fruits from ‘Myrabolan 29/C’ had higher quality than ‘Seedling’ in terms of both physical-chemical characteristics and antioxidant capacity. The choice of rootstock may be important considering the influence of genetic origin, as recently observed by Jakobek et al. (Citation2009), who found different levels of phenolic compounds in sweet cherry on several graft combinations.
This study illustrated the effect of climatic conditions on some qualitative traits of apricot fruit, in both graft combinations; in particular, a drought period during ripening time improved the antioxidant potential of ‘Pisana’ fruits. Moreover, another interesting aspect emerged concerning the storability of fruits as they maintained good levels of antioxidants after storage. This finding could satisfy the demand of consumers who are becoming more interested in healthy fruits.
Acknowledgements
The authors wish to thank Dr T. Lander, Natural History Museum of London, for proofreading the English manuscript.
References
- Andreini L, Viti R, Bartolini S, Ruiz D, Egea J, Campoy JA 2012. The relationship between xylem differentiation and dormancy evolution in apricot flower buds (Prunus armeniaca L.): the influence of environmental conditions in two Mediterranean areas. Trees 26: 919–928. 10.1007/s00468-011-0668-1
- Arts MJTJ, Dallinga JS, Voss HP, Haenen GRMM, Bast AA 2004. New approach to assess the total antioxidant capacity using the TEAC assay. Food Chemistry 88: 567–570. 10.1016/j.foodchem.2004.02.008
- Aubert C, Bony P, Chalot G, Hero V 2010. Changes in physicochemical characteristics and volatile compounds of apricot (Prunus armeniaca L. cv. Bergeron) during storage and postharvest maturation. Food Chemistry 119: 1386–1398. 10.1016/j.foodchem.2009.09.018
- Audergon JM, Giard A, Lambert P, Blanc A, Gilles F, Signoret V et al. 2006. Optimization of apricot breeding by a joint conventional and molecular approach applied to the main agronomic traits – ABRIGEN Project. Acta Horticulturae 701: 317–320.
- Badenes ML, Martinez-Calvo J, Llacer G 1998. Analysis of apricot germplasm from the European ecogeographical group. Euphytica 102: 93–99. 10.1023/A:1018332312570
- Bartolini S, Viti R, Guerriero R 2006. Xylem differentiation and microsporogenesis during dormancy of apricot flower bud. European Journal of Horticultural Science 71: 84–90.
- Bartolini S, Viti R, Zanol G 2004. The involvement of glutathione in flower bud dormancy overcoming in apricot (Prunus armeniaca L.). In: Pandalai SG ed. Recent research developments in agronomy and horticulture. Kerala, India, Research Signpost Press. Pp. 11–28.
- Bassi D, Audergon JM 2006. Apricot breeding: update and perspectives. Acta Horticulturae 701: 279–294.
- Bassi D, Selli R 1990. Evaluation of fruit quality in peach and apricot. Advances in Horticultural Science 2: 107–111.
- Caste WS 1995. Rootstock as a fruit quality factor in citrus and deciduous tree crops. New Zealand Journal of Crop and Horticultural Science 23: 383–394. 10.1080/01140671.1995.9513914
- Cemagref 1981. La qualité gustative des fruits. In: Ministry of Agriculture ed. Methodes pratiques d'analyse. Paris, Ministry of Agriculture. Pp. 150.
- Cinelli F, Viti R 1995. Practical use of root cation exchange capacity as a predictive marker of lime-induced clorosis tolerance in Prunus cerasifera L. rootstocks. Journal of Plant Nutrition 18: 65–75. 10.1080/01904169509364885
- Dragovic-Uzelac V, Levaj B, Mrkic V, Bursac D, Boras M 2007. The content of polyphenols and carotenoids in three apricot cultivars depending on the stage of maturity and geographical origin. Food Chemistry 102: 966–975. 10.1016/j.foodchem.2006.04.001
- Egea J, Ruiz D, Martínez-Gómez P 2004. Influence of rootstock on the productive behaviour of ‘Orange Red’ apricot under Mediterranean conditions. Fruits 59: 367–373. 10.1051/fruits:2004035
- Guerriero R, Martelloni V, Monteleone P, Viti R, Balbi N 2000. Valutazione della capacità di adattamento e di fruttificazione di nuove cultivar di albicocco alle condizioni climatiche del litorale tirrenico. In: ETS ed. Interreg II Toscana-Corsica. Pisa, ETS. Pp 125–133.
- Guerriero R, Massai R, Canterella F, Remorini D 2006. Agronomic behaviour of ‘Pisana’ cultivar on several rootstocks in dry, sandy hills. Acta Horticulturae 717: 163–167.
- Guerriero R, Monteleone P 1992. ‘Pisana’. Frutticoltura 6: 8–11.
- Guerriero R, Viti R, Monteleone P, Gentili M 2002. La valutazione della dormienza nell'albicocco: 3 metodi a confronto. Frutticoltura 3: 73–77.
- Guerriero R, Viti R, Iacona C, Bartolini S 2010. Is apricot germplasm capable of withstanding warmer winters? This is what we learned from last winter. Acta Horticulturae 862: 265–272.
- Hernandez FCA, Pinochet J, Moreno MA, Martınez JJ, Legua P 2010. Performance of Prunus rootstocks for apricot in Mediterranean conditions. Scientia Horticulturae 124: 354–359. 10.1016/j.scienta.2010.01.020
- Jakobek L, Seruga M, Voca S, Sindrak Z, Dobricevic N 2009. Flavonol and phenolic acid composition of sweet cherries (cv. Lapins) produced on six different vegetative rootstocks. Scientia Horticulturae 123: 23–28. 10.1016/j.scienta.2009.07.012
- Leccese A, Bartolini S, Viti R 2008. Total antioxidant capacity and phenolics content in fresh apricots. Acta Alimentaria 37: 65–76. 10.1556/AAlim.37.2008.1.6
- Leccese A, Bartolini S, Viti R 2012a. From genotype to apricot fruit quality: the antioxidant properties contribution. Plant Food for Human Nutrition 67: 317–325. 10.1007/s11130-012-0314-0
- Leccese A, Bartolini S, Viti R 2012b. Genotype, harvest season, and cold storage influence on fruit quality and antioxidant properties of apricot. International Journal of Food Properties 15: 864–879. 10.1080/10942912.2010.506019
- Leccese A, Bartolini S, Viti R, Pirazzini P 2010. Fruit quality performance of organic apricots at harvest and after storage from different environmental conditions. Acta Horticulturae 873: 165–172.
- Lichou J, Jay M, Vaysse P, Lespinasse N 2003. Reconnaître les Varietes d'Abricots. Paris, France, Eds Ctifl. Pp. 17–29.
- Massai R, Loreti F 2009. I portinnesti dell'albicocco. Proceeding of International Conference on Fruit Tree Rootstocks, University of Pisa (Italy), Pisa, 26 June 2009. Pp 137–153.
- Milinovic B, Jelacic T, Halapija Kazija D, Cicek D, Vujevic P, Cmelik Z 2012. The effect of weather conditions on fruit skin colour development and pomological characteristics of four apricot cultivars planted in Donja Zelina. Agriculturae Conspectus Scientificus 77: 191–197.
- Milošević T, Milošević N, Glišić I, Šekularac G 2013. Influence of stock on physical and chemical traits of fresh apricot fruit. International Agrophysics 27: 111–114.
- Moreno MA 2009. Rootstocks for stone and pome fruit tree species in Spain. Proceeding of International Conference on Fruit Tree Rootstocks, University of Pisa (Italy), Pisa, 26 June 2009. Pp. 44–57.
- Nadernejad N, Ahmadimoghadam A, Hossyinifard J, Poorseyedi S 2013. Effect of different rootstocks on PAL activity and phenolic compounds in flowers, leaves, hulls and kernels of three pistachio (Pistacia vera L.) cultivars. Trees 27: 1681–1689. 10.1007/s00468-013-0915-8
- Navarro JM, Pérez-Pérez JG, Romero P, Botia P 2010. Analysis of the changes in quality in mandarin fruit, produced by deficit irrigation treatments. Food Chemistry 119: 1591–1596. 10.1016/j.foodchem.2009.09.048
- Pennone, F, Abbate, V 2006. Preliminary observations on the biological and horticultural behaviour of different apricot rootstocks. Acta Horticulturae 701: 347–350.
- Richardson EA, Seeley SD, Walker RD 1974. A model for estimating the completion of rest for Red Haven and Elberta peach. HortScience 9: 331–332.
- Rodrigo J 2000. Spring frosts in deciduous fruit trees. Morphological damage and flower hardiness. Scientia Horticulturae 85: 155–173. 10.1016/S0304-4238(99)00150-8
- Ruiz D, Egea J 2008. Phenotypic diversity and relationship of fruit quality traits in apricot (Prunus armeniaca L.) germplasm. Euphytica 163: 143–158. 10.1007/s10681-007-9640-y
- Salunke DK, Desai BB. 1998. Effects of agricultural practices, handling, processing, and storage on vegetables. In Karmas E, Harris RS ed. Nutritional evaluation of food processing. New York, AVI. Pp. 23–72.
- Scalzo J, Politi A, Pellegrini N, Mezzetti B, Battino M 2005. Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition 21: 207–213. 10.1016/j.nut.2004.03.025
- Seibert E, Rubio P, Infante R, Nilo R, Orellana A 2010. Intermittent warming heat shock on ‘Pisana’ apricot during postharvest: sensorial quality and proteomic approach. Acta Horticulturae 862: 599–604.
- Szalay L, Papp J, Pedryc A, Szabo Z 2006. Influence of the changing climate on flower bud development of apricot varieties. Acta Horticulturae 717: 75–78.
- Tavarini S, Gil MI, Tomas-Barberan FA, Buendia B, Remorini D, Massai R et al. 2011. Effects of water stress and rootstocks on fruit phenolic composition and physical/chemical quality in Suncrest peach. Annals of Applied Biology 158: 226–233. 10.1111/j.1744-7348.2010.00457.x
- Viti R, Andreini L, Ruiz D, Egea J, Bartolini S, Iacona C et al. 2010a. Effect of climatic conditions on the overcoming of dormancy in apricot flower buds in two Mediterranean areas: Murcia (Spain) and Tuscany (Italy). Scientia Horticulturae 124: 217–224. 10.1016/j.scienta.2010.01.001
- Viti R, Bartolini S, Guerriero R 2006. Apricot floral biology: the evolution of dormancy and the appearance of bud anomalies in several Italian genotypes. Advances in Horticultural Science 20: 267–274.
- Viti R, Bartolini S, Andreini L 2010b. Flower bud frost tolerance of several Italian apricot genotypes. European Journal of Horticultural Science 75: 185–192.
- Viti R, Loreti F, Cinelli F 1989. La valutazione dei portinnesti per la resistenza alla clorosi calcarea. Rivista di Frutticoltura 8–9: 27–32.
- Waterhouse AL 2001. Determination of total phenolics. In: Wroslad RE eds. Current protocols in food analytical chemistry. New York, John Wiley & Sons. Pp. I1.1.1–I1.1.8.
