Abstract
In this study, the possibility of enhancing water stress tolerance of pepper during early growth stages by foliar application of glycinebetaine (GB) was investigated. Pepper seedlings with four true leaves were sprayed with three different concentrations (0, 5 or 25 mM) of GB after which they were subjected to water stress for 10 days. Water stress caused substantial reductions in shoot dry weight, leaf area, chlorophyll content, leaf water potential, gas exchange characteristics and efficiency of photosystem-II while increasing membrane permeability and lipid peroxidation. However, foliar application of GB significantly counteracted the water stress-induced adverse effects by increasing chlorophyll content, stomatal conductance, efficiency of photosystem-II and proline content, while reducing visual damage symptoms, membrane permeability and lipid peroxidation. GB application also improved leaf water potential, relative water content and antioxidant enzymatic activity. Among the GB concentrations applied, enhanced water stress tolerance was obtained with 5 mM GB pre-treatment. Results, therefore, indicate that GB, applied as foliar spray, could be used as an ameliorative agent for pepper seedlings against the deleterious effects of water stress.
Introduction
Abiotic stresses are becoming more widespread as the intensity of agriculture and the demand for farmable land are ever increasing. Faced with dwindling fresh water resources, drought is the single most critical threat to world food security (Farooq et al. Citation2009). It slows growth, reduces canopy absorption of photosynthetically active radiation, induces stomatal closure and therefore lowers photosynthesis (Nemeth et al. Citation2002). Accumulating evidence indicates that membranes are the primary site of desiccation injury to cells and organelles, and it is generally accepted that the maintenance of integrity and stability of membranes under water stress is a major component of drought tolerance in plants (Bajji et al. Citation2002). Severe water stress conditions cause an alteration in the ratio of phospholipids, peroxidation and de-esterifcation of membrane lipids, as well as leading to protein denaturation and mutation of nucleic acids (Mahajan & Tuteja Citation2005). Impaired membrane integrity was suggested to be the cause of increase in electrolyte leakage in cotton (Lv et al. Citation2007), wheat (He et al. Citation2011) and tomato (Roy et al. Citation2009) plants under water stress.
Plants have evolved various mechanisms that allow them to tolerate unfavourable environments for continued growth (Sakamoto & Murata Citation2000). One such mechanism ubiquitous to plants is the accumulation of certain low molecular weight organic metabolites that are known collectively as compatible solutes or osmoprotectants (Bohnert et al. Citation1995). In general, they protect plants from abiotic stress via different means, including contribution to cellular osmotic adjustment, detoxification of harmful reactive oxygen species (ROS), protection of cellular membrane integrity, and stabilization of enzymes/proteins (Yancey et al. Citation1982; Bohnert & Jensen Citation1996; Ashraf & Foolad Citation2007). These solutes include proline, sucrose, polyols, trehalose and quaternary ammonium compounds (QACs) such as choline and various betaines (Rhodes & Hanson Citation1993).
One of the extensively studied QACs is glycinebetaine (GB) (N, N, N-trimethylglycine) which is found in plants, animals and bacteria (Rhodes & Hanson Citation1993). Numerous studies indicate that GB may have a significant role in improving plant tolerance to various abiotic stresses such as drought, salt and extreme temperatures (Ashraf & Foolad Citation2007). Some species, which are known as GB-accumulator plants, accumulate significantly higher amounts of GB under stress conditions (Chen & Murata Citation2008). The elevated levels of endogenous GB are reported to be correlated well with the extent of increased stress tolerance in various crops including sugar beet, wheat, barley, spinach and sorghum (Ashraf & Foolad Citation2007). In plants that do not accumulate GB, exogenous applications elevate tolerance to various abiotic stresses, improving plant growth and survival (Abbas et al. Citation2010; Habib et al. Citation2012). In our previous research, we documented that pre-sowing seed treatment with GB could be used effectively to protect pepper seedlings from the damaging effects of salt stress without any adverse effect on seedling growth (Korkmaz & Şirikçi Citation2011). However, to our knowledge, no information is available regarding the effects of GB on water stress tolerance of pepper seedlings. Therefore, the aims of this experiment were to test the possibility that foliar application of GB would protect pepper seedlings from the damaging effects of water stress and to discuss the possible mechanisms of its protective function.
Materials and methods
Plant material, GB treatments and water stress imposition
Seeds of ‘Sena’ red pepper (Capsicum annuum L.), all from the same seed lot, were obtained from the Agricultural Research Institute, Kahramanmaras, Turkey. The seeds were planted at a depth of 1.0 cm into 5.5 cm-deep flat cells (75 cm3) filled with growth medium consisting of peat and perlite in the ratio of 3:1. The flats were watered regularly with tap water and kept in a growth chamber at 25 ± 1 °C (day/night) under cool fluorescent lamps (250 µmol m−2s−1) for 16 h day−1. The relative humidity inside the growth chamber was maintained around 50%. When the seedlings had fully developed four true leaves (30–34 days after sowing), each seedling was sprayed with 10 mL of 0, 5 or 25 mM GB solutions until both sides of the leaves were completely wet. These GB concentrations were selected after conducting preliminary experiments using a wider range of GB concentrations. One day after the foliar GB application, all plants were watered after which half of them were subjected to water stress while the other half continued to be watered regularly (non-stressed). Water stress was imposed by withholding water for 10 days, then on the 11th day all plants were watered until saturation (Senaratna et al. Citation2000). After the water stress had ended, the moisture content of the growth medium was determined. There was 73%–75% water in the growth medium of non-stressed plants while that of water-stressed plants ranged from 28%–31%. The plants were assessed 48 h after the end of water stress to determine the extent of water stress injury and data were collected. The treatments were replicated four times with 12 plants in each replication and all the treatments were arranged in a randomized complete block design.
Visual damage assessment
All plants were examined visually to determine the extent of water stress injury and classified by using the following scale as shown in : none, no visible symptoms; slight, minor growth restrictions; moderate, wilting on lower leaves; severe, rolling of upper leaves and wilting at whole plant level; extensive, heavy wilting and yellowing, visible necrotic areas on the leaf edges; killed, entire plant necrotic and collapsed. By assigning values of 0, 1, 2, 3, 4 and 5, respectively to each group, the average injury for each treatment was calculated.
Determination of leaf area, shoot dry weight and chlorophyll content
Leaf area of all plants (excluding petioles) was determined with a leaf area meter (LiCor, Model LI-3100C, Lincoln, NE, USA) and all plants were cut at the growth medium surface and their dry weights (dried at 70 °C for 48 h) were recorded.
Chlorophyll content was determined by taking fresh leaf samples (0.5 g) from three randomly selected plants per replicate. The samples were ground with 5 mL of acetone (80% v/v) using a pestle and mortar and filtered through a filter paper (Whatman No. 2). The absorbance was measured with a UV/visible spectrophotometer (Shimadzu UV 1800, Kyoto, Japan) at 663 and 645 nm and chlorophyll concentrations were calculated using the equations proposed by Lichtenthaler (Citation1987) given below. Total chlorophyll content was expressed as Chla + Chlb.
Measurements of gas exchange attributes and chlorophyll fluorescence
Measurements of gas exchange attributes such as photosynthetic rate (A), transpiration (E) and stomatal conductance (gs), were performed on a fully expanded true leaf (from top) on two plants from each replicate using a portable gas exchange system (Model GFS3000, Walz, Effeltrich, Germany). Photosynthetic photon flux density (supplied by red/blue light-emitting diode source) during the measurements was maintained at 750 μmol m−2 s−1 which was the saturating light intensity for photosynthesis. Leaf temperature was maintained at 25 °C and chamber CO2 concentration of 380 ppm was supplied by a CO2 injector. Each leaf was allowed to reach a steady state of CO2 uptake before the measurements were taken. The maximal efficiency of PSII photochemistry (Fv/Fm), which is a direct measure of maximum efficiency of PSII, was determined on the same leaf simultaneously with gas exchange measurements after dark adaptation of the leaf for 30 min. The water use efficiency (WUE) was calculated by the ratio of A to E.
Water relations
To determine relative water content (RWC), two leaf discs (1 cm in diameter) taken randomly from the middle portion of a fully developed third leaf (to exclude the age effect) of different plants were used. Discs were weighed (fresh weight, FW) and then immediately floated on distilled water in a Petri dish for 5 h in the dark. Turgid weights (TW) of leaf discs were obtained after drying excess surface water with paper towels. Dry weights (DW) of discs were measured after drying at 75 °C for 48 h. RWC was calculated using the following formula:
Seedling leaf water potential (Ψw) was determined by using a pressure chamber (Model 1000, PMS Inst, Albany, OR, USA). To measure the solute potential (Ψs), leaf pieces from seedlings were frozen in liquid nitrogen and stored in 2 cm3 polypropyline tubes at −80 °C. Leaf tissue was later thawed and centrifuged at 8000 g for 5 min to extract cell sap. The osmolality of the cell sap was measured using a freezing point depression osmometer (Model 3320, Advanced Instruments Inc, Norwood, MA) and Ψs was calculated from osmolality using the van’t Hoff equation.
Determining membrane permeability, H2O2 and malondialdehyde (MDA) contents
Electrolyte leakage, a measure of membrane permeability, was determined according to Campos et al. (Citation2003) with slight modifications. Freshly cut leaf discs (1 cm in diameter) taken from two randomly chosen plants per replicate were washed with distilled water to remove surface contamination. The discs were placed in individual stoppered vials containing 20 mL of distilled water. After incubating the samples at room temperature on a shaker (150 rpm) for 24 h, the electrical conductivity (EC) of the bathing solution (EC1) was determined. The same samples were then placed in an autoclave at 121 °C for 20 min and a second reading (EC2) was determined after cooling the solution to room temperature. The electrolyte leakage was calculated as EC1 /EC2 and expressed as a percentage.
Hydrogen peroxide (H2O2) content was determined according to Özden et al. (Citation2009) with slight modifications. Fresh leaf tissue (0.25 g) was homogenized in an ice bath with 3 mL of 0.1% (w/v) trichloroacetic acid (TCA). The homogenate was centrifuged at 4 °C and 10,000 g for 10 min and 0.1 mL of the supernatant was added to 0.1 mL of 10 mM potassium phosphate buffer (pH 7.0) and 1.5 mL of 1 M KI. The absorbance of the supernatant was measured at 390 nm. The content of H2O2 was calculated by comparison with a standard calibration curve previously made by using different concentrations of H2O2.
The MDA concentration was determined according to the method of Zhang et al. (Citation2005) with some modification. Homogenization of fresh leaf samples (0.25 g) in 3 mL of 10% TCA was conducted and the samples were centrifuged at 10,000 g for 15 min. The supernatant was collected and 1 mL was mixed with 1 mL of 0.6% thiobarbituric acid. The mixture was placed in a water bath set at 100 °C for 20 min, cooled quickly and centrifuged at 10,000 g for 10 min after which its absorbance was determined at 532, 600 and 450 nm. The MDA concentration was calculated according to the following formula:
Enzyme extractions and assays
Enzyme extractions were performed as described in Seçkin et al. (Citation2010). Fresh leaf samples (0.5 g) were rapidly extracted in a pre-chilled mortar on an ice bath with 1.5 mL of ice cold 50 mM sodium phosphate buffer (pH 7.8) containing 1 mM EDTA-Na2 and 2% (w/v) PVPP. Samples were centrifuged at 14,000 g for 20 min, and supernatants were used for the determination of protein content and enzyme activities. Total soluble protein contents of the enzyme extracts were calculated according to Bradford (Citation1976) using bovine serum albumin (BSA) as a standard and the protein concentration was determined from a BSA standard curve. The specific enzyme activity for all enzymes was expressed as in unit mg−1 protein.
Catalase (CAT, EC 1.11.1.6) activity was determined by the method of Çakmak & Horst (Citation1991), which measures the initial rate of disappearance of H2O2 at 240 nm. The reaction mixture contained 70 µL crude enzyme extract, 930 µL 50 mM Na-phosphate buffer (pH 7.0) with 0.1 mM EDTA and 3% H2O2. The decrease in the absorption was followed for 3 min and 1 mmol H2O2 mL−1 min−1 was defined as 1 unit of CAT.
Peroxidase (POX, EC 1.11.1.7) activity was measured according to the method described by Herzog & Fahimi (Citation1973). The reaction mixture contained 75 µL crude enzyme extract and 925 µL 3,3-diaminobenzidine-tetra hydrochloride dihydrate solution containing 0.1% (w/v) gelatine and 150 mM Na-phosphate-citrate buffer (pH 4.4) and 0.6% H2O2. The increase in the absorbance at 465 nm was followed for 3 min and one unit of POX activity was defined as mmol H2O2 decomposed mL−1 min−1.
Determination of proline and GB contents
Proline content was determined according to the method described by Bates et al. (Citation1973). Fresh leaf material (0.5 g) was homogenized in 10 mL of 3% aqueous sulphosalicylic acid and filtered through Whatman’s No. 2 filter paper. Half a millilitre (0.5 mL) of the filtrate was mixed with 1 mL of acid-ninhydrin and 1 mL of glacial acetic acid in a test tube. The mixture was placed in a water bath for 1 h at 100 °C. The reaction mixture was extracted with 4 mL toluene and the chromophore containing toluene was aspirated, cooled to room temperature, and the absorbance was measured at 520 nm. Appropriate proline standards were included for the calculation of proline in the samples.
Before determining GB content of pepper seedlings, dried shoots of all plants from each replicate were ground together and a working sample was created. GB content was determined by following the method described by Grieve & Grattan (Citation1983) with some modifications. Finely powdered plant material (0.5 g) was mechanically shaken with 20 mL of deionized water for 24 h at 25 °C. The samples were then filtered using Whatman’s No. 2 filter paper and the filtrate was diluted 1:1 with 2 N sulphuric acid. Half a millilitre (0.5 mL) of aliquot was cooled in ice water for 1 h. Cold KI–I2 reagent (0.2 mL) was added and the mixture was vortexed for 5 min. The samples were then stored at 4 °C for 16 h, transferred to centrifuge tubes and centrifuged at 10,000 g for 15 min at 0 °C. The supernatant was discarded and periodite crystals were dissolved in 9 mL of 1,2-dichlor-oethane, and the absorbance was taken at 365 nm. Appropriate GB standards were used for calibration and for the calculation of GB concentration in unknown samples.
Statistical analysis
Data were subjected to analysis of variance (ANOVA) using the MStat statistical software program and mean separation was performed by Fisher’s least significant difference (LSD) test if F test was significant at P = 0.05. Experiments were repeated twice and since there were no significant differences between the results of the two experiments, the data from both experiments were combined and the mean values were presented (n = 8).
Results
Visual damage index
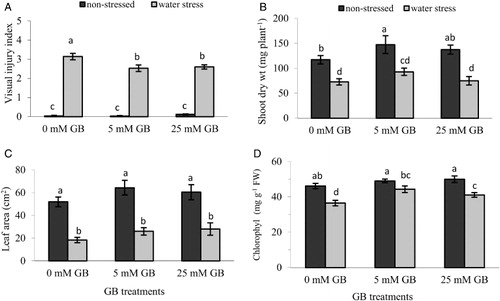
Water stress markedly suppressed pepper seedling growth, causing severe to extensive damage on the seedlings; however, foliar application of GB was effective in reducing visual injury symptoms (). After exposure to water stress for 10 days, plants not treated with GB (0 mM) exhibited typical water stress injury symptoms at severe to extensive levels while GB-treated seedlings were only moderately damaged. The non-GB-treated seedlings had wilted leaves and lost a considerable portion of their foliage due to necrotic areas compared with GB-treated seedlings. There were no visual differences observed between plants treated with 5 and 25 mM GB, exhibiting similar damage symptoms. In plants not exposed to water stress conditions, however, GB applications had no apparent effect on the visual appearance of the pepper seedlings.
Leaf area, plant mass and chlorophyll content
Plants subjected to water stress were half the size of those grown under optimum conditions and had reduced chlorophyll content. GB application had no significant effect on seedling leaf area and dry weight under water stress conditions even though 5 mM GB application increased shoot dry weight by 27% and leaf area by 42% compared with non-GB-treated plants (, ). Under optimum conditions, however, 5 mM GB application significantly affected plant dry mass causing 25% increase compared with non-GB-applied plants. Foliar application of GB positively affected leaf chlorophyll content under water stress conditions and plants treated with 5 mM GB had 21% greater total chlorophyll content than non-GB-treated plants (). On the contrary, GB application caused slight but insignificant enhancement in chlorophyll content of plants not exposed to water stress.
Membrane leakage and lipid peroxidation
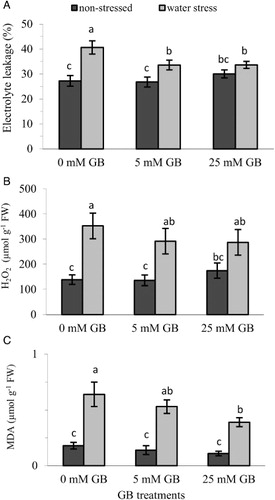
Water stress severely affected membrane integrity due to increased H2O2 content and lipid peroxidation measured as MDA levels, all of which ultimately resulted in pronounced increases in electrolyte leakage. Even though foliar application of 25 mM GB slightly increased H2O2 content and electrolyte leakage under non-stress conditions compared with 0 mM GB treatment, this effect was found statistically insignificant (, ). Under water stress conditions, however, GB application in both concentrations significantly improved membrane integrity expressed as reduced electrolyte leakage (). Treatment of plants with GB caused slight but insignificant decrease in H2O2 content (), whereas 25 mM GB application significantly lowered MDA levels compared with control seedlings ().
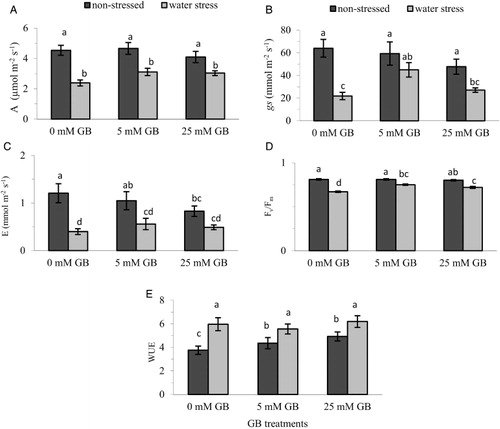
Gas exchange parameters and chlorophyll fluorescence
Water stress suppressed all photosynthetic parameters, resulting in significant reductions in A (), gs () and E (). Foliar application of GB decreased E considerably while slightly reducing A and gs of plants grown under optimum conditions; however, it significantly improved the photosynthetic capacity of water-stressed plants (–). Even though 5 mM GB treatment increased A and E of pepper seedlings under water stress by 30% and 40%, respectively, compared with the control plants, these enhancements were statistically insignificant. By contrast, 5 mM GB-treated plants had significantly higher gs, exhibiting twice as high stomatal conductance rates as the control plants. Efficiency of photosystem-II (PSII), determined as the Fv/Fm ratio, was also significantly affected by water stress as well by foliar application of GB which resulted in as much as a 12% increase in the Fv/Fm ratio () compared with control plants. Water stress significantly improved WUE of pepper seedlings under non-stress conditions but no change was observed in WUE due to GB applications under stress conditions ().
GB and proline contents
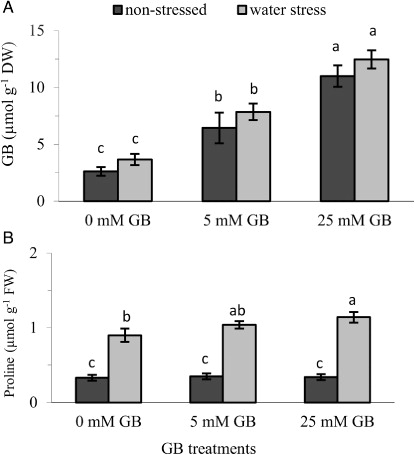
GB accumulation in the leaves of pepper seedlings was not affected by water stress (), but proline content was considerably enhanced due to water stress (). Leaf GB content increased significantly with increasing concentration of exogenously applied GB under both stress and non-stress conditions. Proline content was also boosted significantly by GB applications under water stress conditions while, on the other hand, remained unchanged under non-stress conditions.
Water relations
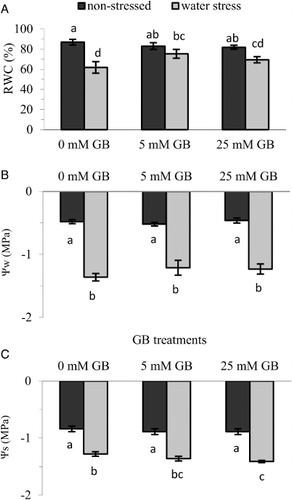
Water stress caused a significant reduction in all water relation parameters such as RWC, water potential and osmotic potential of pepper seedlings. Even though exogenously applied GB did not change the seedling’s leaf water status under non-stress conditions, it improved the seedling’s leaf water status under stress conditions compared with control plants (–). There was a significant change in leaf RWC between control and GB-applied plants and GB application caused a substantial (up to 22%) increase in RWC of water-stressed plants (). On the other hand, GB application did not cause a significant change in leaf Ψw despite an increase of up to 11% with 5 mM GB application (). In contrast, exogenous application of GB significantly reduced leaf Ψs, which may be the major factor responsible for increasing RWC under water stress conditions ().
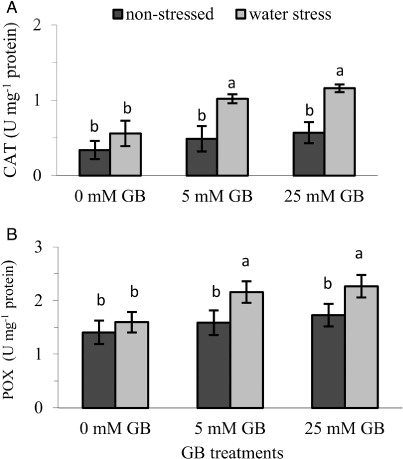
Antioxidant enzyme activity
Under non-stress conditions, activities of CAT and POX were increased slightly by GB application; however, exogenous application of GB significantly affected the CAT and POX activities causing as much as 102% and 42% increase in the activity of CAT and POX, respectively (, ) under water stress conditions.
Discussion
Data presented in this study indicated that a marked decline in growth of pepper seedlings due to water stress was observed and that application of GB through foliar spray protected the seedlings from the damaging effects of water stress. Of the GB concentrations, 5 mM treatment seemed to be more effective in enhancing tolerance to water stress since it affected positively more variables compared with 25 mM GB concentration. Plants pre-treated with GB exhibited moderate injury symptoms and had small necrotic areas on leaf edges, but those that were not sprayed with GB had severe damage and lost considerable portions of their foliage (). GB-treated plants were also considerably larger in size as indicated by increased leaf area () and seedling dry mass () and had significantly higher chlorophyll content () than those that were not pre-treated with GB. These results correlate well with the findings of Hussain et al. (Citation2009) who found that foliar application of 100 mM GB at the flowering stage significantly improved the leaf area index, leaf area duration and crop growth rate of hybrid sunflower plants grown under water deficient conditions. Ma et al. (Citation2007) also reported that tobacco seedlings pre-treated with 80 mM GB through foliar application had significantly higher chlorophyll contents than control plants under water deficit conditions that lasted for 15 days.
Cellular membranes can be damaged by environmental stresses such as drought, salinity and extreme temperatures and become more permeable due to increased solubilization and the peroxidation of membrane lipids under stress conditions (Farooq et al. Citation2009; Gill & Tuteja Citation2010). The decomposition of polyunsaturated fatty acids in the membranes by peroxidation generates MDA, a toxic byproduct. Elevated electrolyte leakage and MDA levels are considered as symptoms of stress-induced damage caused by ROS such as H2O2. In this study, membrane stability was significantly reduced by water stress as demonstrated by increased levels of electrolyte leakage and higher H2O2 and MDA contents, indicating that a definite oxidative stress was taking place in the leaves. However, foliar application of GB ameliorated the negative effects of water stress exerted on the membranes, which were obvious in terms of markedly enhanced membrane stability as evident from decreased electrolyte leakage () and reduced leaf H2O2 () and MDA () contents. Improved membrane permeability and reduced MDA and H2O2 levels have also been reported in tobacco (Ma et al. Citation2007), maize (Xin et al. Citation2011) and rice (Farooq et al. Citation2008) plants subjected to drought stress by exogenous application of GB.
Many studies have demonstrated that exogenous application of GB or overproduction of GB enhanced the photosynthetic capacity of crop plants under drought stress conditions. For example, Anjum et al. (Citation2011) reported that GB-treated maize plants maintained considerably higher gas exchange rate and chlorophyll content during drought stress than non-GB-treated plants. Foliar application of GB at a rate of 100 mM increased A of drought stressed wheat (Ma et al. Citation2006) and tomato (Mäkelä et al. Citation1998), and the improvement in A was reported to be due to increased gs and improved Fv/Fm values. In addition, by introducing betA gene into wheat, the GB-overaccumulating transgenic lines had elevated levels of chlorophyll content and exhibited higher levels of gs, A and Fv/Fm values compared with wild type plants under drought stress (He et al. Citation2011). In accordance with these previous findings, we found in the present study that water stress significantly reduced the gas exchange attributes such as A (), gs () and E () of pepper seedlings but GB application alleviated these disturbances. Improved stomatal conductance along with increased chlorophyll content () might be the reason for the maintaining of a relatively higher photosynthesis rate under water stress conditions and the mitigation of negative effects. Continued growth of pepper seedlings under water stress as demonstrated by higher leaf area () and seedling mass () may also be associated with improved A, since GB application moderated the decrease in photosynthetic capacity.
The factors that can reduce the photosynthetic rate under water stress conditions include stomatal closure, damage to the photosynthetic apparatus and reduced leaf area (Farooq et. al. Citation2009). Stomatal closure has a direct effect on CO2 entering cells, whereas long-term moderate or severe stress can be detrimental to the photosynthetic apparatus. The mechanisms by which GB application enhances the stomatal conductance are not clear. It has been reported that GB increases the proportion of bound water in the cell structures, which increases the turgor pressure in the stomatal guard cells and leads to increased stomatal conductance (Timasheff Citation1992). Yang & Lu (Citation2005) reported that increased stomatal conductance in salt-stressed plants induced by GB application was due to increased turgor pressure in maize guard cells. It was also suggested that GB regulates the movements of stomata by prolonging stomatal opening under relatively high leaf turgor as a consequence of increased osmoprotective activity, and stomatal closure occurs when a threshold turgor due to water loss is established (Mahouachi et al. Citation2012).
GB application also significantly improved the actual efficiency of PSII (Fv/Fm value) (), which is the most frequently used parameter to indicate the damage to PSII due to stress factors including drought (He et al. Citation2011). The increased Fv/Fm values in GB-treated plants under photo-inhibitory conditions suggested that the PSII complex is better suited to endure photo-induced inactivation and that exogenous GB is able to alleviate photo-inhibition in the leaves of plants under drought stress combined with high irradiance (Ma et al. Citation2007).
Some species are able to accumulate GB, proline and other compatible solutes in response to environmental stress and this is considered to be one of the protective mechanisms allowing plants to tolerate unfavourable environmental conditions (Sakamoto & Murata Citation2002; Ashraf & Foolad Citation2007). Pepper, however, is not a GB-accumulating species and can naturally synthesize GB in very small amounts. However, consistent with findings in other crop plants (Mäkelä et al. Citation1996; Ma et al. Citation2007) the increased GB concentration in GB-pre-treated plants observed here () suggests that foliar-applied GB can be readily absorbed by leaves and transported to other organs, where it can contribute to improved stress tolerance by boosting the ability of plant cells to osmotically adjust, maintaining stabilization and integrity of cell membranes. Elevated levels of proline accumulation due to GB application in crops under drought stress as observed in the present study () have also been reported (Farooq et al. Citation2008; Farooq et al. Citation2010) and this may be attributed to the protective role of GB on some key enzymes that take an active role in the biosynthesis of soluble sugars and free proline (Liang et al. Citation2009).
GB is known to act as a compatible solute; thus, GB application should reduce Ψs and improve the water status of plants under stress. The results of this research demonstrated that when the pepper seedlings were subjected to water stress, Ψw became more negative and the foliar application of GB reversed the effects of water stress on leaf water status considerably. RWC was significantly higher in GB-treated plants (), while GB application increased leaf Ψw () moderately (less negative) and lowered Ψs (). These results are in agreement with previous studies in which exogenous application of GB has been reported to improve Ψw of maize plants under salt stress (Nawaz & Ashraf Citation2007) and RWC of tobacco seedlings under drought stress (Ma et al. Citation2007).
Lowering of Ψs is generally considered an adaptation of plants to maintain turgor in a water limited environment which results from accumulation of compatible solutes such as GB and proline in the tissues (Ludlow & Muchow Citation1990; Ashraf & Foolad Citation2007). There are conflicting reports on the effect of exogenous application of GB on the Ψs of plants under osmotic stress. Farooq et al. (Citation2008) found that foliar application of 100 mM GB helped rice plants to maintain Ψw at relatively less negative levels under water deficit conditions by increasing Ψs. The authors concluded that accumulation of compatible solutes such as GB and proline at higher levels in the tissues lowered Ψs which enabled plants to take up additional water from the soil, thereby offsetting the immediate effect of water shortages on the tissues. On the contrary, non-significant differences were observed in leaf Ψs of GB-treated and untreated plants of tomato (Mäkelä et al. Citation1998) and sunflower (Iqbal et al. Citation2008) plants grown under water limited conditions.
An inevitable aftermath of drought stress is the overproduction of various ROS in plant tissues and plants are endowed with an array of antioxidant enzymes such as enzyme superoxide dismutase (SOD), CAT and POX and some non-enzymatic molecules such as glutathione, ascorbic acid, α-tocopherol and carotenoids to cope with ROS (Mittler Citation2002; Ma et al. Citation2006). The antioxidant enzymes along with non-enzymatic antioxidant molecules protect cellular membranes and organelles from the deleterious effects of toxic concentrations of ROS and help retain their integrity and stability under drought stress conditions (Arora et al. Citation2002). Additionally, drought tolerance in various species has been associated with the capacity to adjust the activity of the antioxidant system (Farooq et al. Citation2009; Gill & Tuteja Citation2010). Our findings indicated that antioxidant enzyme activities of pepper seedlings were substantially elevated by GB application under water stress conditions (, ) and that lower H2O2 () and MDA () contents determined in these plants may be due to boosted enzymatic activity. Similar results were reported by different studies in which exogenous application of GB has been found to stimulate the activities of antioxidative enzymes in rice (Farooq et al. Citation2008), wheat (Ma et al. Citation2006), maize (Xin et al. Citation2011) and tobacco (Ma et al. Citation2007) seedlings under water deficit conditions. Moreover, it was also reported that transgenic wheat plants with betA, a choline dehydrogenase gene to biosynthesize more GB, exhibited significantly higher antioxidant enzyme activity compared with non-transgenic (wild type) plants under drought stress (He et al. Citation2011).
Conclusions
The present study showed that water stress severely hampered the growth and metabolic functions of pepper seedlings, and that pre-treatment with GB through foliar spray was effective in inducing water stress tolerance by mitigating the negative effects of water stress. Our results have also revealed that enhanced water stress tolerance due to GB application was found to be related to improved photosynthetic capacity and water relations as well as reduced lipid peroxidation-linked membrane deterioration due to boosted antioxidant enzymatic activity. Thus, our finding that foliar application of 5 mM GB could be used as an ameliorative agent for protecting pepper seedlings against the detrimental effects of water stress may have significant practical applications; however, more research is necessary to determine the effects of GB application on the yield potential of pepper plants under water-limited conditions.
References
- Abbas A, Ashraf M, Akram NA 2010. Alleviation of salt-induced adverse effects in eggplant (Solanum melongena L.) by glycinebetaine and sugar beet extracts. Scientia Horticulturae 125: 188–195. 10.1016/j.scienta.2010.04.008
- Anjum, SA, Farooq M, Wang LC, Xue LL, Wang SG, Wang L et al. 2011. Gas exchange and chlorophyll synthesis of maize cultivars are enhanced by exogenously-applied glycinebetaine under drought conditions. Plant Soil and Environment 57: 326–331.
- Ashraf M, Foolad MA 2007. Improving plant abiotic-stress resistance by exogenous application of osmoprotectants glycine betaine and proline. Environmental and Experimental Botany 59: 206–216. 10.1016/j.envexpbot.2005.12.006
- Arora A, Sairam RK, Srivastava GC 2002. Oxidative stress and antioxidative systems in plants. Current Science 82: 1227–1238.
- Bajji M, Kinet J, Lutts S 2002. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regulation 36: 61–70. 10.1023/A:1014732714549
- Bates LS, Waldren RP, Teare ID 1973. Rapid determination of free proline for water-stress studies. Plant and Soil 39: 205–207. 10.1007/BF00018060
- Bohnert HJ, Jensen RG 1996. Strategies for engineering water-stress tolerance in plants. Trends in Biotechnology 14: 89–97. 10.1016/0167-7799(96)80929-2
- Bohnert HJ, Nelson DE, Jensen RG 1995. Adaptations to environmental stresses. The Plant Cell 7: 1099–1111. 10.1105/tpc.7.7.1099
- Bradford MM 1976. A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of the protein-dye binding. Analytical Biochemistry 72: 248–254. 10.1016/0003-2697(76)90527-3
- Çakmak I, Horst WJ 1991. Effect of aluminum on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiologia Plantarum 83: 463–468. 10.1111/j.1399-3054.1991.tb00121.x
- Campos PS, Quartin V, Ramalho JC, Nunes MA 2003. Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. plants. Journal of Plant Physiology 160: 283–292. 10.1078/0176-1617-00833
- Chen TH, Murata N 2008. Glycinebetaine: an effective protectant against abiotic stress in plants. Trends in Plant Science 13: 499–505. 10.1016/j.tplants.2008.06.007
- Farooq M, Basra SMA, Wahid A, Cheema ZA, Cheema MA, Khaliq A 2008. Physiological role of exogenously applied glycinebetaine to improve drought tolerance in fine grain aromatic rice (Oryza sativa L.). Journal of Agronomy and Crop Science 194: 325–333. 10.1111/j.1439-037X.2008.00323.x
- Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA 2009. Plant drought stress: effects, mechanisms and management. Agronomy for Sustainable Development 29: 185–212. 10.1051/agro:2008021
- Farooq M, Wahid A, Lee DJ, Cheema SA, Aziz T 2010. Comparative time course action of the foliar applied glycinebetaine, salicylic acid, nitrous oxide, brassinosteroids and spermine in improving drought resistance of rice. Journal of Agronomy and Crop Science 196: 336–345. 10.1111/j.1439-037X.2010.00422.x
- Gill SS, Tuteja N 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry 48: 909–930. 10.1016/j.plaphy.2010.08.016
- Grieve CM, Grattan SR 1983. Rapid assay for the determination of water soluble quaternary ammonium compounds. Plant and Soil 70: 303–307. 10.1007/BF02374789
- Habib N, Ashraf M, Ali Q, Perveen, R 2012. Response of salt stressed okra (Abelmoschus esculentus Moench.) plants to foliar-applied glycinebetaine and glycinebetaine containing sugar beet extract. South African Journal of Botany 83: 151–158. 10.1016/j.sajb.2012.08.005
- He C, Zhang W, Gao Q, Yang A, Hu X, Zhang J 2011. Enhancement of drought resistance and biomass by increasing the amount of glycine betaine in wheat seedlings. Euphytica 177: 151–167. 10.1007/s10681-010-0263-3
- Herzog V, Fahimi H 1973. Determination of the activity of peroxidase. Analytical Biochemistry 55: 554–562. 10.1016/0003-2697(73)90144-9
- Hussain M, Malik MA, Farooq M, Khan MB, Akram M, Saleem MF 2009. Exogenous glycinebetaine and salicylic acid application improves water relations, allometry and quality of hybrid sunflower under water deficit conditions. Journal of Agronomy and Crop Science 195: 98–109. 10.1111/j.1439-037X.2008.00354.x
- Iqbal N, Ashraf M, Ashraf MY 2008. Glycinebetaine an osmolyte of interest to improve water stress tolerance in sunflower (Helianthus annuus L.): water relations and yield. South African Journal of Botany 74: 274–281. 10.1016/j.sajb.2007.11.016
- Korkmaz A, Şirikçi R 2011. Improving salinity tolerance of germinating seeds by exogenous application of glycinebetaine in pepper. Seed Science and Technology 39: 377–388.
- Liang C, Zhang XY, Luo Y, Wang GP, Zou Q, Wang W 2009. Overaccumulation of glycinebetaine alleviates the negative effects of salt stress in wheat. Russian Journal of Plant Physiology 56: 370–376. 10.1134/S1021443709030108
- Lichtenthaler HK 1987. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods in Enzymology 148: 350–382.
- Ludlow MM, Muchow RC 1990. A critical evaluation of traits for improving crop yield in water limited environments. Advances in Agronomy 43: 107–153.
- Lv S, Yang A, Zhang K, Wang L, Zhang L 2007. Increase of glycinebetaine synthesis improves drought tolerance in cotton. Molecular Breeding 20: 233–248. 10.1007/s11032-007-9086-x
- Ma QQ, Wei W, Li YH, Li DQ, Zou Q 2006. Alleviation of photoinhibition in drought-stressed wheat (Triticum aestivum) by foliar-applied glycinebetaine. Journal of Plant Physiology 163: 165–175. 10.1016/j.jplph.2005.04.023
- Ma XL, Wang YJ, Xie SL, Wang C, Wang W 2007. Glycinebetaine application ameliorates negative effects of drought stress in tobacco. Russian Journal of Plant Physiology 54: 472–479. 10.1134/S1021443707040061
- Mahajan S, Tuteja N 2005. Cold, salinity and drought stresses: an overview. Archives of Biochemistry and Biophysics 444: 139–158. 10.1016/j.abb.2005.10.018
- Mäkelä P, Peltonen-Sainio P, Jokinen K, Pehu E, Setällä H, Hinkkanen R et al. 1996. Uptake and translocation of foliar-applied glycinebetaine in crop plants. Plant Science 121: 221–230. 10.1016/S0168-9452(96)04527-X
- Mäkelä P, Jokinen K, Kontturi M, Peltonen-Sainio P, Pehu E, Somersalo S 1998. Foliar application of glycine betaine – a novel product from sugarbeet – as an approach to increase tomato yield. Industrial Crops and Products 7: 139–148. 10.1016/S0926-6690(97)00042-3
- Mahouachi J, Argamasilla R, Gomez-Cadenas A 2012. Influence of exogenous glycinebetaine and abscisic acid on papaya in responses to water-deficit stress. Journal of Plant Growth Regulation 31: 1–10. 10.1007/s00344-011-9214-z
- Mittler R 2002. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science 7: 405–410. 10.1016/S1360-1385(02)02312-9
- Nawaz K, Ashraf M 2007. Improvement in salt tolerance of maize by exogenous application of glycinebetaine: growth and water relations. Pakistan Journal of Botany 39: 1647–1653.
- Nemeth M, Janda T, Horvath E, Paldi E, Gabrella S 2002. Exogenous salicylic acid increases polyamine content but may decrease drought tolerance in maize. Plant Science 162: 569–574. 10.1016/S0168-9452(01)00593-3
- Özden M, Demirel U, Kahraman A 2009. Effects of proline on antioxidant system in leaves of grapevine (Vitis vinifera L.) exposed to oxidative stress by H2O2. Scientia Horticulturae 119: 163–168. 10.1016/j.scienta.2008.07.031
- Rhodes D, Hanson AD 1993. Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annual Review of Plant Physiology and Plant Molecular Biology 44: 357–384. 10.1146/annurev.pp.44.060193.002041
- Roy R, Agrawal V, Gupta SC 2009. Comparison of drought-induced polypeptides and ion leakage in three tomato cultivars. Biologia Plantarum 53: 685–690. 10.1007/s10535-009-0123-y
- Sakamoto A, Murata N 2000. Genetic engineering of glycinebetaine synthesis in plants: current status and implication for enhancement of stress tolerance. Journal of Experimental Botany 51: 81–88. 10.1093/jexbot/51.342.81
- Sakamoto A, Murata N 2002. The role of glycinebetaine in the protection of plants from stress: clues from transgenic plants. Plant, Cell and Environment 25: 163–171. 10.1046/j.0016-8025.2001.00790.x
- Seçkin B, Türkan I, Sekmen AH, Ozfidan C 2010. The role of antioxidant defense systems at differential salt tolerance of Hordeum marinum Huds. (sea barley grass) and Hordeum vulgare L. (cultivated barley). Environmental and Experimental Botany 69: 76–85. 10.1016/j.envexpbot.2010.02.013
- Senaratna T, Touchell D, Bunn E, Dixon K 2000. Acetyl salicylic acid (Aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. Plant Growth Regulation 30: 157–161. 10.1023/A:1006386800974
- Timasheff, S 1992. A physicochemical basis for the selection of osmolytes by nature. In: Somero GN, Osmond CB, Bolis CL eds. Water and life: comparative analysis of water relationships at the organisms, cellular and molecular levels. Berlin, Springer. Pp. 70–84.
- Xin ZL, Mei G, Shiqing L, Shengxiu L, Zongsuo L 2011. Modulation of plant growth, water status and antioxidantive system of two maize (Zea may L.) cultivars induced by exogenous glycinebetaine under long term mild drought stress. Pakistan Journal of Botany 43: 1587–1594.
- Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN 1982. Living with water stress: evolution of osmolyte systems. Science 217: 1212–1222. 10.1126/science.7112124
- Yang X, Lu C 2005. Photosynthesis is improved by exogenous glycinebetaine in salt-stressed maize plants. Physiologia Plantarum 124: 343–352. 10.1111/j.1399-3054.2005.00518.x
- Zhang JH, Huang WD, Liu YP, Pan QH 2005. Effects of temperature acclimation pretreatment on the ultrastructure of mesophyll cells in young grape plants (Vitis vinifera L. cv. Jingxiu) under cross-temperature stresses. Journal of Integrative Plant Biology 47: 959–970. 10.1111/j.1744-7909.2005.00109.x