Abstract
Garlic is an important bulb vegetable because of its medicinal and nutritional values. Selenium, an important trace element, and humic acid, a soil amendment, can positively affect garlic's nutritional values. To evaluate the impact of selenium and humic acid on antioxidant activity and phenol, flavonoid and allicin contents in garlic, Na2SeO4 solution was sprayed in concentrations of 0, 10, 20 and 30 μg Se/mL, and humic acid was used in fertigation at rates of 0, 10 and 20 kg/ha. Results showed the applied treatments had positive effects on the total antioxidant activity in garlic. The application of low concentrations of Se with moderate amounts of humic acid caused the highest antioxidant activity, as did the application of high Se concentrations with no humic acid. Humic acid at the rate of 10 kg/ha contributed more to the lower total phenol content than did the 20 kg/ha (H20) rate. The Se treatment decreased flavonoid content, and the control plants (Se0) had the highest amount of flavonoids. The results regarding sulphur and allicin contents were greatly similar in that they were both at their maximum level in the Se0H20 treatment. Se0 led to the highest allicin content, and its difference from other Se treatments was significant (P < 0.01). A strong negative correlation was found between selenium concentration and allicin content (R2 = 0.881). The findings showed that humic acid positively affects most tested traits, but negative or no effects were seen for the Se treatment. Therefore, if the production of Se-enriched garlic is the aim, a decrease in some nutritional values must be accepted.
Introduction
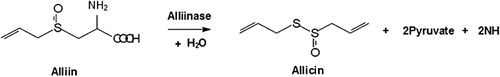
Garlic (Allium sativum L.), which is cultivated and consumed worldwide, is an important bulb vegetable because of its medicinal and nutritional values (Nosraty Citation2004). It has many medicinal effects: it lowers blood cholesterol levels and antiplatelet aggregation, produces anti-inflammatory activity and inhibits cholesterol synthesis. Moreover, it has long been known to have antibacterial, antifungal, anticancer, antioxidant and antiviral activities (Lawrence & Lawrence Citation2011). Allicin, the main biologically active component of freshly crushed garlic cloves, is produced by the degradation of alliin, which results from alliinase activity (Bocchini et al. Citation2001; Rahman et al. Citation2012).
According to Benkeblia (Citation2005), the Allium species contain powerful antioxidants, sulphur (S) and numerous phenolic compounds, which make them substantial and interesting for food industries. The natural antioxidants present in the edible species are very important in increasing health benefits and resistance to oxidative stress in humans (Dimitrios Citation2006).
Othman et al. (Citation2011) reported that garlic expresses higher free radical scavenging effects than onion. They also claimed that a poor relationship existed between total phenolic content and antioxidant activity, indicating that the antioxidant activity of garlic is not solely due to its phenolic compounds.
Selenium (Se) is a trace mineral that is an essential micronutrient for animals and humans (Young Citation1981). Selenium has been shown to reduce tumour growth in laboratory tests and may provide protection against specific cancers in humans (Ip Citation1998). Based on recommendations by the World Health Organization, an adult needs 50 μg Selenium per day. Selenium is closely related to sulphur (an element highly absorbable by garlic) and can substitute for it in different metabolic pathways (Morris Citation1970). Garlic is a selenium hyper-accumulator and absorbs this element effectively; thus, cultivating it in soil containing high selenium concentrations can produce selenium-enriched garlic. High-Se garlic has been studied extensively because of its impact on reducing tumour growth in laboratory animals (Ip Citation1998). Poldma et al. (Citation2011) reported that foliar Se fertilisation of garlic can be recommended to increase the number of large bulbs and bulb antioxidant capacity.
Many reports have shown that humic substances influence respiration, protein synthesis and enzyme activity in higher plants (Nardi et al. Citation2007; Carletti et al. Citation2008). Based on Aiken et al. (Citation1985), humic substances are defined as ‘a category of naturally occurring, biogenic, heterogeneous organic substances that can generally be characterised as being yellow to blank in colour, of high molecular weight, and refractory’. Humic substances have positive effects on nutrient uptake, especially that of major inorganic elements such as nitrogen, phosphorus, potassium and sulphur (Trevisan et al. Citation2010). Moreover, these substances are able to produce various morphological, physiological and biochemical effects on higher plants. The fraction of humic substances precipitated from the aqueous solution in pH levels below 2 is considered a humic acid (Liu et al. Citation1998). Increasing cell membrane permeability, oxygen uptake, respiration, photosynthesis, phosphate uptake and root cell elongation are possible mechanisms of humic acid and its positive impact on plant growth (Ameri & Tehranifar Citation2012).
The current study hypothesised that selenium as an antioxidant and humic acid used as a soil amendment can have positive impacts on the nutritional aspects of garlic. Therefore, this study investigated the effects of humic acid and selenium as two important treatments for increasing the quality and quantity of garlic in terms of its nutritional values.
Materials and methods
Plant growth conditions and treatment application
The experimental field was located in Tirtash, Mazandaran province, Iran, which has a Mediterranean climate, according to the de Martonne climate classification system. The experimental site was geographically located at 36°45′ latitude, 53°44′ longitude and +14 m altitude. The main physicochemical properties of the soil were: pH, 7.5; organic matter, 1.83%; electrical conductivity, 0.54 mS/m; saturation percentage, 49%; soil texture, silt-loam; total nitrogen (N), 0.11%; absorbable phosphorus (P), 24 ppm; and absorbable potassium (K), 210 ppm.
Garlic cloves (Allium sativum cv. Mazand) were planted in the second week of November with distances of 15 cm between plants in each row and 25 cm between rows. When the plants had six to seven leaves (3April 2013), Na2SeO4 solution was sprayed at concentrations of 0, 10, 20 and 30 μg Se/mL at a rate of 50 mL/m2 (treatments S0, S10, S20 and S30, respectively). Humic acid was used in fertigation at rates of 0, 10 and 20 kg/ha on 28 March 2013 (treatments H0, H10 and H20, respectively). All plants were harvested per plot at maturity on 21May 2013.
Determination of total antioxidant, phenol and flavonoids
DPPH radical-scavenging activity
The stable DPPH radical method was used to determine the free radical scavenging activity of the extracts, which could show the antioxidant activities of the samples. A known amount of sample was extracted from three plants using the maceration method. Absolute methanol was used as a solvent. The collected substance was concentrated until a crude solid extract was obtained. DPPH radical-scavenging activity was determined using a slightly modified version of the method described by Ebrahimzadeh et al. (Citation2010). A concentration of 800 μg extract/mL methanol was added to the DPPH (100 μM) methanol solution. After 15 min at room temperature, absorbance was recorded at 517 nm. Finally, the percentage of inhibition that showed the scavenging ability of the DPPH free radicals was calculated.
Determination of total phenol and flavonoid contents
Extracts were prepared using the maceration method and absolute methanol. The total phenolic compound contents were determined using the Folin-Ciocalteu method (Nabavi et al. Citation2008). The extract samples (0.5 mL) were mixed with 2.5 mL 0.2 N Folin-Ciocalteu reagents for 5 min, and then 2.0 mL 75 g–1sodium carbonate was added. The absorbance reaction was measured at 760 nm after incubation at room temperature. Results were expressed as gallic acid equivalents. Total flavonoids were estimated according to Nabavi et al. (Citation2008). Briefly, a 0.5 mL solution of each extract in methanol was separately mixed with 1.5 mL methanol, 0.1 mL 10% aluminium chloride, 0.1 mL 1M potassium acetate and 2.8 mL distilled water and left at room temperature for 30 min. The absorbance of the reaction mixture was measured at 415 nm with a double beam spectrophotometer (Perkin Elmer). Total flavonoid contents were calculated as quercetin equivalent by performing a calibration curve.
Determination of allicin content
The allicin content of the garlic extract was quantitatively determined using high-performance liquid chromatography (HPLC) as described in detail by Arzanlou & Bohlooli (Citation2010). To extract the juice, 2 g of cloves (collected from three plants) were crushed manually using a mortar and pestle, homogenised using a homogeniser (Heidolph Silentcrush M, Schwabach, Germany) and sonicated continuously for 5 min at 100% amplitude using an ultrasonicator (UP200H, Hielscher Ultrasonics, Teltow, Germany) with 60 mL distilled water in an ice container. The obtained mash was squeezed through five layers of cheesecloth, and the suspension was transferred into a 50 mL falcon tube and centrifuged (with an Eppendorf 5810R) at 1258 g for 20 min at 4 °C in order to separate the remaining debris from the liquid. The supernatant was transferred into a second sterile 50 mL falcon tube and sealed. The resultant extract was either used immediately or stored at –20 °C until analysis.
Quantitative analysis of allicin by analytical HPLC
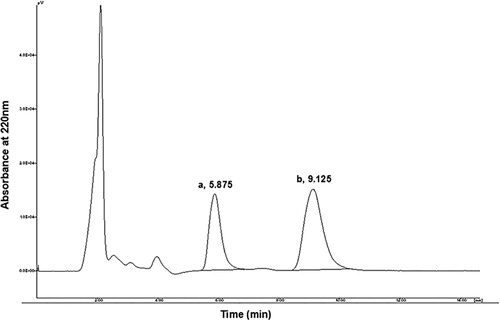
Briefly, 100 μL of internal standard (Ethylparaben, 150 μg/mL in mobile phase) solution (final concentration 15 µg/mL) was added to 10 μL of purified allicin, and the final volume was adjusted to 1 mL by mobile phase, vortexed and centrifuged at 15,294 g for 10 min. 20μL of supernatant was injected on to an HPLC system (Jasco liquid chromatography system, Tokyo, Japan) equipped with a C18, Nucleosil 100 ODS (5 µm) analytical column measuring 4.6 × 150 mm (Alltech Grom GmbH, Rottenburg, Germany). The mobile phase was methanol–water (50:50, v/v) with a flow rate of 1 mL/min. The allicin in the effluents was detected at 220 nm and quantified by comparing the peak area produced by authentic allicin.
Determining total sulphur
Total sulphur from clove material was measured in the digest as described by Quin & Wood (Citation1976). Garlic samples (0.1 g) were analysed for sulphur after magnesium nitrate and perchloric digestion. Barium chlorate was added to the mixture and it was left overnight, following which the absorbance of the final reaction mixture was measured at 420 nm. Results are expressed as percentage of total S in dry matter.
Statistical analysis
The experiment was arranged in factorial format based on a completely randomised block design with three replications. A statistical analysis was performed using analysis of variance in the SAS 9.1 software, and means were compared using Duncan's multiple range tests at a 5% level.
Results
Total antioxidant activity, phenol and flavonoid contents
Scavenging the stable DPPH radical is a method widely used to evaluate the free radical scavenging ability of various samples. The lowest values of antioxidant activity were recorded in samples of Se0H0 (control) and Se10H0, for which the amounts of both Se and humic acid were minimised (). Applying low Se concentrations accompanied by moderate amounts of humic acid (Se0H10 and Se10H10) caused the highest rate of antioxidant activity; correspondingly, high Se concentrations with no humic acid (Se20H0 and Se30H0) had the same effect. When selenium was used in low concentrations (0 and 10 μg/mL), applying humic acid at the rate of 10 kg/ha led to a higher rate of antioxidant activity. In high concentrations of Se (20 and 30 μg/mL), humic acid, even at the rate of 10 kg/ha, caused a decrease in antioxidant activity.
Table 1 Some nutritional values of garlic under selenium and humic acid treatments.
Total phenol compounds were reported as gallic acid equivalents by reference to standard curve (y = 0.0063x, r2 = 0.987), and flavonoid content by reference to standard curve (y = 0.0067x + 0.0132, r2 = 0.999). The effect of humic acid and Se interaction on phenol and flavonoid contents was not significant (P ≤ 0.05); however, significant differences were observed in the effects of humic acid on phenol and Se on flavonoid (, ). Humic acid at a rate of 20 kg/ha contributed to the highest total phenol content, which did not differ from that of the control, and at a rate of 10 kg/ha, humic acid resulted in the lowest total phenol content. Selenium decreased flavonoid contents. The highest amount of flavonoids was observed in the control plants (S0).
Table 2 Total phenol content of garlic under humic acid treatment.
Table 3 Flavonoids content of garlic under selenium treatment.
Total sulphur and allicin contents
As seen in the data given in , total sulphur content in the garlic bulbs ranged from 0.309% to 0.506% dry matter. The highest sulphur content was found in the Se0H20 treatment, which was significantly different from the other treatments (P < 0.05).
Allicin content was quantitatively determined using HPLC. Typical representative chromatograms of garlic extract are shown in . As can be seen in the chromatograms, there were no interfering peaks between allicin and the internal standard in other components of the extract. Retention times for allicin and the internal standard were approximately 5.9 and 9.1 min, respectively. Total HPLC run time for each sample was approximately 12 min. As shown in , the allicin content of the garlic bulbs ranged from 2.461 to 3.323 mg/mL in this experiment; it was at its highest level in the Se0H20 treatment.
Discussion
Total antioxidant activity
The results of the current study show the positive effects of applied treatments on the total antioxidant activity of garlic. Aminifard et al. (Citation2012) and Poldma et al. (Citation2011) reported the same results for humic acid and selenium, respectively. The Se0H10 and Se10H10 treatment caused the highest antioxidant activity. A further increase in the soil's humic acid concentration, followed by an H20 treatment, contributed to the lowest amount of antioxidant activity. According to different studies, high amounts of humic acid could have negative effects on plant growth (Atiyeh et al. Citation2002), root hydraulic conductivity (Asli & Neumann Citation2010) and some mineral nutrient concentrations such as calcium (Ca), copper (Cu) and nitrogen (N) (Liu et al. Citation1998). These negative effects could be the consequence of humic acid accumulation at the root cell wall (Asli & Neumann Citation2010), which causes disorder in metabolic pathways. Because of such negative impacts, especially on mineral nutrition which is closely linked with antioxidant capacity, the treatment of a high rate of humic acid resulted in decreased antioxidant activity.
High Se concentrations with no humic acid (Se20H0 and Se30H0) had the same effect (high antioxidant activity) since these treatments were not significantly different from Se0H10 and Se10H10. The antioxidant role of selenium may be decreased by the high uptake of mineral nutrients following an application of humic acid and the interference of these mineral elements in Se. Numerous studies have shown an increase in the absorption of mineral nutrients after an application of humic acid (Arslan & Pehlivan Citation2008; Asik et al. Citation2009).
Phenol and flavonoid contents
Humic acid at the rate of 10 kg/ha contributed to the lowest phenolic compounds. A moderate amount of humic acid could probably ameliorate stress impacts; because of this, treated plants need lower amounts of phenols, which are known to be important radical scavengers. Our findings are in agreement with those of Asik et al. (Citation2009), who demonstrated that humic substances have anti-stress effects under abiotic stressful conditions.
The highest amount of phenols was recorded in garlic treated with humic acid at the rate of 20 kg/ha. Humic acid can generate reactive oxygen species (ROS) which act as messengers and induce physiological effects (Cordeiro et al. Citation2011). According to Close & McArthur (Citation2002), who formulated the ‘oxidative pressure hypothesis’, the biosynthesis of phenolic compounds follows oxidative stress. Therefore, it can be concluded that the generation of ROS by humic acid led to an increase in total phenol content in this study. Total phenol content in the control plants was not significantly different from that in H20 treated plants. This result could be related to natural stress found in the experimental field.
As noted above, treating the plants with Se increased antioxidant activity, but decreased flavonoid contents. Consequently, increasing garlic antioxidant activity with a Se treatment was not related to flavonoids: other mechanisms such as Se antioxidant impact were responsible for the increased antioxidant activity. As a flavonoid is a secondary metabolite, its biosynthesis requires photosynthetic products and is limited by carbon supply and energy. Excessive plant growth consumes more photosynthetic products, thereby reducing the synthesis of secondary metabolites, including flavonoids (Treutter Citation2006). Accordingly, a possible reason for the decrease in flavonoids in Se treatments could be the decrease in photosynthetic products following the high growth of selenium-treated garlic. Increased bulb growth of selenium-treated garlic was demonstrated by Poldma et al. (Citation2011).
Total sulphur and allicin contents
As garlic produces a lot of organosulphur compounds, sulphur plays a crucial role in the growth of this bulbous vegetable, and garlic has high demands for this mineral element. The results of the current study clearly show that Se0 and H20 interactions cause high sulphur uptake. This finding is in accordance with Poldma et al. (Citation2011) and Liu et al. (Citation1998), who reported the negative effect of Se treatment on S content and the increase in S uptake after an application of humic acid. Poldma et al. (Citation2011) found a significant negative correlation between S and Se content in garlic affected by selenium treatments. They interpreted their results to mean that selenium replaced sulphur in plant metabolism and could settle in the S amino acids of cysteine and methionine structures. Liu et al. (Citation1998) also pointed out that the sulphur content increased significantly in response to a humic acid treatment.
Allicin (allyl 2-propenethiosulphinate) is responsible for the usual odour of fresh-cut garlic and is commonly used to measure garlic quality. The sulphur and allicin contents were greatly similar in that both of them reached maximum levels with the Se0H20 treatment. The role of sulphur in the biosynthesis of alliin can shed light on why this similarity was found. Allicin is produced in crushed garlic cloves through the rapid lysis of alliin by alliinase (). There are two sulphur atoms in the chemical structure of allicin; as a result, sulphur is a key to allicin biosynthesis. As explained above, humic acid at the rate of 20 kg/ha increased sulphur uptake, but this effect was seen alongside Se0. In other combined treatments, however, humic acid did not increase sulphur uptake. Most likely, Se is replaced by S in plant metabolism, and as a result it disrupts normal biochemical reactions (Mikkelsen et al. Citation1989).
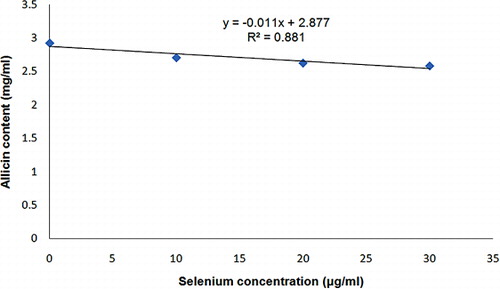
shows that the effect of Se treatments on allicin content could be beneficial. As shown in this figure, Se0 led to the highest allicin content, and its difference with other Se treatments was significant at a 1% probability level. A strong negative correlation was found between selenium concentration and allicin content (, r2 = 0.881). This negative correlation could prove the adverse effect of Se treatment on allicin content following Se replacement with S in the organosulphur compounds of garlic. shows the selenium concentration of garlic cloves in different Se treatments; the results indicate the possible harmful selenium concentrations for allicin biosynthesis.
Table 4 Selenium concentration of garlic cloves in different Se treatments.
Conclusion
The results obtained in the current study show that treating garlic plants with humic acid can positively affect most tested traits, while selenium has negative or no effects on them. Low selenium concentrations accompanied by a moderate amount of humic acid led to the highest total antioxidant activity. The negative correlation between selenium concentration and allicin content is one of the most important effects of selenium in garlic. Therefore, if producing Se-enriched garlic is the aim, a decrease in some nutritional values must be accepted.
Acknowledgements
The authors would like to express their appreciation to Mr Gholami for his kind support and assistance in conducting this experiment.
References
- Aiken GR, Mc Knight DM, Wershaw RL, Mc Carthy P 1985. Humic substances in soil, sediment and water. New York, A Wiley-Interscience Publication. 692 p.
- Ameri A, Tehranifar A 2012. Effect of humic acid on nutrient uptake and physiological characteristic Fragaria ananassa var: Camarosa. Journal of Biological and Environmental Sciences 6: 77–79.
- Aminifard MH, Aroiee H, Azizi M, Nemati H, Jaafar HZE 2012. Effect of humic acid on antioxidant activities and fruit quality of hot pepper (Capsicum annuum L.). Journal of Herbs, Spices & Medicinal Plants 18: 360–369.10.1080/10496475.2012.713905
- Arslan G, Pehlivan E 2008. Uptake of Cr3+ from aqueous solution by lignite-based humic acids. Bioresource Technology 99: 7597–7605.10.1016/j.biortech.2008.02.007
- Arzanlou M, Bohlooli S 2010. Introducing of green garlic plant as a new source of allicin. Food Chemistry 6: 12–15.
- Asik BB, Turan MA, Celik H, Katkat AV 2009. Effects of humic substances on plant growth and mineral nutrients uptake of wheat (Triticum durum cv. Salihli) under conditions of salinity. Asian Journal of Crop Science 1: 87–95.10.3923/ajcs.2009.87.95
- Asli S, Neumann PM 2010. Rhizosphere humic acid interacts with root cell walls to reduce hydraulic conductivity and plant development. Plant and Soil 336: 313–322.10.1007/s11104-010-0483-2
- Atiyeh RM, Lee S, Edwards CA, Arancon NQ, Metzger JD 2002. The influence of humic acids derived from earthworm processed organic wastes on plant growth. Bioresource Technology 84: 7–14.10.1016/S0960-8524(02)00017-2
- Benkeblia N 2005. Free-radical scavenging capacity and antioxidant properties of some selected onions (Allium cepa L.) and garlic (Allium sativum L.) extracts. Brazilian Archives of Biology and Technology 48: 753–759.10.1590/S1516-89132005000600011
- Bocchini P, Andalò C, Pozzi R, Galletti GC, Antonelli A 2001. Determination of diallyl thiosulfinate (allicin) in garlic (Allium sativum L.) by high-performance liquid chromatography with a post-column photochemical reactor. Analytica Chimica Acta 441: 37–43.10.1016/S0003-2670(01)01104-7
- Carletti P, Masi A, Spolaore B 2008. Protein expression changes in maize roots in response to humic substances. Journal of Chemical Ecology 34: 804–818.10.1007/s10886-008-9477-4
- Close DC, McArthur C 2002. Rethinking the role of many plant phenolics-protection from photodamage not herbivores. Oikos 99: 166–172.10.1034/j.1600-0706.2002.990117.x
- Cordeiro FC, Santa-Catarina C, Silveira V, Souza SR 2011. Humic acid effect on catalase activity and the generation of reactive oxygen species in corn (Zea mays). Bioscience, Biotechnology and Biochemistry 75: 70–74.10.1271/bbb.100553
- Dimitrios B 2006. Sources of natural phenolic antioxidants. Trends in Food Science and Technology 17: 505–512.10.1016/j.tifs.2006.04.004
- Ebrahimzadeh MA, Nabavi SF, Nabavi SM, Eslami B 2010. Antihemolytic and antioxidant activities of Allium paradoxum. Central European Journal of Biology 5: 338–345.10.2478/s11535-010-0013-5
- Ip C 1998. Lessons from basic research in selenium and cancer prevention. Journal of Nutrition 128: 1845–1854.
- Lawrence R, Lawrence K 2011. Antioxidant activity of garlic essential oil (Allium sativum) grown in north Indian plains. Asian Pacific Journal of Tropical Biomedicine 1: 51–54.10.1016/S2221-1691(11)60122-6
- Liu C, Cooper RJ, Bowman DC 1998. Humic acid application affects photosynthesis, root development and nutrient content of creeping bentgrass. Hortscience 33: 1023–1025.
- Mikkelsen RL, Page AL, Bingham FT 1989. Factors affecting selenium accumulation by agricultural crops. In: Jacobs LW ed. Selenium in agriculture and environment special publication. Madison, WI, Soil Science Society of America. Pp. 65–94.
- Morris VC 1970. Selenium content of foods. Journal of Nutrition 100: 1385–1386.
- Nabavi SM, Ebrahimzadeh MA, Nabavi SF, Hamidinia A, Bekhradnia AR 2008. Determination of antioxidant activity, phenol and flavonoids content of Parrotia persica Mey. Pharmacologyonline 2: 560–567.
- Nardi S, Muscolo A, Vaccaro S, Baiano S, Spaccini R, Piccolo A 2007. Relationship between molecular characteristics of soil humic fractions and glycolytic pathway and Krebs cycle in maize seedlings. Soil Biology and Biochemistry 39: 3138–3146.10.1016/j.soilbio.2007.07.006
- Nosraty AE 2004. Effect of planting method, plant density and seed clove size on yield of Hamedan garlic. Seed and Plant 20: 401–404.
- Othman SFC, Idid SZ, Koya MS, Rehan AM, Kamarudin KR 2011. Antioxidant study of garlic and red onion: a comparative study. Pertanika Journal of Tropical Agricultural Science 34: 253–261.
- Poldma P, Tonutare T, Viitak A, Luik A, Moor U 2011. Effect of selenium treatment on mineral nutrition, bulb size, and antioxidant properties of garlic (Allium sativum L.). Journal of Agriculture and Food Chemistry 59: 5498–5503.10.1021/jf200226p
- Quin BF, Wood PH 1976. Rapid manual determination of sulfur and phosphorous in plant material. Communication in Soil Science and Plant Analysis 7: 415–426.10.1080/00103627609366652
- Rahman MM, Fazlic V, Saad NW 2012. Antioxidant properties of raw garlic (Allium sativum) extract. International Food Research Journal 19: 589–591.
- Treutter D 2006. Significance of flavonoids in plant resistance a review. Environmental Chemistry Letters 4: 147–157.10.1007/s10311-006-0068-8
- Trevisan S, Francioso O, Quaggiotti S, Nardi S 2010. Humic substances biological activity at the plant-soil interface. Plant Signaling and Behavior 5: 635–643.10.4161/psb.5.6.11211
- Young VR 1981. Selenium: a case for its essentiality in man. New England Journal of Medicine 304: 1228–1230.10.1056/NEJM198105143042010