Abstract
Infection by Botrytis cinerea is a major cause of postharvest decay in strawberries. The efficacy of baby corn fermented vinegar (BFV) for growth inhibition of B. cinerea was investigated by diffusing 0%–0.225% acetic acid from BFV or glacial acetic acid into potato dextrose agar. BFV containing 0.225% acetic acid completely inhibited the growth of B. cinerea. Strawberry-flavoured baby corn fermented vinegar (SF-BFV) was developed to mask unfavourable smells caused by BFV. The effectiveness of BFV liquid and vapour for controlling postharvest decay in strawberries was evaluated along with associated sensory analyses. Taste panellists readily accepted fruit that had been sprayed with SF-BFV or exposed to BFV vapour, both treatments that significantly reduced fruit decay. Strawberries inoculated with B. cinerea were also tested. Shelf life at 4 °C of strawberries sprayed with SF-BFV was extended to 7 days while that of fruit exposed to BFV vapour was extended to 11 days.
Introduction
Strawberry is a very important commercial table fruit. It is grown extensively in many parts of the world, including Thailand. It is a physically fragile fruit (strictly a pseudocarp) with a short postharvest life. It is also very vulnerable to infection by the fungus Botrytis cinerea, which is one of the major causes of postharvest decay of strawberry. B. cinerea often infects strawberry flowers but then becomes dormant, as a potential source of inoculum. These dormant infections can reactivate later in the season, either just before or after harvest when sugar levels are high and weather conditions are favourable for disease development (Goetz et al. Citation1999; Ayala-Zavala et al. Citation2004). A variety of chemicals have been used to control postharvest fruit diseases, in order to help retain quality during transport and storage. Some chemicals used in the past have posed risks to consumer health, so considerable effort has been made to identify alternatives. Many natural compounds that offer promising levels of postharvest disease control have recently been identified (Xu et al. Citation2007; Tzortzakis Citation2010; Sellamuthu et al. Citation2013).
Fermented vinegar (FV) is a natural food, containing acetic acid from the fermentation of diluted alcohols from wine or other sources including cereals and a range of fruits. The detailed composition of an FV varies with both the alcohol source and the production method. The main organic component of FV, acetic acid, is well known for its safety and for its antimicrobial properties—it is a traditional kitchen food preservative. It can also be used as a sanitiser for reducing bacterial and fungal contamination, and thus postharvest decay, in fresh produce such as apples, tomatoes, carrots, stone fruit, lettuce and strawberries (Sholberg et al. Citation2000; Sengun & Karapinar Citation2004; Kilonzo-Nthenge et al. Citation2006; Chang & Fang Citation2007). As with liquid vinegar, vinegar vapour has also been shown to have antibacterial and antifungal properties in many food products such as eggs, tomatoes, apples, apricots, lettuce and strawberries (Sholberg et al. Citation2000; Tzortzakis Citation2010; Krusong et al. Citation2012). Therefore, FV can be seen as an effective, safe, traditional alternative to synthetic chemicals for controlling postharvest decay in strawberries.
Postharvest heat treatments have also been used for control and these have been applied using different media: hot water (Wszelaki & Mitcham Citation2003), hot acetic acid (Farahnaky & Afshari-Jouybari Citation2011), hot acetic-acid vapour (Radi et al. Citation2010) and hot air (Yahia et al. Citation2007; Shao et al. Citation2009). However, these can have negative effects on fruit quality such as: discoloration, shrivelling and loss of lustre (Wszelaki & Mitcham Citation2003), skin browning (Woolf & Laing Citation1996), heat injury, mass loss (Zhou et al. Citation2002) and accelerated fruit ripening (Jacobi et al. Citation2001). Therefore, use of room-temperature FV vapour has the potential to be a useful postharvest control strategy for strawberries that eliminates the risk of heat damage.
In this study, baby corn fermented vinegar (BFV), produced from baby corn blanching water—a by-product of the baby corn canning industry—was used as the postharvest treatment compound. The aims were: (1) to determine the efficacy of BFV as a growth inhibitor of B. cinerea; and (2) to determine the shelf life extension obtained in strawberries by spraying them with strawberry-flavoured baby corn fermented vinegar (SF-BFV) to help mask the disagreeable ‘vinegary’ smell associated with surface sprays of FV or exposure to BFV vapour. The strawberries tested had previously been inoculated with B. cinerea and were subsequently monitored for postharvest decay during storage at 4 °C.
Materials and methods
Sources of fruits
Fresh strawberry fruits, Fragaria × ananassa Duch. (cultivar Prarajchatan 60) were purchased from a local commercial orchard in Chiang Mai, Thailand, and were taken immediately (within about 7 h of harvest) to the laboratory at the Division of Fermentation Technology, Faculty of Agro-Industry, King Mongkut's Institute of Technology Ladkrabang (KMITL), Bangkok, Thailand, where they were packed in corrugated cartons and kept at 4 °C.
Baby corn fermented vinegar
BFV, from baby corn blanching water, was produced as described by Krusong et al. (Citation2007, Citation2010). The acetic acid content of BFV is 10 ± 0.1%.
Fungal culture and conidial suspensions
The B. cinerea isolate was produced from naturally decayed strawberries by the Department of Plant Protection, Faculty of Agricultural Product, Maejo University, Chiang Mai, Thailand. It was cultured on potato dextrose agar (PDA, Himedia, India) at 30 °C for 2 weeks before the conidia were harvested. The conidia were then washed in 5 mL of sterile distilled water and stored in screw-cap test tubes. The concentrations of the conidial suspensions were adjusted to (105 conidia/mL) using a haemocytometer as recommended by Sholberg et al. (Citation2000).
BFV effect on B. cinerea growth
The evaluation of BFV and glacial acetic acid (AA) containing various concentrations of acetic acid on the growth of B. cinerea was conducted using PDA. The PDA was acidified using BFV or AA to give a range of 10 acetic-acid concentrations between 0% and 0.225% (V/V) with increments of 0.025% (v/v). Discs of agar with mycelia grown for 48 h on PDA plates were harvested with a sterile cork borer (diameter 5 mm) and placed on acidified PDA plates (method modified from Lee et al. Citation2007). The diameters of the resulting colonies were measured at 3, 5 and 7 days after inoculation and incubation at 30 °C for BFV. To determine the effect of the fruit phytochemicals and trace components present in BFV on B. cinerea growth, inhibition of the pathogen at 7 days by BFV was compared with that of AA.
SF-BFV spray effects on spoilage of fresh strawberries and sensory evaluation
BFV sprays leave residual odours that are unacceptable to taste panellists (W. Krusong, unpubl. data). To improve sensory impressions and thus consumer acceptance, a strawberry-flavoured BFV was developed (SF-BFV). The SF-BFV was prepared by soaking 200 g fresh fruit for 15 days in 800 mL BFV containing 4% acetic acid. The flavour attribute and some phytochemicals were extracted from the strawberry into the BFV. Vinegars contain hundreds of trace components from the raw materials, fermentation process, brewing conditions and storage time, which contribute to the smell and taste of vinegar as reported by Zhang et al. (Citation2006). The resulting SF-BFV contained 2.98% acetic acid (pH 2.98). Based on studies reported in Krusong et al. (Citation2012), this mixture was evaluated as follows. Fresh strawberries were washed gently in tap water for 1 min, then rinsed three times in sterile distilled water for 1 min each and dried in laminar air flow for 5 min. Prior to determining the effectiveness of SF-BFV sprays, the residual microorganism loading of the fruit surfaces was monitored by a swab test.
A set of 20 fresh fruit was sprayed to run-off with 10 mL SF-BFV and dried in laminar air for 5 min. The spray treatment was repeated twice (modified from Yu et al. Citation2008). Another set of 20 fresh fruit was sprayed with sterile water as control. After SF-BFV treatment, the treated and control fruits were stored separately in a refrigerator at 4 °C and relative humidity (RH) 80% ± 2%. The fruits were divided at random into two groups to determine the proportions (%) of decayed fruit and for sensory evaluation. The samples of fruit stored at 4 °C were examined for spoilage after 0, 3, 5, 7, 9, 11 and 15 days. The experiment was of completely randomised design with three replications per treatment and 20 fruit per replicate. Each fruit was packed separately in a small plastic box for protection from neighbouring fruit that might have spoiled during the study. The sensory attributes of the treated fruit were analysed because, in addition to analytical chemistry, sensory analysis can be used as a tool to determine vinegar ingredients (Zhang et al. Citation2006).
BFV vapour effect on fruit spoilage and sensory evaluation
Washed, fresh fruit samples were prepared as above and placed in a plastic box (25 × 30 × 25 cm) with a vent valve to prevent pressure build-up during vapour treatment. BFV vapour was prepared by bubbling external air through BFV (acetic acid content 10%) contained in a 500 mL bottle at room temperature. The BFV vapour in the headspace of the bottle was delivered to the box (SF Fig. 1). The rate of vapour production was calculated from the rate of weight loss of the BFV as 0.042 ± 0.002 g BFV/min. Five durations of vapour treatment were used: 0, 5, 10, 15 and 20 min. After vapour treatment, fruit samples were stored at 4 °C and RH 80% ± 2% for 15 days. Samples were examined for evidence of fungal decay after 0, 3, 5, 7, 9, 11, 13 and 15 days and the proportions (%) of decayed fruits were recorded. The experiment was conducted as a completely randomised design with three replicates per treatment. Sensory evaluation was carried out after 2 days of storage.
SF-BFV and BFV vapour effects on fruit inoculated with B. cinerea
Washed, fresh fruits were prepared as described above. A sterile needle was used to make three wounds in each fruit. The wounds were each immediately inoculated with 10 µL of a conidial suspension (105 conidia/mL) of B. cinerea. One hour later, fruits were sprayed with SF-BFV containing 4% acetic acid or treated with BFV vapour for 20 min as described above. Samples were then stored at 4 °C and RH 80% ± 2%. Fruits inoculated with B. cinerea but with no other treatment (controls) were stored separately either at room temperature or in a refrigerator at 4 °C. The proportions of decayed fruit (%) were determined after 0, 3, 5, 7, 9, 11, 13 and 15 days of storage. Each treatment was applied to three replications of 20 fruit samples.
Sensory analysis
Taste panel candidates were pre-screened for their ability to discriminate basic tastes (Gunness et al. Citation2009) using the following solutions: quinine hydrochloride hydrate (0.025–0.1 g/L), tartaric acid (0.175–1.5 g/L), sucrose (1–10 g/L) and NaCl (0.25–1 g/L) which are compounds for bitterness, sourness, sweetness and saltiness, respectively (Ferrer-Gallego et al. Citation2014). The panellists (aged 21–45 years, 10 males and 20 females) included staff members and students of the Division of Food Science (KMITL, Thailand). They were trained in sensory evaluation with various concentrations of acetic acid (1%– 4%) in BFV and sensory attributes of strawberry (general appearance, colour, odour, texture and taste) at 10 training sessions (2 h per day) over a period of a month. The members of the sensory panel were familiar with the characteristics of BFV and strawberry and the rating of the sensory attributes. The panel size of 30 persons was modified from a method of sensory analysis of wine vinegars (Gerbi et al. Citation1997). The size of the panel was relatively large, to reduce the impact of the aggressive smell and taste of vinegar on the sensory impressions (hedonic responses) of individual members of the panel.
Two series of tests were carried out, with and without spraying with SF-BFV or exposure to BFV vapour. Half-fruit samples of 15 fruits and one-tenth samples of five fruit were prepared for the sensory evaluation. The panellists were given a half-fruit sample for evaluating general appearance, colour, odour and overall acceptance and a one-tenth sample for evaluating texture and taste. The cut samples, coded with three-digit numbers, were immediately distributed in a completely randomised order. Sensory evaluation was conducted in separate booths at room temperature. The sensory attributes assessed were: general appearance, colour, odour, texture, taste and overall acceptance (Gol et al. Citation2013). A five-point hedonic scale (1 = dislike very much, 2 = dislike, 3 = neutral, 4 = like, 5 = like very much) was used to score the samples, according to Kappel et al. (Citation1995) with some modifications.
Statistical analyses
The data obtained from inhibition of B. cinerea growth and fruit decay were analysed by one-way analysis of variance (ANOVA) and the sensory score was analysed separately for each attribute with two-way ANOVA, using storage time and treatment as fixed factors. When significant differences were observed among treatments, a means comparison test was performed using Tukey's test (P ≤ 0.05). SPSS Version 10.0 for Windows pocket program was used for these analyses.
Results
Inhibition of B. cinerea growth by BFV and acetic acid
Colony diameters of B. cinerea cultured on PDA acidified with BFV or AA of various AA concentrations are presented in . The acidity of BFV and AA solutions, containing 0% to 0.225% acetic acid, ranged from pH 5.50 to 3.93 and 5.50 to 3.28, respectively (). B. cinerea colony diameters on PDA acidified with BFV were determined after 3, 5 and 7 days of incubation. The results indicate that for any duration of incubation, colony diameter reduced as BFV acetic acid concentration increased. Also, colony diameter increased with incubation time. Therefore, higher concentrations of AA are required to fully inhibit B. cinerea growth. At the end of the experiment, a treatment with FV containing 0.225% acetic acid (pH 3.93) completely inhibited B. cinerea growth after incubation at 30 °C throughout the experimental period while at the same concentration of 0.225% AA, B. cinerea was still able to grow a little.
Table 1 Diameters of Botrytis cinerea colonies recorded at 30 °C following inoculation on PDA plates acidified with BFV (after 3, 5 and 7 days) and AA (after 7 days) containing acetic acid concentrations ranging between 0% to 0.225%.
SF-BFV spraying effects on fruit spoilage and sensory evaluation
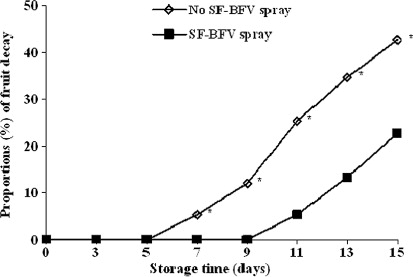
shows the positive effect of SF-BFV sprays on reducing fruit decay during storage at 4 °C. Severe decay of control fruit (i.e. without SF-BFV) was observed within 5 days of storage, while the shelf life of the SF-BFV sprayed fruits was extended beyond the controls by 4 days.
Significant differences between storage times were detected at a very low level of P-value (P < 0.0001) for all attributes (SF Table 1). Also, a significant effect of SF-BFV spray was found in odour, taste and overall acceptance. However, a two-way ANOVA model also revealed significant storage time–SF-BFV spray interactions for taste and overall acceptance. Results of sensory evaluation of fruit treated with and without SF-BFV during storage at 4 °C for 15 days are shown in . There were no significant differences (P ≤ 0.05) in colour and texture of fruit under either treatment. The trained panellists were able to detect slight differences in flavour and taste at the beginning of the study, but not after longer periods of storage. However, the overall acceptability of both samples decreased with increasing storage due to gradual deterioration of texture and all attributes.
Table 2 Sensory evaluations of strawberries treated with SF-BFV (controls without treatment) prior to storage at 4 °C for 15 days. Scores are based on a five-point hedonic scale.*
BFV vapour effect on spoilage and sensory evaluation
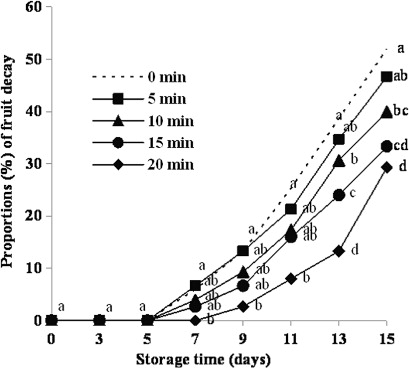
shows the efficacy of BFV vapour (air from the headspace above FV containing 10% acetic acid) after different exposure times. Exposure to BFV vapour for 20 min (the longest exposure period) was most effective in controlling fruit decay. It is clear that fruit decay decreased with increasing BFV treatment times.
According to the ANOVA results, all sensory attributes were affected by storage time, but they were not affected by BFV vapour treatment. The interaction between storage time and treatment was not significantly different (SF Table 2). Sensory analyses of fruit treated with only BFV and BFV vapour after storage at 4 °C for 15 days are shown in . There were no significant differences between control and BFV vapour within a time point.
Table 3 Sensory evaluations for strawberries treated with BFV vapour prior to storage at 4 °C for 15 days. Scores are on a five-point hedonic scale.*
SF-BFV spray and BFV vapour treatment effects on B. cinerea-inoculated fruit
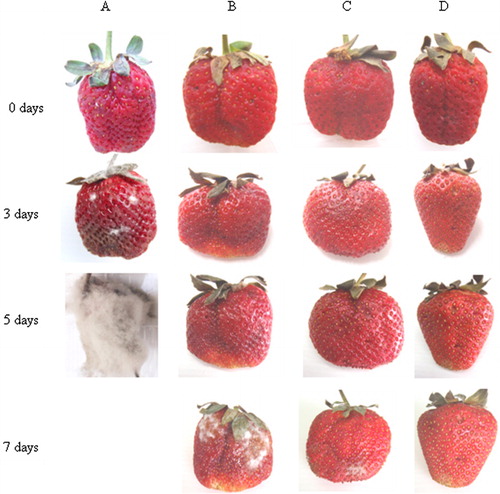
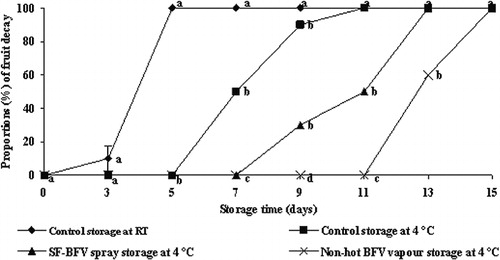
As shown in –, control fruits inoculated with B. cinerea but without remedial treatment showed rapid spoilage after only 3 days at room temperature and after 5 days at 4 °C. Shelf life was extended to 7 days at 4 °C when fruit were treated with SF-BFV spray and this was extended to 11 days when fruit were treated with BFV vapour. BFV vapour was clearly the more effective of the two treatments.
Discussion and conclusions
Strawberries have a very short shelf life due, among other factors, to the growth of grey mould (B. cinerea). It is interesting that AA treatment inhibited the growth of B. cinerea slightly less than BFV (P ≥ 0.05). This suggests that other compounds than AA in BFV also inhibit B. cinerea. However, the main factor responsible for the growth inhibition of B. cinerea was the concentration of acetic acid in the BFV (). Acetic acid is thus a valid candidate for controlling postharvest decay in fruit and, with its ready consumer acceptance, has the potential to replace synthetic chemicals for this purpose. Acetic acid has already been shown to have antimicrobial activity (Adams & Hall Citation1988). Acetic acid is a weak acid and so (by definition) it disassociates incompletely in water. Its antimicrobial properties are based on the percentage of undissociated molecules (this proportion varies with concentration and with pH). Undissociated molecules (less so, dissociated ones) can pass readily through conidial membranes, where they lower cell pH, inactivate enzymes and thus cause death of the pathogen (Casal et al. Citation1996).
Postharvest fruit decay can be significantly reduced with acetic acid. A number of alternative strategies for controlling disease in stored fruit using acetic acid have been developed. These include hot acetic acid dips (Radi et al. Citation2010), acetic acid vapour (Sholberg et al. Citation2004; Venditti et al. Citation2009) and acetic acid vapour derived from vinegar (Sholberg et al. Citation2000; Tzortzakis Citation2010; Krusong et al. Citation2012). In this study, liquid and vapour-phase acetic-acid treatments based on BFV were applied to strawberries to inhibit fruit decay during postharvest storage. SF-BFV sprays significantly delayed decay when fruit were stored at 4 °C (). The data in indicate that panellists noticed a significant effect of SF-BFV spray in odour and taste at the beginning of the experimental storage period (within 0–3 days), but these effects were lost at subsequent time points. Thus, considerable care is needed with the application of SF-BFV. Further optimisation of SF-BFV is also required to improve the intensity of the strawberry flavour to prevent detectable residual odour, which is of significant importance to consumer acceptability. This might also provide a stronger argument for the use of vapour over spray, since these effects were not detected with vapour. The reduced decay with SF-BFV sprays is attributed mainly to the acetic acid content of the BFV. However, some phytochemicals in the fruit and trace components of BFV slightly enhance the inhibitory effect on the growth B. cinerea, which confirms that fruits and vegetables provide a number of constitutive and inducible compounds as well as volatile compounds that have antimicrobial properties (Tripathi & Dubey Citation2004). In the studies of Jetti et al. (Citation2007) and Vandendriessche et al. (Citation2013), aldehydes and alcohols such as hexanol, 2-hexanol and 3-hexanol are important for the green, unripe notes in strawberry aroma. Their concentrations depend on both cultivar and degree of ripeness. Volatile compounds released from raspberry and strawberry fruit during ripening, 1-hexanol, E-2-hexanol and 2-non-anone have been reported to have antifungal activities including B. cinerea (Vaughn et al. Citation1993). Control of B. cinerea and Rhizopus stolonifer, commonly found in strawberry, was achieved by treating the fruit with acetaldehyde (Avissar & Pesis Citation1991). Moreover, hexanal vapour was reported to inhibit growth of Penicillium expansum and B. cinerea in vitro and on apple slices (Song et al. Citation1996).
Many reports provide evidence for the advantages of using acetic acid solutions as antimicrobial agents. These solutions are also useful as anti-browning agents both for fruit (Son et al. Citation2001) and for vegetables (Castañer et al. Citation1997). It has been suggested that in the latter case the mechanism involved is the lowering of cell pH to values below those necessary for polyphenol oxidase activity—this is well known to result in the accumulation of brown pigments in fruit and vegetables (McEvily et al. Citation1992). The 20 min BFV vapour treatment provided the greatest reduction in the level of decay (). The study shows that BFV vapour treatment reduces decay more effectively than the SF-BFV spray (). This suggests that undissociated acetic-acid molecules predominate in the BFV vapour more than in the solution (Casal et al. Citation1996). The advantage of using BFV is the antimicrobial activity of its vapour phase. A further advantage of the vapour-phase treatment is its rapid diffusion (gases are much more rapidly diffusive than liquids) which allows deep penetration of undissociated acetic acid molecules into spaces inaccessible to the liquid phase.
Our study shows that both SF-BFV spray and BFV vapour treatments delay decay in strawberries. However, BFV vapour treatment extends their shelf life more effectively than SF-BFV sprays. A better understanding of the mechanisms involved should enable further refinement and fine-tuning of BFV vapour treatments for application in a range of sectors of the agro-industry.
Supplementary files
Supplementary file 1: Figure 1. Procedure for exposing strawberries to BFV vapour. Abbreviations: AA, acetic acid; BFV, baby corn fermented vinegar.
Supplementary file 2: Table 1. F and P-value for all sensory attributes of strawberry in the two-way ANOVA corresponding to storage time and SF-BFV spray vs. control.
Supplementary file 3: Table 2. F and P-value for all sensory attributes of strawberry in the two-way ANOVA corresponding to storage time and BFV vapour exposure vs. control.
Krusong_SF3.doc
Download MS Word (47 KB)Krusong_SF2.doc
Download MS Word (46.5 KB)Krusong_SF1.tif
Download TIFF Image (436.1 KB)Acknowledgements
This research was supported financially by the Faculty of Agro-Industry, King Mongkut's Institute of Technology Ladkrabang, Thailand. The researchers are grateful to Ms Pattarapan Jaroonrattanasakul for laboratory assistance.
References
- Adams MR, Hall CJ 1988. Growth inhibition of food-borne pathogens by lactic and acetic acids and their mixtures. International Journal of Food Science & Technology 23: 287–292. 10.1111/j.1365-2621.1988.tb00581.x
- Avissar I, Pesis E 1991. The control of postharvest decay in table grapes using acetaldehyde vapors. Annals of Applied Biology 118: 229–237. 10.1111/j.1744-7348.1991.tb06101.x
- Ayala-Zavala JF, Wang SY, Wang CY, Gonzalez-Aguilarc GA 2004. Effect of storage temperatures on antioxidant capacity and aroma compounds in strawberry fruit. LWT-Food Science and Technology 37: 687–695. 10.1016/j.lwt.2004.03.002
- Casal M, Cardoso H, Leão C 1996. Mechanisms regulating the transport of acetic acid in Saccharomyces cerevisiae. Microbiology 142: 1385–1390. 10.1099/13500872-142-6-1385
- Castañer M, Gil MI, Artés F 1997. Organic acids as browning inhibitors on harvested “Baby” lettuce and endive. Zeitschrift für Lebensmitteluntersuchung und -Forschung A 205: 375–379.
- Chang JM, Fang TJ 2007. Survival of Escherichia coli O157:H7 and Salmonella enterica serovars Typhimurium in iceberg lettuce and the antimicrobial effect of rice vinegar against E. coli O157:H7. International Journal of Food Microbiology 24: 745–751. 10.1016/j.fm.2007.03.005
- Farahnaky A, Afshari-Jouybari H 2011. Physicochemical changes in Mazafati date fruits incubated in hot acetic acid for accelerated ripening to prevent diseases and decay. Scientia Horticulturae 127: 313–317. 10.1016/j.scienta.2010.10.019
- Ferrer-Gallego R, Hernández-Hierro JM, Rivas-Gonzalo JC, Escribano-Bailón MT 2014. Sensory evaluation of bitterness and astringency sub-qualities of wine phenolic compounds: synergistic effect and modulation by aromas. Food Research International 62: 1100–1107. 10.1016/j.foodres.2014.05.049
- Gerbi V, Zeppa G, Antonelli A, Carnacini A 1997. Sensory characterization of wine vinegars. Food Quality and Preference 8: 27–34. 10.1016/S0950-3293(96)00003-1
- Goetz G, Fkyerat A, Métais N, Kunz M, Tabacchi R, Pezet R et al. 1999. Resistance factors to grey mould in grape berries: identification of some phenolics inhibitors of Botrytis cinerea stilbene oxidase. Phytochemistry 52: 759–767. 10.1016/S0031-9422(99)00351-9
- Gol NB, Patel PR, Rao TVR 2013. Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biology and Technology 85: 185–195. 10.1016/j.postharvbio.2013.06.008
- Gunness P, Kravchuk O, Nottingham SM, D’Arcy BR, Gidley MJ 2009. Sensory analysis of individual strawberry fruit and comparison with instrumental analysis. Postharvest Biology and Technology 52: 164–172. 10.1016/j.postharvbio.2008.11.006
- Jacobi KK, MacRae EA, Hetherington SE 2001. Loss of heat tolerance in ‘Kensington’ mango fruit following heat treatments. Postharvest Biology and Technology 21: 321–330. 10.1016/S0925-5214(00)00180-0
- Jetti RR, Yang E, Kurnianta A, Finn C, Qian MC 2007. Quantification of selected aroma-active compounds in strawberries by headspace solid-phase microextraction gas chromatography and correlation with sensory descriptive analysis. Journal of Food Science 72: 5487–5496. 10.1111/j.1750-3841.2007.00445.x
- Kappel F, Fisher-Fleming R, Hogue EJ 1995. Ideal pear sensory attributes and fruit characteristics. Scientia Horticulturae 30: 988–993.
- Kilonzo-Nthenge A, Chen F, Godwin SL 2006. Efficacy of home washing methods in controlling surface microbial contamination on fresh produce. Journal of Food Protection 69: 330–334.
- Krusong W, Dansai P, Itharat A 2012. Combination impact of turmeric extract and fermented vinegar on reduction of inoculated Salmonella Typhimurium on fresh lettuce. KMITL Science and Technology 12: 77–84.
- Krusong W, Petch-nom P, Pinviset P 2010. Semi-continuous production process of corn vinegar in stirred tank reactor using fixation of Acetobacter aceti WK on surface of loffa sponge. Kasetsart Journal (Natural Science) 44: 201–207.
- Krusong W, Vichitraka A, Pornpakdeewattana S 2007. Luffa sponge as supporting material of Acetobacter aceti WK for corn vinegar production in semi-continuous process. KMITL Science Journal 7: 63–68.
- Lee WH, Han SK, Kim BK, Shrestha B, Lee SY, Ko CS et al. 2007. Proliferation of Tricholoma matsutake mycelial mats in pine forest using mass liquid inoculum. Mycobiology 35: 54–61. 10.4489/MYCO.2007.35.2.054
- McEvily AJ, Iyengar R, Otwell WS 1992. Inhibition of enzymatic browning in foods and beverages. Critical Reviews in Food Science and Nutrition 32: 253–273.
- Radi M, Jouybari HA, Mesbahi G, Farahnaky A, Amiri S 2010. Effect of hot acetic acid solutions on postharvest decay caused by Penicillium expansum on Red Delicious apples. Scientia Horticulturae 126: 421–425. 10.1016/j.scienta.2010.06.023
- Sellamuthu PS, Mafune M, Sivakumar D, Soundy P 2013. Thyme oil vapour and modified atmosphere packing reduce anthracnose incidence and maintain fruit quality in avocado. Journal of the Science of Food and Agriculture 93: 3024–3031. 10.1002/jsfa.6135
- Sengun IY, Karapinar M 2004. Effectiveness of lemon juice, vinegar and their mixture in elimination of Salmonella typhimurium on carrots. International Journal of Food Microbiology 96: 301–305. 10.1016/j.ijfoodmicro.2004.04.010
- Shao X, Tu K, Tu S, Zhao Y 2009. Increased residual activity controls disease in ‘Red Fuji’ apples (Malus domestica) following postharvest heat treatment. New Zealand Journal of Crop and Horticultural Science 37: 375–381. 10.1080/01140671.2009.9687593
- Sholberg P, Haag P, Hocking R, Bedford K 2000. The use of vinegar vapor to reduce postharvest decay of harvested fruit. HortScience 35: 898–903.
- Sholberg PL, Shephard T, Randall P, Moyls L 2004. Use of measured concentrations of acetic acid vapour to control postharvest decay in d’Anjou pears. Postharvest Biology and Technology 32: 89–98. 10.1016/j.postharvbio.2003.09.014
- Son SM, Moon KD, Lee CY 2001. Inhibitory effects of various anti-browning agents on apple slices. Food Chemistry 73: 23–30. 10.1016/S0308-8146(00)00274-0
- Song J, Leepipattanawit R, Deng W, Beaudry RM 1996. Hexanol vapor is a natural, metabolizable fungicide: inhibition of fungal activity and enhancement of aroma biosynthesis in apple slices. Journal of the American Society for Horticultural Science 121: 937–942.
- Tripathi P, Dubey NK 2004. Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biology and Technology 32: 235–245. 10.1016/j.postharvbio.2003.11.005
- Tzortzakis NG 2010. Ethanol, vinegar and Origanum vulgare oil vapour suppress the development of anthracnose rot in tomato fruit. International Journal of Food Microbiology 142: 14–18. 10.1016/j.ijfoodmicro.2010.05.005
- Vandendriessche T, Vermeir S, Martinez CM, Hendrickx Y, Lammertyn J, Nicolai BM et al. 2013. Effect of ripening and inter-cultivar differences on strawberry quality. LWT-Food Science and Technology 52: 62–70. 10.1016/j.lwt.2011.12.037
- Vaughn SF, Spencer GF, Shasha BS 1993. Volatile compounds from raspberry and strawberry fruit inhibit postharvest decay fungi. Journal of Food Science 58: 793–796. 10.1111/j.1365-2621.1993.tb09360.x
- Venditti T, Dore A, Molinu MG, Agabbio M, D’hallewin G 2009. Combined effect of curing followed by acetic acid vapor treatments improves postharvest control of Penicillium digitatum on mandarins. Postharvest Biology and Technology 54: 111–114. 10.1016/j.postharvbio.2009.06.002
- Woolf AB, Laing WA 1996. Avocado fruit skin fluorescence following hot water treatments and pretreatments. Journal of American Society for Horticultural Science 121: 147–151.
- Wszelaki AL, Mitcham EJ 2003. Effect of combinations of hot water dips, biological control and controlled atmospheres for control of gray mold on harvested strawberries. Postharvest Biology and Technology 27: 255–264. 10.1016/S0925-5214(02)00095-9
- Xu W-T, Huang K-L, Guo F, Qu W, Yang J-J, Liang Z-H et al. 2007. Postharvest grapefruit seed extract and chitosan treatments of table grapes to control Botrytis cinerea. Postharvest Biology and Technology 46: 86–94. 10.1016/j.postharvbio.2007.03.019
- Yahia EM, Soto-Zamora G, Brecht JK, Gardea A 2007. Postharvest hot air treatment effects on the antioxidant system in stored mature-green tomatoes. Postharvest Biology and Technology 44: 107–115. 10.1016/j.postharvbio.2006.11.017
- Yu T, Zhang H, Li X, Zheng X 2008. Biocontrol of Botrytis cinerea in apple fruit by Cryptococcus laurentii and indole-3-acetic acid. Biological Control 46: 171–177. 10.1016/j.biocontrol.2008.04.008
- Zhang Q, Zhang S, Xie C, Zeng D, Fan C, Li D et al. 2006. Characterization of Chinese vinegars by electronic nose. Sensors and Actuators 119: 538–546. 10.1016/j.snb.2006.01.007
- Zhou T, Xu S, Sun D-W, Wang Z 2002. Effects of heat treatment on postharvest quality of peaches. Journal of Food Engineering 54: 17–22. 10.1016/S0260-8774(01)00179-0