ABSTRACT
Small, dark brown leaf spots consistent with symptoms caused by Phoma sp. were observed on kiwifruit (Actinidia deliciosa cv. Hayward) at an orchard in Sacheon, South Korea. The causal agent was isolated from the spots and identified by morphological and molecular characteristics by sequencing the internal transcribed spacer region. To establish Koch’s postulates, the pathogen was inoculated on to leaves in a pathogenicity test and reisolated from the developed lesions. Phoma sp. has been reported as a causal agent of kiwifruit leaf spot disease in New Zealand and Italy. However, this is the first report of leaf spot disease caused by Phoma sp. in Korea.
Introduction
Kiwifruit (Actinidia spp.) is an economically promising crop in Korea. Kiwifruit has been cultivated in Korea since the early 1980s (Koh et al. Citation2003). Green-fleshed Actinidia deliciosa cv. Hayward is the most common cultivar (Beutel Citation1997). Kiwifruit is native to the Yangtze Valley and coastal region of Zhejiang Province of China. Seeds were subsequently carried to other regions of the world, most notably New Zealand where the fruit received its characteristic name (Ferguson Citation1991). Kiwifruit is now widespread and a number of leaf spot pathogens have been reported, including Phomopsis sp., Colletotrichum acutatum, Glomerella cingulata, Pestalotiopsis sp. and Alternaria alternata (Jeong et al. Citation2008). Although leaf spot diseases might not kill kiwifruit vines, they may cause defoliation and reduce yield (Schneider et al. Citation1975; Campbell & Duthie Citation1990). Phoma sp. has been associated with postharvest disease such as fruit rot as well as leaf spot disease of kiwifruit (Hawthorne & Otto Citation1986). Occurrence of leaf spot disease caused by Phoma sp. has been reported in New Zealand (Hawthorne & Otto Citation1986). However, leaf spot resulting from Phoma sp. has not yet been reported in South Korea.
Materials and methods
Fungal isolations were made from symptomatic kiwifruit (Actinidia deliciosa cv. Hayward) leaves. The leaves were immersed in 70% ethanol for 1 min then 1% sodium hypochlorite solution for 1 min then the lesions were rinsed twice in sterile water. The rinsed lesions were transferred to water agar (WA) and incubated at 27 °C for 3 days. Colonies on WA were transferred to potato dextrose agar (PDA) and incubated at 27 °C for 7 days. A single-spore isolate was used for DNA extraction. The DNA extraction protocol was described by Graham et al. (Citation2003). The internal transcribed spacer (ITS) region was amplified by ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′), ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) primers (White et al. 1990). Each 20 µL reaction contained: 2 µL of 10 × reaction buffer, 100 ng of genomic DNA, 1 µL of dNTP and 1 µL of 10pmol ITS1, ITS4 primers were used amplified of ITS region by polymerase chain reaction (PCR). PCR conditions were 94 °C for 5 min for pre-denaturation, repeat 30 cycles at 95 °C for 30 s, 50 °C for 30 s, 72 °C for 30 s and final extension at 72 °C for 10 min. The amplicons were sequenced in Solgent (Daegeon). The sequence was deposited at Genbank (NCBI Accession No. KT239669). The isolate was also deposited in the Korea Agricultural Culture Collection (Accession No. KACC47997). Pathogenicity tests were conducted with 7-week-old wounded and non-wounded detached leaves, inoculated with 6 mm diameter mycelial plugs of Phoma sp. cultivated on PDA (Cui et al. Citation2015). Control leaves were mock inoculated with sterile PDA plugs. Each leaf was inoculated at five different sites. The pathogenicity tests were performed three times. Inoculated leaves were incubated at 25 °C in a 90% relative humidity chamber for 7 days with a 12 h photoperiod. In addition, leaves were also inoculated by injecting a conidial suspension containing 2 × 106 cfu/mL. The incubating conditions were the same as described above. The fungus was reisolated from lesions of inoculated leaves to confirm Koch’s postulates.
Results and discussion
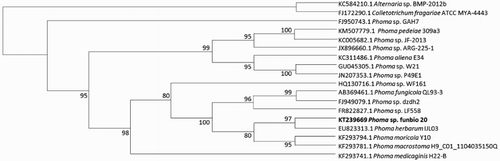
In May 2015, dozens of kiwifruit (Actinidia deliciosa cv. Hayward) were observed suffering from leaf spot similar to those caused by Phoma sp. with approximately 70% incidence in an orchard in Sacheon (34°56′59.6″N, 128°03′26.6″E), South Korea. The disease showed small, dark brown spots on leaves. The leaf lesions were circular to irregular (A). The lesion size varied (1–20 mm) and average lesion diameter was 8 mm. As the symptoms progressed, lesion size increased, and lesion centre faded to light brown and plants defoliated. The disease was more severe following heavy rains accompanied by winds and high humidity. The isolated fungi exhibited a dense and white villous mycelium, which changed to greyish and brownish from the centre of the colony after 2 weeks on PDA (B). Pycnidia were observed after 2 weeks of cultivation; they were round, fruiting structures which measured 185–240 µm to 145–235 µm (data not shown). Conidia were hyaline, ellipsoidal, round and 3–7 µm in diameter (D). Morphological characteristics of this isolate were identified as Phoma sp. (Aveskamp et al. Citation2009). Molecular identification by sequencing of ITS region showed 99% similarity and 99% coverage with Phoma sp. (Genbank Accession Nos. KC005682.1, GU045305.1 and JN207353.1). A total of seven isolates were characterised; their morphology and their molecular characteristics were identical to each other. According to a phylogenetic tree, this isolate was closely grouped with many Phoma spp. (). All injected conidial suspension and inoculated mycelial plugs with wound regions were observes disease symptoms. However, no disease symptoms were visible in the absence of a wound (C). Therefore, in leaf spot disease caused by Phoma sp. it is assumed that the pathogen infects the leaf through a wound. Therefore, it is necessary to manage the crop during the heavy rain and typhoon season to prevent leaf spot disease.
Figure 1. Disease symptoms and morphological characteristics of Phoma sp. in kiwifruit. A, Typical leaf spot symptoms on kiwifruit leaves under field conditions; B, pathogen colony morphology on PDA media; C, leaf spot symptoms artificially induced by inoculation Phoma sp. with and without wound; D, conidia of isolated Phoma sp. Bar indicates 10 µm.

Figure 2. Phylogenetic identification of Phoma sp. (funbio 20) based on ITS sequence. The values on the phylogenetic tree are bootstrap values, which is the percentage calculated from 1000 replicates in a bootstrap analysis. The phylogenetic tree was constructed by MEGA 4 and calculated by the neighbour-joining method. Colletotrichum fragariae and Alternaria sp. were assembled in a different group from Phoma sp.
Leaf spot on kiwifruit caused by Phoma sp. has been reported in northern Italy and New Zealand (Hawthorne & Otto Citation1986). However, to our knowledge, this study is the first report of leaf spot on the most common kiwifruit cultivar, Hayward, caused by Phoma sp. in Korea. This disease could be a potential threat to the Korean kiwifruit industry.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aveskamp MM, Verkley GJM, Gruyter J, Murace MA, Perello A, Woundenberg JHC, Groenewald JZ, Crous PW. 2009. DNA phylogeny reveals polyphyly of Phoma section Peyronellaea and multiple taxonomic novelties. Mycologia. 101:363–382. doi: 10.3852/08-199
- Beutel JA. 1997. Kiwifruit. In: Janick J, Simon JE editors. Advances in new crops. Portland, OR: Timber Press; p. 309–316.
- Campbell CL, Duthie JA. 1990. Impact of leaf spot diseases on yield and quality of alfalfa in North Carolina. Am Pat Soc Plant Disease. 74:241–245. doi: 10.1094/PD-74-0241
- Cui Y, Gong G, Yu X, Xu J, Wen X, Zhang M, Chen H, Zheng X, Zhou Y, Chang X. 2015. First report of brown leaf spot on kiwifruit caused by Corynespora cassiicola in Sichuan, China. Plant Dis. 99:725. doi: 10.1094/PDIS-08-14-0808-PDN
- Ferguson AR. 1991. Actinidia arguta—the hardy kiwifruit. New Zeal Kiwifruit. 81:23–24.
- Graham J, Marshall B, Squire G. 2003. Genetic differentiation over a spatial environmental gradient in wild Rubus idaeus populations. New Phytol. 157:667–675. doi: 10.1046/j.1469-8137.2003.00693.x
- Hawthorne BT, Otto C. 1986. Pathogenicity of fungi associated with leaf spots of kiwifruit. New Zeal J Agri Res. 29:533–538. doi: 10.1080/00288233.1986.10423506
- Hawthorne BT, Rees-George J, Samuels GJ. 1982. Fungi associated with leaf spots and postharvest fruit rots of kiwifruit (Actinidia chinensis) in New Zealand. New Zeal J Bot. 20:143–150. doi: 10.1080/0028825X.1982.10428835
- Jeong IH, Lim MT, Kim GH, Han TW, Kim HC, Kim MJ, Park HS, Shin SH, Hur JS, Shin JS, Koh YJ. 2008. Incidences of leaf spots and blights on kiwifruit in Korea. Plant Pathol J. 24:125–130. doi: 10.5423/PPJ.2008.24.2.125
- Koh YJ, Jung JS, Hur JS. 2003. Current status of occurrence of major diseases on kiwifruits and their control in Korea. Acta Hort. 610:437–443. doi: 10.17660/ActaHortic.2003.610.58
- Schneider RW, Williams RJ, Sinclair JB. 1975. Cercospora leaf spot of cowpea: Models for estimating yield loss. Phytopathology. 66:384–388. doi: 10.1094/Phyto-66-384
- West JS, Kharbanda PD, Barbetti MJ, Fitt BDL. 2001. Epidemiology and management of Leptosphaeria maculans (Phoma stem canker) on oilseed rape in Australia, Canada and Europe. Plant Pathol. 50:10–27. doi: 10.1046/j.1365-3059.2001.00546.x
