ABSTRACT
Application of an edible coating is a technique that can be used to increase fruit storability. Tomato fruit were coated with 0%, 5%, 10%, 15% and 20% Aloe vera gel and fruit quality maintenance was examined up to 14 days at 11 °C and 90% relative humidity. Results showed that 10% and 15% A. vera coating reduced fruit ethylene production. The ripening index (total soluble solids/titratable acidity) decreased after 7 days of storage in 10% Aloe-coated fruits, maintaining the overall quality of the tomato fruit. Lycopene and β-carotene content were reduced with 20% A. vera in both examined storage periods. Ascorbic acid content was increased in 10% Aloe-coated fruits. Total phenolics and antioxidative status were increased in 20% coated fruits after 14 days of storage. Fruit firmness, titratable acidity, weight loss, respiration rate and fruit colour (L*, a*, b*) did not differ among treatments. Thus, an edible coating of 10% A. vera could be considered as a promising treatment to maintain tomato quality during postharvest storage.
Introduction
Tomato (Solanum lycopersicum Mill.) postharvest life as a climacteric fruit is relatively short since many processes cause loss of quality and storability, including high respiration rates, transpiration, postharvest diseases and acceleration in ripening process and senescence (Zapata et al. Citation2008). Tomato quality changes continuously after harvesting. Fruit quality aspects include firmness, flavour, colour and nutritional value, as well as shelf life, processing attributes and resistance to pathogens (Ju et al. Citation2000). Tomatoes deteriorate rapidly after harvest and in some cases during or after transport and marketing.
Due to the economic impacts of spoiled foods and consumers’ concerns over the safety of foods containing synthetic chemicals, a lot of attention has been paid to naturally derived compounds or natural products (Stavropoulou et al. Citation2014). Edible coatings using natural biomaterials are being explored as a safer alternative to extend the shelf life of perishable food crops and improve food appearance (Mahfoudhi et al. Citation2015). Different compounds have been used as edible coatings to prevent commodity weight loss, including wax, milk proteins, celluloses, lipids, starch, zein and alginate (Cha & Chinnan Citation2004). Aloe vera gel has been identified as a novel coating agent with good antimicrobial properties (Castillo et al. Citation2010; Navarro et al. Citation2011; Nejatzadeh-Barandozi Citation2013).
In recent years, the use of A. vera gel has gained much attention for use as a safe and environment-friendly postharvest treatment. Aloe vera gel has been applied as edible coating material for raw produce including nectarines (Ahmed et al. Citation2009; Navarro et al. Citation2011; Palanides et al. Citation2014), mangoes (Dang et al. Citation2008), apples (Ergun & Satici Citation2012), strawberries (Singh et al. Citation2011), cherries (Martinez-Romero et al. Citation2006; Palanides et al. Citation2014), papayas (Marpudi et al. Citation2011), peaches and plums (Guillen et al. Citation2013; Palanides et al. Citation2014), tomatoes (Athmaselvi et al. Citation2013; Chauhan et al. Citation2015) and table grapes (Serrano et al. Citation2006). Typically, the A. vera concentration used in these studies ranged between 50% and 100%, although it was much lower for apples (0%–10%). The results of these studies have indicated that A. vera reduces the respiration rate, ethylene production, weight loss, softening, total acidity and prevents colour development. Interestingly, the antifungal activity of Aloe gel from several species has been correlated with the content of aloin, one of the major phenolic compounds of Aloe leaves (Zapata et al. Citation2013).
For centuries, A. vera has been used for its medicinal and therapeutic qualities (Eshun & He Citation2004). The two major liquid sources of A. vera are a yellow latex (exudate) and clear gel (mucilage), both of which are produced from the large leaf parenchymatic cells (Ni et al. Citation2004). Currently, there is increasing interest in the use of A. vera gel as a source of functional ingredients in drinks, ice creams and beverages as well as being applied as an edible coating (Martinez-Romero et al. Citation2006). Aloe vera contains malic acid-acetylated carbohydrates (including β-1,4-glucomannans) which exhibit anti-inflammatory activity and antibiotic actions (Eshun & He Citation2004).
The aim of this work was to study the effect of A. vera coatings on the change in physicochemical parameters related to tomato fruit quality during storage.
Materials and methods
Plant material and experimental design
Tomato fruit (cv. Dafni) were obtained from local fields (crops cultivated for 6 months under commercial conditions and standard cultural practices in a clay loam soil, frequently irrigated according to crop needs during spring with a temperature range of 19–29 °C) in Limassol, Cyprus. Fruits were harvested at the third inflorescences of the plants. In the laboratory, fruits were selected to obtain homogeneous batches based on colour, size, ripeness (red-ripe stage) and absence of defect or injury.
Fresh A. vera gel was prepared according to previous reports (Navaro et al. Citation2011). Briefly, for each leaf the spikes along the margins were removed before longitudinally slicing to separate the rind from the inner leaf gel. The gel fillets were crushed to yield a mucilaginous gel which was filtered to discard the fibrous fraction. The gel was diluted with distilled water for the A. vera 5%, 10%, 15% and 20% (v/v) treatments. Before the coating application, fruit were washed with a solution of sodium hypochlorite (0.05%) for 5 min, rewashed with distilled water and air-dried at ambient temperature.
Twenty-four fruit were immersed in each concentration of A. vera gel coating solution (5%, 10%, 15% and 20%) for 10 min. The coating solution was applied uniformly on the whole fruit surface, while control fruit were dipped in purified water. After treatment, all fruits were air-dried for 30 min at room temperature. After coating, pairs of fruit were placed into 1 L polystyrene containers with snap-on lids, resulting in six containers (biological replications) per treatment for each of the two storage periods (7 and 14 days) at 11 °C and 90% relative humidity (RH) in darkness.
In summary, the experimental set-up consisted of five treatments × six replications (two fruits per replication) × two storage periods (plus day 0) using a total of 132 fruits. Filter paper moistened with water was put in a small beaker, placed into each container and remoistened every second day to maintain high RH during the storage period, as described by Tzortzakis et al. (Citation2007). Twelve samples of treated and control fruits were taken after 7 and 14 days for immediate analysis. For day 0 measurements, fruits were used after chlorine washing (six containers).
Decay evaluation
The severity of fruit decay (in individual fruits in each container; total 12 fruits per treatment per storage period) was evaluated visually after 7 and 14 days of storage at 11 °C. Tomato fruit showing surface mycelia development was considered to be decayed. The degree of infection on fruit was rated using a scale of 1 to 5, where: 1 – clean, no infection; 2 – trace infection; 3 – slight infection; 4 – moderate infection; and 5 – severe infection. Rots were distinguished by tomato tissue subculture on to potato dextrose agar media as described previously (Tzortzakis et al. Citation2008).
Respiration rate and ethylene emission
The CO2 and ethylene production were measured by placing each tomato in a 1 L glass jar hermetically sealed with a rubber stopper for 1 h at ambient room temperature. For respiration rate determination, the holder atmosphere was sucked out by a dual gas analyser (International Control Analyser Ltd) for 30 s. Results were the means of two determinations for each jar (six jars per treatment and storage period; n = 6) expressed as millilitres of CO2 per kilogram per hour. Ethylene was quantified by using an ethylene analyser (ICA 56 Analyser, International Control Analyser Ltd); the container air sample was sucked out for 30 s. Results were the means of two determinations for each jar and expressed as microlitres of ethylene per kilogram per hour (six jars per treatment and storage period; n = 6).
Weight loss, colour and fruit firmness
Weights of individual tomatoes were recorded on the day of harvesting (day 0) and after the different sampling dates. Weight loss was calculated for each fruit (n = 6) per treatment and storage time as follows: weight loss % = 100 (Wo – Wf) / Wo, where Wo is the initial weight and Wf is the final weight of the fruit.
Colour was determined using the Hunter Lab System and a Minolta colorimeter model CR400 (Konica Minolta). Following the recording of individual L*, a* and b* parameters, results were the means of determinations made on four points for each fruit along the equatorial axis. Twenty-four measurements for each treatment and storage time were made.
Fruit firmness was measured at two points on the shoulder of each tomato fruit (1 cm2 of skin removed) for each treatment by applying a plunger 8 mm in diameter, using a texturometer FT 011 (TR Scientific Instruments). The amount of force (measured in Newtons [N]) required to break the radial pericarp (i.e. surface) of each tomato was recorded at ambient (22–24 °C) temperature. Twelve measurements for each treatment and storage time were made.
Soluble solids, titratable acidity, ascorbic acid and carotenoids
Total soluble solids concentration (TSS) was determined in triplicate from the juice obtained from two pooled tomatoes for each replication (n = 6) with a temperature-compensated digital refractometer (model Atago PR-101, Atago Co Ltd) at 20 °C, and the results were expressed in Brix. The titratable acidity (TA) was measured by potentiometric titration (Mettler Toledo DL22) of 5 mL supernatant diluted to 50 mL with distilled water using 0.1 N NaOH up to pH 8.1. Results were expressed as a percentage of citric acid. Eighteen measurements for each treatment and storage time were made.
Ascorbic acid (comprising the major part of vitamin C) in six independent pools of tomato juice was determined by the 2,6-dichloroindophenol titrimetric method (AOAC Citation1995). An aliquot of 5 mL of pooled tomato juice was diluted with 5 mL of water and titrated by the dye solution until the colour changed. Data were expressed as mg of ascorbic acid per gram of fresh weight.
Carotenoids (lycopene and β-carotene) were determined according to the Nagata & Yamashita (Citation1992) method after modification. Six individual samples (two fruits pooled in each sample) were examined per treatment and storage period. Thus, for each sample, 1 g of blended tomatoes was placed in a 50 mL falcon and stored at −20 °C until analysis (within 48 h). A volume of 16 mL of acetone:hexane 4:6 (v:v) was added to each sample, the samples shaken vigorously and the two phases separated automatically. An aliquot was taken from the upper solution for measurement of optical density at 663, 645, 505 and 453 nm in a spectrophotometer, using a reference acetone:hexane (4:6) ratio. Lycopene and β-carotene contents were calculated according to the Nagata & Yamashita (Citation1992) equations:
Results were expressed as nmol per gram of fresh weight.
Polyphenol extraction and analysis
Preparation of extracts
Six individual samples (two fruits pooled in each sample) were examined per treatment and storage period. Samples of 5 g were milled in a T25 digital ultra-turrax (IKA) with 10 mL methanol (50% v/v) for 30 s, and polyphenol extraction was assisted with ultrasound (Ultrasonic cleaning baths-150, Raypa) for 5 min. The slurry was centrifuged for 30 min at 5000 rpm at 4 °C (Sigma 3-18 K, Sigma Labs). The supernatant was transferred to a 15 mL falcon tube and stored at 4 °C until analysis (within 48 h) for evaluation of total phenolic content and total antioxidant activity.
Total phenolic content
The total phenolic content of the methanol extracts was determined by using Folin-Ciocalteu reagent (Merck), according to the procedure described by Tzortzakis et al. (Citation2007). Briefly, 125 μL of plant extract was mixed with 125 μL of Folin reagent. The mixture was shaken, before the addition of 1.25 mL of 7% Na2CO3, adjusted with distilled water to a final volume of 3 mL and mixed thoroughly. After incubation in the dark for 90 min, the absorbance at 755 nm was measured and compared with the prepared blank. Total phenolic content was expressed as μmol of gallic acid equivalents per gram of fresh weight (μmol GAE g−1 FW), through a calibration curve with gallic acid. All samples were analysed in triplicate.
Determination of antioxidant capacity by ferric-reducing antioxidant power (FRAP) assay
A 3 mL sample of freshly prepared FRAP solution (0.3 mol L−1 acetate buffer, pH 3.6) containing 10 mmol L−1 TPTZ (tripyridil-s-triazine) and 40 mmol L−1 FeCl3·10H2O and 20 μL extract (50 mg mL−1) was incubated at 37 °C for 4 min and the absorbance was measured at 593 nm. The absorbance change was converted into a FRAP value, by relating the change of absorbance at 593 nm of the test sample to that of the standard solution of trolox ([±]-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid). A standard curve was prepared using different concentrations of trolox, and the results were expressed as mg trolox per gram of fresh weight (Pantelidis et al. Citation2007). All samples were analysed in triplicate.
Determination of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging activity
Radical scavenging activity was determined according to Wojdylo et al. (Citation2007) with some modifications. The DPPH radical scavenging activity of the plant extracts was measured from the bleaching of the purple-coloured 0.3 mM solution of DPPH. One millilitre of the DPPH solution in ethanol, 1.98 mL (50% v/v) methanol and 0.02 mL plant extract were mixed. After shaking, the mixture was incubated at room temperature in the dark for 30 min, and then the absorbance was measured at 517 nm. The results were expressed in mg trolox per gram of fresh weight. All samples were analysed in triplicate.
Statistical analysis
Data were first tested for normality and then subjected to analysis of variance (ANOVA). Sources of variation were time of storage and treatments. Significant differences between mean values were determined using Tukey’s HSD test (P = 0.05) following one-way ANOVA. Significant differences on percentage values (weight loss) were logarithmic transformed prior to using ANOVA. Statistical analyses were performed using SPSS (SPSS Inc) and graphs were produced using Prism v.2.0 (Graph Pad Inc). All the assumption of analyses were checked to ensure validity of statistical analysis.
Results and discussion
At the market interface, only produce that corresponds to the expectations of the consumer is acceptable. Thus, it is vital to assess the effects of potentially innovative practices on sensory and organoleptic properties of fruit and vegetables. Postharvest fruit quality attributes are influenced by several traits such as weight loss, colour, firmness, total soluble solids and total acidity and their changes during storage.
Decay percentage
There was no visible sign of decay in coated or control fruit until day 7 of the storage period. Indeed, at the end of the experiment (day 14), only the control and the 5% Aloe-treated fruit had signs (evaluated as 2.25 and 2.16, respectively, according to the 1–5 scale) of decay (mainly symptoms of anthracnose rot [caused by Colletotrichum coccodes] and secondary symptoms of black spot [caused by Alternaria alternata]) as presented in .
Table 1. Effect of A. vera coating on tomato fruit decay during storage (11 oC, 90% RH).
Respiration and ethylene production rates
The CO2 production rate in control and Aloe-treated tomatoes was significantly increased during storage (), while the A. vera coating did not affect the respiration rate throughout storage period; it averaged at 1.817 mL CO2 kg−1 h−1 and 4.041 mL CO2 kg−1 h−1 at 7 and 14 days, respectively. Martinez-Romero et al. (Citation2006) reported that when cherries were coated with 33% A. vera gel, the CO2 production rate was increased during cold storage and this increase was higher in the control than in the Aloe-treated fruit. This contradictory observation might be the result of the higher percentage A. vera gel used for the cherry coating compared with the present study. Paladines et al. (Citation2014) reported that the respiration rate decreased in Aloe-treated stone fruits (cherries, nectarines and plums) but no significant differences were obtained between control and Aloe-treated peaches, highlighting the variable observations related to species and/or climatic and growth conditions.
Figure 1. Respiration rate (A) and ethylene emission (B) during storage (11 °C, 90% RH) of control and A. vera-coated tomatoes. Values are the means of six samples (n = 6) per treatment and storage period. Values followed by the same letter in each column do not differ significantly (P < 0.05). * or ns indicate significance or not, respectively, among controls through storage period.
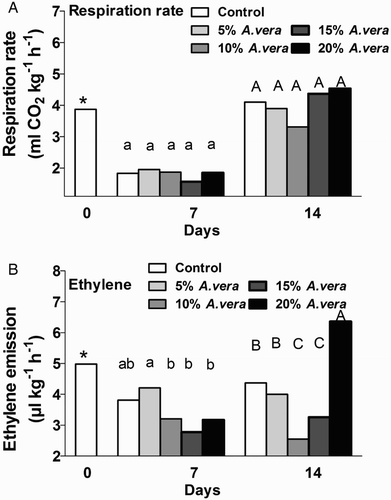
The ethylene production rate was reduced in 10%, 15% and 20% Aloe-treated tomatoes after 7 days of storage compared with the 5% Aloe-treated tomatoes, and was evident for 10% and 15% coated tomatoes only after day 14 (). A steady increase in ethylene emission was found in 20% Aloe-treated tomatoes after 14 days of storage; it is possible that increased stress in the fruit speeded up the fruit ripening process. The increase in ethylene production was delayed in Aloe-treated stone fruits (Navarro et al. Citation2011; Paladines et al. Citation2014). Despite the unchanged respiration rates in Aloe-treated fruits, the decrease in ethylene production has been attributed to the fact that A. vera gel acts as an edible coating and initiates a decrease in gas permeability through the fruit surface. This leads to modification of the internal atmosphere with enhanced CO2 and diminution of O2 concentration, in accordance with results found with other coatings (Valero et al. Citation2013). However, a detailed study at molecular level is required to examine the impact of A. vera gel on gene and/or protein expression involved in metabolic pathways, i.e. the ethylene biosynthetic pathway, which is related to fruit ripening, especially for climacteric fruit such as the tomato.
Tomato quality parameters and bioactive compounds
In the present study, the Aloe-coating on tomatoes was ineffective, with no significant effect on weight loss during 7 or 14 days’ storage compared with the control samples (). Opposite findings were reported in nectarines treated with 2.5% gel (Ahmed et al. Citation2009), in cherries treated with 33% gel (Martinez-Romero et al. Citation2006), in table grapes treated with 33% gel (Castillo et al. Citation2010) and in ‘Granny Smith’ (but not in ‘Red Smith’) apples treated up to 10% gel (Ergun & Satici Citation2012) as A. vera coating suppressed the increase in weight loss.
Figure 2. Effect of A. vera coating on fruit weight loss (A) and firmness (B) of tomatoes during storage (11 °C, 90% RH). Values are the means of six samples (n = 6) per treatment and storage period. Values followed by the same letter in each column do not differ significantly (P < 0.05). * or ns indicate significance or not, respectively, among controls through storage period.
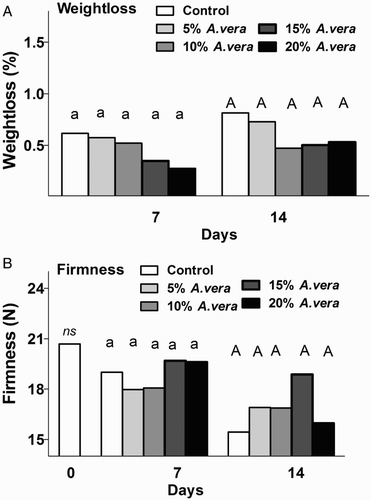
With respect to firmness, tomatoes softened during cold storage for both treated and control fruit (). At the end of the cold storage, control fruit showed flesh firmness levels of 15.44 ± 0.63 N, whereas in 15% Aloe-treated fruits, flesh firmness was 18.88 ± 0.94 N. Martinez-Romero et al. (Citation2006) reported that a higher A. vera concentration (33% v/v) significantly reduced the firmness losses (more than 50%) during cold storage in cherries. Moreover, Aloe gel treatment (100%) suppressed the firmness loss in mangoes ripened at 21 °C for 19 days (Dang et al. Citation2008). This marked effect could be due to the higher Aloe gel ratio and/or higher storage temperature employed for the mangoes compared with our experiment. Interestingly, the double application of A. vera on table grapes was more effective in fruit firmness retention than a sole application (Castillo et al. Citation2010).
In general, Aloe coating had no profound effect on fruit colour. The lightness (L* value) was decreased, while the redness (a* value) was increased during storage in both coated and uncoated fruit (data not presented). The highest decrease in lightness was observed in 20% coated fruit after 14 days of storage. No significant changes were found for the b* values among treatments.
The TSS was significantly lower (P < 0.05) in 10% and 15% Aloe-coated fruits after 7 days of storage, but this effect was not evident after 14 days of storage (). The titratable acidity values of coated and uncoated fruits did not differ after 7 or 14 days of storage. In fact, the ripening index (TSS/TA ratio) was decreased in 10% coated fruits (average 6.25) compared with control treatments and/or 5%, 15% and 20% coated fruits. This indicates that the control fruit exhibited a more pronounced ripening development than the 10% Aloe-coated tomatoes. The titratable acidity of raspberries treated with A. vera gel showed no significant difference compared with untreated fruits after 8 days of storage (Hassanpour Citation2015), which is in agreement with the present findings for 7 and 14 days of storage.
Table 2. Effect of A. vera coating on fruit total soluble solids (TSS, in oBrix), titratable acidity (TA, in % citric acid) and ripening index (TSS/TA ratio) during storage (11 oC, 90% RH).
During fruit development the pigment content is changing, whereas during ripening the chlorophyll content decreases and there is a prompt synthesis of carotenoids and thus the red pigment lycopene as well as β-carotene are synthesised. Both lycopene and β-carotene content decreased in Aloe-coated fruits greater than 10% of coating at 7 days and in 20% coated fruits at 14 days of storage, compared with the untreated fruit (). The decrease in carotenoids is possibly related to the reduced ethylene emission rates and slows down the ripening process for the 10% and 15% Aloe-coated fruits.
Figure 3. Effect of A. vera coating on β-carotene (nmol g−1 f Fwt) (A), lycopene (nmol g−1 Fwt) (B) and ascorbic acid (mg g-1 Fwt) (CSBOLDSTARTCCSBOLDEND) content in tomato fruit during storage (11 oC, 90% RH). Values are the means of six pooled samples (n = 6) per treatment and storage period. Values followed by the same letter in each column do not differ significantly (P < 0.05). * Or ns indicate significance or not, respectively, among controls through storage period.
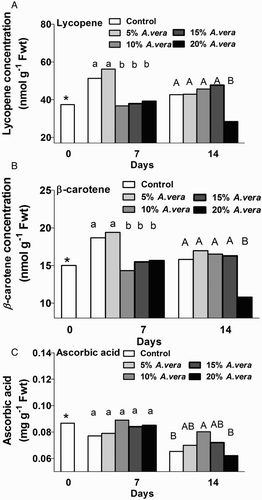
Ascorbic acid content usually decreases as the ripening stage advances. Thus, an Aloe coating significantly (P < 0.05) delayed the decline of ascorbic acid content in coated tomatoes in comparison with control fruits, which can be considered a positive effect of edible coatings. In detail, the ascorbic acid content of 10% Aloe-coated fruit was higher after 14 days of storage compared with the untreated fruits and the 20% Aloe-coated tomatoes, while no significant differences were observed among 5% and 15% Aloe coatings (). Similarly, formulated Aloe coatings on tomato, including antioxidants and plasticisers, have shown that ascorbic acid content increased during the breaker fruit stage, followed by a slight fall during the light red stage, and then a slight increase during the red stage (Athmaselvi et al. Citation2013). Hassanpour (Citation2015) reported that A. vera coating was more effective in the retention of TSS, TA and ascorbic acid levels because of the resultant lower gas permeability which inhibited the respiratory rate and retarded the overall metabolic activity of raspberry fruits during storage. However, respiration rates in the present study were not affected, indicating a small effect of Aloe coating on fruit metabolism. Vitamin C (ascorbic and L-dehydroascorbic acid) has high antioxidant activity, providing protection against the presence of free radicals and consequently participating in the prevention of many degenerative diseases, as well as being an essential nutrient for humans.
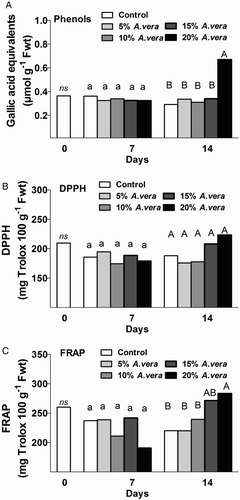
The content of total phenolics as well as the antioxidant activity, indicated by FRAP and DPPH assays, are presented in . On the day of harvesting, the total phenolic content was 0.364 ± 0.024 μmol equiv. gallic acid per g fresh weight. Total phenolics remained stable during storage for both coated and uncoated fruits with exception of 20% Aloe-coated fruits in which the content of phenolics increased two-fold, compared with the control fruits and ≤15% Aloe-coated fruits. Similar observations were made of the antioxidant status by FRAP assay at 14 days of storage. The increased content of phenolics and antioxidant status in 20% Aloe-coated fruits may be attributed to the increased ethylene emission rates after 14 days of storage. The evolution of total phenolics of fruits during storage could be different; depending on the species, cultivar, temperature and climactic and environmental conditions during the growth period (Kalt Citation2005).
Figure 4. Effect of A. vera coating on total phenols content (GAE μmol g−1 Fwt) (CSBOLDSTARTACSBOLDEND) and antioxidant activity (mg trolox g-1 Fwt) (B, C) in tomato fruit during storage (11 °C, 90% RH). Values are the means of six pooled samples (n = 6) per treatment and storage period. Values followed by the same letter in each column do not differ significantly (P < 0.05). *Or ns indicate significance or not, respectively, among controls through storage period.
The effects of applying A. vera edible coatings on delaying the postharvest ripening process and parameters related to fruit quality of tomatoes were similar to those previously observed for sweet and sour cherry, table grape, strawberry, peach, plum, papaya and nectarine coated with A. vera (Martinez-Romero et al. Citation2006; Serrano et al. Citation2006; Ahmed et al. Citation2009; Castillo et al. Citation2010; Marpudi et al. Citation2011; Navarro et al. Citation2011; Singh et al. Citation2011; Guillen et al. Citation2013; Ravanfar et al. Citation2014). This effect in climacteric fruit was attributed to the inhibition of ethylene production, as found in the present study.
Aloe vera gel has been used as an edible coating for many fruits and vegetables. Edible coatings have various favourable effects on fruit such as imparting a glossy appearance and better colour, retarding weight loss or prolonging storage/shelf life by preventing microbial spoilage (Dang et al. Citation2008). Aloe leaves are rich in bioactive compounds some of which are antioxidants and are used in food engineering as preservatives, such as mannans, antrachinon, c-glycoside, antron, antrakuinon and lectine (Eshun & He Citation2004). The gel of A. vera and other Aloe spp. is mainly composed of polysaccharides and soluble sugars followed by proteins, vitamins and minerals (Eshun & He Citation2004), but are very low in lipid content, ranging from 0.07%–0.42% depending on the Aloe spp. and climatic conditions during the growth cycle (Zapata et al. Citation2013). Thus, the gas barrier and hydrophobic properties of Aloe-based edible coatings could be improved with the addition of lipids, since the increase of lipid content in the composition of edible coatings leads to higher hydrophobic properties and barrier efficacy (Morillon et al. Citation2002).
Conclusion
The results of this study indicate that tomato fruit coated with 10% or 15% A. vera gel showed a significant delay in the change of ethylene emission during storage at 11 °C, compared with uncoated control fruit. In addition, the ripening index was decreased after 7 days of storage and the ascorbic acid content was increased in 10% coated fruits. As a consequence, the overall quality of the tomato fruit was maintained during storage. Further studies should be conducted on the coating properties. These studies may be related to the improvement of hydrophobic properties of the A. vera coatings and the gaseous exchange towards fruits. Thus new formulation and application developments to different climacteric and non-climacteric fruit and vegetables may be explored. Additional investigation is needed to elucidate the underlying relationship between A. vera gel treatment and antioxidant capacity in tomato fruits.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ahmed MJ, Singh Z, Khan AS. 2009. Postharvest Aloe vera gel-coating modulates fruit ripening and quality of ‘Arctic Snow’ nectarine kept in ambient and cold storage. Int J Food Sci Tech. 44:1024–1033. doi: 10.1111/j.1365-2621.2008.01873.x
- AOAC. 1995. Official method 967.21 Ascorbic acid in vitamin preparations and juices. 16th ed. Volume II. Washington, DC: Association of Official Analytical Chemists.
- Athmaselvi KA, Sumitha P, Revathy B. 2013. Development of Aloe vera based edible coating for tomato. Int Agrophys. 27:369–375. doi: 10.2478/intag-2013-0006
- Castillo S, Navarro D, Zapata PJ, Guillen F, Valero D, Serrano M, Martinez-Romero D. 2010. Antifungal efficacy of Aloe vera in vitro and its use as a preharvest treatment to maintain postharvest table grape quality. Postharvest Biol Tec. 57:183–188. doi: 10.1016/j.postharvbio.2010.04.006
- Cha DS, Chinnan M. 2004. Biopolymer-based antimicrobial packaging: a review. Crit Rev Food Sci. 44:223–237. doi: 10.1080/10408690490464276
- Chauhan OP, Nanjappa C, Ashok N, Ravi N, Roopa N, Raju PS. 2015. Shellac and Aloe vera gel based surface coating for shelf life extension of tomatoes. J Food Sci Tech. 52:1200–1205. doi: 10.1007/s13197-013-1035-6
- Dang KTH, Singh Z, Swinny EE. 2008. Edible coatings influence fruit ripening, quality, and aroma biosynthesis in mango fruit. J Agr Food Chem. 56:1361–1370. doi: 10.1021/jf072208a
- Ergun M, Satici F. 2012. Use of Aloe vera gel as biopreservative for ‘Granny Smith’ and ‘Red Chief’ apples. J Anim Plant Sci. 22:363–368.
- Eshun K, He Q. 2004. Aloe vera: a valuable ingredient for the food, pharmaceutical and cosmetic industries: a review. Crit Rev Food Sci. 44:91–96. doi: 10.1080/10408690490424694
- Guillen F, Diaz-Mula HM, Zapata PJ, Valero D, Serrano M, Castillo S, Martinez-Romero D. 2013. Aloe arborescens and Aloe vera gels as coatings in delaying postharvest ripening in peach and plum fruit. Postharvest Biol Tec. 83:54–57. doi: 10.1016/j.postharvbio.2013.03.011
- Hassanpour H. 2015. Effect of Aloe vera gel coating on antioxidant capacity, antioxidant enzyme activities and decay in raspberry fruit. LWT - Food Sci Technol. 60:495–501. doi: 10.1016/j.lwt.2014.07.049
- Ju Z, Duan Y, Ju Z. 2000. Plant oil emulsion modifies internal atmosphere, delays fruit ripening, and inhibits internal browning in Chinese pears. Postharvest Biol Tec. 20:243–250. doi: 10.1016/S0925-5214(00)00120-4
- Kalt W. 2005. Effects of production and processing factors on major fruit and vegetables antioxidants. J Food Sci. 47:4639–4644.
- Mahfoudhi N, Chouaibi M, Hamdi S. 2015. Effectiveness of almond gum trees exudate as a novel edible coating for improving postharvest quality of tomato (Solanum lycopersicum L.) fruits. Food Sci Technol Int. 20:33–43. doi: 10.1177/1082013212469617
- Marpudi SL, Abirami LS, Pushkala R, Srividya N. 2011. Enhancement of storage life and quality maintenance of papaya fruits using Aloe vera based antimicrobial coating. Indian J Biotechnol 10:83–89.
- Martínez-Romero D, Alburquerque N, Valverde JM, Guillén F, Castillo S, Valero D, Serrano M. 2006. Postharvest sweet cherry quality and safety maintenance by Aloe vera treatment: a new edible coating. Postharvest Biol Tec. 39:93–100. doi: 10.1016/j.postharvbio.2005.09.006
- Morillon V, Debeaufort F, Blond G, Capelle M, Voilley A. 2002. Factors affecting the moisture permeability of lipid-based edible films: a review. Criti Rev Food Sci. 42:67–89. doi: 10.1080/10408690290825466
- Nagata M, Yamashita I. 1992. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. Nippon Shokuhin Kogyo Gakkaish. 39:925–928. doi: 10.3136/nskkk1962.39.925
- Navarro D, Diaz-Mula HM, Guillen F, Zapata PJ, Castillo S, Serrano M, Valero D, Martinez-Romero D. 2011. Reduction of nectarine decay caused by Rhizopus stolonifer, Botrytis cinerea and Penicillium digitatum with Aloe vera gel alone or with the addition of thymol. Int J Food Microbiol. 151:241–246. doi: 10.1016/j.ijfoodmicro.2011.09.009
- Nejatzadeh-Barandozi F. 2013. Antibacterial activities and antioxidant capacity of Aloe vera. Org Med Chem Lett. 3:1–8. doi: 10.1186/2191-2858-3-5
- Ni Y, Turner D, Yates KM, Tizard I. 2004. Isolation and characterization of structural components of Aloe vera L. leaf pulp. Int Immunopharmacol. 4:1745–1755. doi: 10.1016/j.intimp.2004.07.006
- Paladines D, Valero D, Valverde JM, Diaz-Mula H, Serrano M, Martinez-Romero D. 2014. The addition of rosehip oil improves the beneficial effect of Aloe vera gel on delaying ripening and maintaining postharvest quality of several stone fruit. Postharvest Biol Tec. 92:23–28. doi: 10.1016/j.postharvbio.2014.01.014
- Pantelidis GE, Vasilakakis M, Manganaris GA, Diamantidis G. 2007. Antioxidant capacity, phenol, anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants, gouseberries and Cornelian cherries. Food Chem. 102:777–783. doi: 10.1016/j.foodchem.2006.06.021
- Ravanfar R, Niakousari M, Maftoonazad N. 2014. Postharvest sour cherry quality and safety maintenance by exposure to Hot- water or treatment with fresh Aloe vera gel. J Food Sci Tech. 51:2872–2876. doi: 10.1007/s13197-012-0767-z
- Serrano M, Valverde JM, Guillen F, Castillo S, Martinez-Romero D, Valero D. 2006. Use of Aloe vera gel coating preserves the functional properties of table grapes. J Agr Food Chem. 54:3882–3886. doi: 10.1021/jf060168p
- Singh DB, Singh R, Kingsly ARP, Sharma RR. 2011. Effect of Aloe vera coatings on fruit quality and storability of strawberry (Fragaria × ananassa). Indian J Agr Sci. 81:407–412.
- Stavropoulou A, Loulakakis K, Magan N, Tzortzakis N. 2014. Origanum dictamnus oil vapour suppress the development of grey mould in eggplant fruit and in vitro. Biomed Res Int. Article 562679. doi:10.1155/2014/562679.
- Tzortzakis NG, Borland A, Singleton I, Barnes JD. 2007. Impact of atmospheric ozone-enrichment on quality-related attributes of tomato fruit. Postharvest Biol Tec. 45:317–325. doi: 10.1016/j.postharvbio.2007.03.004
- Tzortzakis NG, Singleton I, Barnes JD. 2008. Impacts of low-level atmospheric ozone-enrichment on black spot and anthracnose rot of tomato fruit. Postharvest Biol Tec. 47:1–9. doi: 10.1016/j.postharvbio.2007.06.004
- Valero D, Diaz-Mula M, Zapata PJ, Guillen F, Martinez-Romero D, Castillo S, Serrano M. 2013. Effects of alginate edible coating on preserving fruit quality in four plum cultivars during postharvest storage. Postharvest Biol Tec. 77:1–6. doi: 10.1016/j.postharvbio.2012.10.011
- Wojdylo A, Oszmiański J, Czemerys R. 2007. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 105:940–949. doi: 10.1016/j.foodchem.2007.04.038
- Zapata PJ, Guillen F, Martinez-Romero D, Castillo S, Valero D, Serrano M. 2008. Use of alginate or zein as edible coatings to delay postharvest ripening process and to maintain tomato (Solanum lycopersicon Mill) quality. J Sci Food Agr. 88:1287–1293. doi: 10.1002/jsfa.3220
- Zapata PJ, Navarro D, Guillen F, Castillo S, Martinez-Romero D, Valero D, Serrano M. 2013. Characterization of gels from different Aloe spp. as antifungal treatment: potential crops for industrial application. Ind Crop Prod. 42:223–230. doi: 10.1016/j.indcrop.2012.06.002
