ABSTRACT
This study examined the effects of organically-derived plant growth promoters (PGPs) on vegetative growth of Pinot noir cuttings under glasshouse conditions for 8 weeks. Solutions of 10 products were applied four times, 2 weeks apart, as a soil drench. In standard potting mix, none of the products caused significant increases in vegetative growth. In low-nutrient potting mix, three products (Synerlogic, Actavize, Just Fish) increased aspects of growth, such as leaf number, shoot length and shoot and root dry weight. These products were relatively high in NPK, and therefore cutting growth was positively correlated to concentrations of NPK in the added solutions. The results imply that addition of PGPs does not by default enhance growth of grape cuttings, and that the mechanism of growth enhancement appears to be simple nutrient provision, rather than by a more complex physiological route involving plant hormones or hormone mimics.
Introduction
The use of plant growth promoters (PGPs) or ‘bio-stimulants’ based on organic by-products such as compost, seaweed, fish waste and ‘blood and bone’ is now common place in horticultural settings and in vineyards employing organic or ‘biodynamic’ management strategies (e.g. du Jardin Citation2015). In support of these practices, the literature contains numerous reports from grape growers and viticulture researchers indicating these products can increase plant growth and improve plant health, and enhance the yield and quality of grape berry juice (e.g. Norrie & Keathley Citation2006; Holden et al. Citation2008; Wiens & Reynolds Citation2008; Strydom Citation2013). However, the effectiveness of these products in improving plant performance remains vague. Edmeades (Citation2002) summarised the results of field trials on 28 liquid fertilisers derived from organic materials. He reported there were approximately equal numbers of positive and negative effects of these products on plant performance, and that the number of trials resulting in statistically significant positive effects was in line with that expected by chance. Edmeades (Citation2002) concluded that the products he examined did not contain sufficient quantities of plant nutrients or organic matter to improve plant growth when applied at the recommended concentrations. McHugh (Citation2003) reiterated that many (seaweed derived) products would not meet the nutrient requirements for enhanced plant growth and suggested the mechanisms behind any observed plant improvements were not completely understood.
It is often claimed that seaweed-based products benefit plants not by providing nutrients or organic biomass, but by the actions of phytohormones, such as auxins, cytokinins, gibberellins, or by hormone mimics that influence plant physiological pathways (Edmeades Citation2002; Stirk et al. Citation2014). Craigie (Citation2011) suggested the bio-stimulant effects of plant hormones in seaweed extracts may require revision, and that bioactivity may be due to larger molecules such as oligomers and polysaccharide elicitors.
The use of cuttings to study grapevine responses to growing conditions and pest resistance has proved to be a valuable model system, as less room is taken up for trial work, there is greater uniformity in starting material, and environmental conditions (such as base nutrition level in the growing media) can be controlled to a greater degree than in field studies (Mullins & Rajasekaran Citation1981). The aim of this investigation was to examine the effects of readily available PGPs on the early development of Pinot noir grape cuttings under glasshouse conditions. The effects of 10 PGP products on the growth of foliage and roots were compared to those obtained using an inorganic fertiliser and to a no-fertiliser control. Six of the PGPs were assessed when cuttings were potted in both standard and low-nutrient potting mix to examine whether there were any interactions between fertiliser treatment and initial soil nutrient levels.
Methods
Cuttings of Pinot noir (clone 115; approx. 30 cm long) were taken (12 July 2014) from a commercial vineyard in Canterbury New Zealand and stored at 4°C until July 28. The cuttings were cultivated in large trays filled with pumice, maintained on a heating pad in a shaded outdoor facility. This cultivation method promotes root development by applying warmth to the growing medium, and delays bud development by exposing the above-soil portion of the cutting to cooler temperatures.
On 20 October 2014, the cuttings were potted into plastic pots (8 × 8 cm2; 10 cm deep) using two types of growing media: a standard mix (80% compost and bark, 20% pumice, Osmocote® slow release fertiliser) and a low-nutrient mix (80% compost and bark, 20% pumice, no fertiliser). In an attempt to standardise cuttings at this stage, roots were trimmed to approximately 10 cm and new vegetative growth pruned down to one node.
Growth of the cuttings was subsequently monitored from 20 October to 15 December 2014, a period of 8 weeks, under glasshouse conditions (mean temperature: 20.0 ± 2°C) at Lincoln University, Canterbury, New Zealand. A total of 10 organically derived plant promoting products were tested, with water used as the primary control. ‘Phostrogen’, a conventional inorganic plant fertiliser (N:P:K 14:10:27), was used as a positive control (). The class of product (e.g. seaweed; fish; blood and bone), organic status and recommended dilution were obtained from information on product packaging. Solutions were applied four times over the course of the trial at approximately 2-week intervals, starting from first day of observation (20 October). On each occasion, 50 mL of solution was applied to each pot as a soil drench, which was a recommended application method for all products used. All pots were placed on to individual plastic trays (10 cm diameter) so that any excess solutions that drained through the potting mix could be re-absorbed.
Table 1. Products tested in this trial: class of products, organic certification and dilution used (based on information provided on the manufacturers’ labels).
All cuttings were lightly watered each day but not to the extent where the drip trays were overflowing. Cuttings were not watered on the day and subsequent day the solutions of plant growth products were added. At harvest, the number of leaves, shoot length (mm) and shoot fresh weight (FW g) were measured. Shoots and roots from each plant, and the woody part of the cutting, were dried for four days at 65°C and then reweighed. Percentage dry matter of the foliage was then calculated using shoot FW (g) and dry weight (DW g).
There were 7 replicates of each of the 12 treatments in the low-nutrient soil. In the standard potting mix, only eight treatments were used, again with seven replicates (see ). This combination of potting mix and growth products therefore produced 20 treatments, with a total of 140 cuttings. The cuttings were arranged on glasshouse benches in a complete randomised block design. Thus, there were 7 blocks with 1 replicate of each of the 20 treatments assigned to a randomised position.
Table 2. Summary (mean; n = 7) of growth parameters for Pinot noir cuttings maintained under glasshouse conditions for 8 weeks in pots containing either standard potting mix or low-nutrient potting mix with the addition of various plant growth promoters.
To establish the nutrient content of each product, the nitrogen (N), phosphate (P) and potassium (K) concentrations of the applied solutions were measured. N was determined by using a Dumas style elemental analyser (Elementar Vario-Max CN), where the sample is first combusted at 900°C in an oxygen atmosphere. The combustion process converts any elemental carbon and nitrogen into CO2, N2 and NOx, and the NOx species are subsequently reduced to N2. These gases are passed through a TC (thermal conductivity) cell to determine CO2 and N2 concentrations and the %C and %N calculated from the sample weights. Determination of P and K was made by first digesting the sample with nitric acid and then analysing on an Inductively Coupled Plasma Optical Emission Spectrophotometer (ICP-OES; Agilent).
All statistical analysis and the arrangement of treatments for the complete randomised block design were performed on Genstat v15. As the design was not completely factorial, the treatments in standard potting mix and in the low-nutrient potting mix were analysed separately. Fertiliser treatments were compared using one-way ANOVA, incorporating spatial block as a random factor and including the DW of the woody tissue as a covariate to account for any differences in growth due to the size of the cutting. Treatments were compared to the control in a pairwise manner using protected least significant differences (LSDs) (P < .05). Standardised effect sizes of treatments were calculated as [(treatment − control)/control] × 100%.
The concentrations of NPK in the different products exhibited bimodal distributions. Therefore, the relationships between aspects of cutting growth and concentrations of NPK in the added solutions were investigated using Spearman’s rank correlation.
Results
When the cuttings were grown in the standard potting mix, the ANOVA procedures suggested there were no statistically significant differences among the treatments for any of the growth variables measured (). Conversely, when the cuttings were maintained in the low-nutrient potting mix, statistically significant differences were found among the treatments for all variables (). Five compounds significantly increased at least one component of cutting growth compared to the water control treatment. Phostrogen, the positive control treatment, increased root DW (g), leaf number and shoot FW (g) and DW (g). Of the organically derived PGPs, Synerlogic, Actavize and Just Fish increased all the growth parameters except shoot DW (%). Although Bounty also increased all growth parameters, these effects were smaller than the above-mentioned products and only root DW (g) could be statistically separated from the control treatment. Neither of the two certified organic products, Vertesea and Biofeed, induced significant changes in the growth of the cuttings.
Some of the largest effects of the PGPs were observed on root growth: Just Fish increased root growth by 133% compared to the control treatment, Actavize by 124% and Bounty by 78%. Increases in shoot length (e.g. Actavize 50.6%; Just Fish 47.9%) and shoot weight (DW; e.g. Actavize 58.8%; Synerlogic 50.6%) were smaller than the effects seen for root growth but nevertheless still significant considering the duration of the trial. Shoot DW (%) was only significantly affected by Synerlogic, which caused a decrease of 7.1% compared to the control treatment.
At the recommended dilutions used in this experiment, the levels of N, P and K in the various products were all highly correlated (rs > 0.7, P < .01; ). Therefore, some products, such as Phostrogen, Synerlogic, Actavize and Just Fish were relatively high in all three nutrients, whereas other products, such as the seaweed-based products, Vertesea, Yates Seaweed and Seasol, contained levels of N and P only slightly higher than that found in the water control. Plant growth, in terms of the number of new leaves, root DW (g), shoot FW (g) and shoot DW (g) were all positively correlated to the concentrations of NPK in the added solutions, whereas shoot length (mm) was only significantly correlated to concentrations of N and P (; ). Shoot DW (%) exhibited a negative correlation with concentrations of N and P (; ).
Figure 1. Scatterplots of growth parameters of Pinot noir cuttings with concentrations of NPK in solutions of plant growth promoters added to a low-nutrient potting mix. Points are based on the mean values obtained for each parameter for each plant growth promoter. Plots include points for the water and ‘Phostrogen’ control treatments.
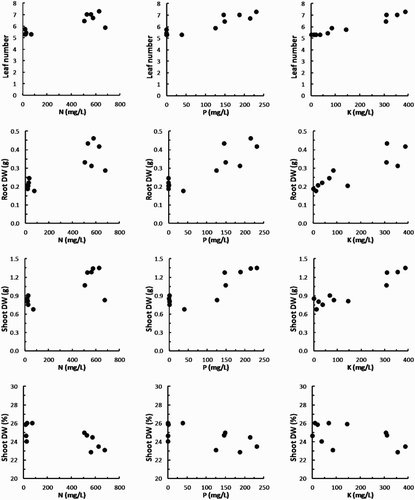
Table 3. Values of Spearman’s rank correlation coefficient indicating strength of relationships between growth parameters of Pinot noir cuttings with concentrations of NPK in solutions of plant growth promoters added to low-nutrient potting mix.
Discussion
In our study, no product had any statistically significant effects on plant growth in the high-nutrient growing medium, although clear effects on cutting development were seen when the products were applied to the low-nutrient potting mix. The results of this study agree with the conclusions of Edmeades (Citation2002), in that application of organically derived PGPs does not, by default, increase the growth of plants, and that most of the PGPs we studied lack sufficient levels of major nutrients to influence plant growth. The products that enhanced growth of Pinot noir cuttings in our trial (Synerlogic, Actavize, Just Fish) all had relatively high levels of NPK, but even these products only affected plant growth when the growing medium was initially low in nutrient levels.
Although there are numerous reports of PGPs producing positive effects on grape performance (e.g. Norrie et al. Citation2002; Holden et al. Citation2008; Strydom Citation2013), there are other examples that report no observable effects, such as the work of Martin (Citation2012) where application of algal extracts did not have any significant effects on multiple aspects of yield or components of juice quality in Syrah grapes. It should be noted that the vines used in that study had a sufficient level of NPK, as indicated through petiole testing, thereby highlighting the importance of the nutrient status of the starting material prior to interpreting published treatment effects.
Additionally, some studies that report beneficial effects of adding organically derived plant growth products also incorporated some form of additional nutrients supplementation. For example, the positive effects described by Anderson (Citation2009) during field trials on Cabernet Sauvignon and Cabernet Franc grapes in Australia were produced by application of a seaweed product (‘Natrakelp’) fortified with NPK. Khan et al. (Citation2012) reported that a foliar spray with an amino acid solution plus seaweed extract increased a number of growth parameters (leaf area, grapes per bunch, berry size) in grapes cv. ‘Perlette’, but they did not examine the effects of the seaweed extract in the absence of the amino acid solution. However, Sabir et al. (Citation2014) did use a factorial experimental design to investigate the effects of a seaweed extract and a fertiliser on the performance of ‘Narince’ grapes in Turkey, and found that, for some parameters (e.g. leaf FW, chlorophyll content, berry weight), the seaweed had an effect in isolation of the fertiliser treatment.
We concede that making strong conclusions from the results of our study is hindered by a number of experimental constraints. Not all products were tested in the high-nutrient potting mix, and the duration of the study was limited to 8 weeks as we focussed on the effects of these products on the early growth of cuttings, rather than on established plants. Additionally, some organically derived PGPs are claimed to improve plant vigour by stimulating beneficial soil microorganisms (e.g. Chen et al. Citation2002; Alam et al. Citation2014), and these effects may only manifest when the products are applied to natural soils rather than glasshouse potting mix. The labelling on some products indicates that ‘activity’ may wane if products are stored incorrectly (i.e. in warm, light conditions) or stored for too long a period. In this study, all products were stored in a cool, dark cupboard in their original packaging and used within six months of purchase. However, most products were bought from local stores, and no information was gathered about when the products had been supplied to the stores, or the time spent on store shelves, although this likely represents a typical situation for most purchasers unless they are obtaining supplies directly from the manufacturer.
In this study, we applied the products only as soil drenches, primarily because there was so little foliage on the cuttings through which nutrients could have been absorbed, and all the products tested suggested that application can be effective both by foliar spray and by soil drench. It is possible that some nutrients may be absorbed better through the foliage rather than roots (see Mengel Citation2002; Fernández & Brown Citation2013), and therefore that some of these products may have induced more noticeable changes in plant growth if they had been applied as a foliar spray rather than a root drench. Comparisons of effects of foliar spray versus those observed when products are applied as a root drench, and further examination of how the concentration and frequency of PGP application affect grape performance, should be considered in future studies.
Many mechanisms have been suggested as to why organically derived PGPs have beneficial effects on quantitative and qualitative effects on plant growth, such as amelioration of abiotic and biotic stresses and increased capability of nutrient assimilation (Edmeades Citation2002; McHugh Citation2003). However, in our trial, there was no exposure to insect pests or plant pathogens, no induction of drought stress, and enhanced growth was only observed if the growing medium was nutrient deficient. The results we obtained strongly suggest that the mechanism behind increased growth of the grape cuttings after application of some PGPs was straightforward nutrient provision, rather than increased soil-nutrient extraction by the plant due to some more complex physiological route. Although it is a considerable stretch to extrapolate these findings to full-scale grape production, grape growers may benefit from exercising some caution, and seek evidence of efficacy when considering the use of organically derived PGPs in their commercial systems.
Acknowledgements
The authors wish to thank Brent Richards and Leona Meachen for plant care and Roger Cresswell for organising the nutrient analysis of the plant growth products.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
S. Hodge http://orcid.org/0000-0001-6933-5253
References
- Alam MZ, Braun G, Norrie J, Mark Hodges D. 2014. Ascophyllum extract application can promote plant growth and root yield in carrot associated with increased root-zone soil microbial activity. Canadian Journal of Plant Science. 94:337–348. doi: 10.4141/cjps2013-135
- Anderson G. 2009. Seaweed extract shows improved fruit quality at McLaren Vale vineyard trial. Australian and New Zealand Grapegrower and Winemaker. 548:17–22.
- Chen SK, Subler S, Edwards CA. 2002. Effects of agricultural biostimulants on soil microbial activity and nitrogen dynamics. Applied Soil Ecology. 19:249–259. doi: 10.1016/S0929-1393(02)00002-1
- Craigie JS. 2011. Seaweed extract stimuli in plant science and agriculture. Journal of Applied Phycology. 23:371–393. doi: 10.1007/s10811-010-9560-4
- Edmeades DC. 2002. The effect of liquid fertilisers derived from natural products on crop, pasture and animal production. A review. Australian Journal of Agricultural Research. 53:965–976. doi: 10.1071/AR01176
- Fernández V, Brown PH. 2013. From plant surface to plant metabolism: the uncertain fate of foliar-applied nutrients. Frontiers in Plant Science. 4:289. doi:10.3389/fpls.2013.00289
- Holden D, Johnson H, Ocafrain M, Norrie J. 2008. Effect of seaweed extract on fruit set, yield, and quality in Pinot noir winegrapes. Proceedings of the 35th Annual Meeting Plant Growth Regulation Society America; 2008 August 3–7; San Francisco (USA), Article 22.
- du Jardin P. 2015. Plant biostimulants: definition, concept, main categories and regulation. Scientia Horticulturae. 196:3–14. doi: 10.1016/j.scienta.2015.09.021
- Khan AS, Ahmad B, Jaskani MJ, Ahmad R, Malik AU. 2012. Foliar application of mixture of amino acids and seaweed (Ascophylum nodosum) extract improve growth and physico-chemical properties of grapes. International Journal of Agriculture and Biology. 14:383–388.
- Martin J. 2012. Impact of marine extracts applications on cv. Syrah grape (Vitis vinifera L.) yield components, harvest juice quality parameters, and nutrient uptake [Unpublished MSc thesis]. San Luis Obispo: California Polytechnic State University.
- McHugh DJ. 2003. A guide to the seaweed industry. FAO Fisheries Technical Paper T441. FAOUN.
- Mengel K. 2002. Alternative or complementary role of foliar supply in mineral nutrition. Acta Horticulturae. 594:33–47. doi: 10.17660/ActaHortic.2002.594.1
- Mullins MG, Rajasekaran K. 1981. Fruiting cuttings: revised method for producing test plants of grapevine cultivars. American Journal of Enology and Viticulture. 32:35–40.
- Norrie J, Branson T, Keathley PE. 2002. Marine plant extracts impact on grape yield and quality. Acta Horticulturae. 594:315–319. doi: 10.17660/ActaHortic.2002.594.38
- Norrie J, Keathley JP. 2006. Benefits of Ascophyllum nodosum marine-plant extract applications to ‘Thompson Seedless’ grape production. Acta Horticulturae. 727:243–248. doi: 10.17660/ActaHortic.2006.727.27
- Sabir A, Yazar K, Sabir F, Kara Z, Yazici MA, Goksu N. 2014. Vine growth, yield, berry quality attributes and leaf nutrient content of grapevines as influenced by seaweed extract (Ascophyllum nodosum) and nanosize fertiliser pulverizations. Scientia Horticulturae. 175:1–8. doi: 10.1016/j.scienta.2014.05.021
- Stirk WA, Tarkowská D, Turečová V, Strnad M, van Staden J. 2014. Abscisic acid, gibberellins and brassinosteroids in Kelpak®, a commercial seaweed extract made from Ecklonia maxima. Journal of Applied Phycology. 26:561–567. doi: 10.1007/s10811-013-0062-z
- Strydom J. 2013. Effect of CPPU (N-(2-Chloro-4-Pyridinyl)-N’- Phenylurea) and a seaweed extract on Flame Seedless, Redglobe and Crimson Seedless grape quality. South African Journal of Enology and Viticulture. 34:233–240.
- Wiens G, Reynolds AG. 2008. Efficacy testing of organic nutritional products for Ontario Canada vineyards. International Journal of Fruit Science. 8:125–145. doi: 10.1080/15538360802368040
