ABSTRACT
The aim of this research was to determine the interaction between fertigation with KIO3 and pre-sowing application of exogenous humic and fulvic acids (used in the form of the commercial concentrate Humistar, HU) on yield, iodine biofortification and chemical composition of spinach cv. ‘Olbrzym zimowy’. The plants were cultivated in a pot experiment with the following treatments: control, pre-sowing HU application at doses of 0.0125, 0.05 and 0.2 cm3 HU dm−3 of soil, KIO3 fertigation and KIO3 fertigation with pre-sowing HU application at doses of 0.0125, 0.05 and 0.2 cm3 HU dm−3 of soil, fertigation at a dose of 0.0004% I. The use of HU and fertigation with KIO3 caused an increase of iodine content and iodine transfer factor (TF) from the soil to the plants; with the highest extent in the pot treated with KIO3 + 0.2 cm3 HU dm−3 of soil.
Introduction
Iodine is an important element for the proper thyroid functioning, development of nervous tissues and brain development in the prenatal period and in the first year after birth. It is also involved in the mechanism of resistance to ionising radiation (Charlton et al. Citation2010; Melse-Boonstra & Jaiswal Citation2010).
Despite the broad application of domestic salt iodisation, pathological symptoms related to inadequate iodine intake are diagnosed in 30–38% of the population (White & Broadley Citation2009). The percentage of people with insufficient iodine supply in Europe is 59.9% for children and 56.9% for adults (Andersson et al. Citation2007).
Biofortification of crops with iodine is proposed as an alternative method of introduction of this element into the food chain (White & Broadley Citation2009). Before its promotion, optimal agro-technical methods of iodine application need to be developed, which is particularly important as this element is not a plant nutrient.
Numerous studies on iodine biofortification of plants have been conducted in soil-less and hydroponic systems. With these methods, plants take up and accumulate iodine to a considerably greater extent than when grown in soil. Irrespective of the chemical form of iodine, it is strongly fixed in the soil as soon as a few dozen hours to a few days from the application. For iodine sorption, mineral nutrients are responsible, among others Fe and Al sesquioxides – essentially with pH < 6.0–6.9 (Muramatsu et al. Citation1990; Yoshida et al. Citation1992) and Cu/Al or Cu/Cr hydroxides (Pless et al. Citation2007). A substantial amount of iodine in soils is also fixed with soil organic matter – mainly with well-decomposed soil humus, but, interestingly, not with humus freshly introduced into soil (Muramatsu et al. Citation1996). Fixation occurs through the formation of covalent bonds with aromatic compounds containing double bonds (e.g. phenolic and polyphenolic compounds), which are part of humified organic matter, including fulvic acids and humins (Maillant et al. Citation2005). On the basis of such a mechanism, iodine is strongly fixed with soil humus (Muramatsu et al. Citation1996; Schlegel et al. Citation2006). It needs to be underlined that iodine desorption in soil is a very slow process (Yamaguchi et al. Citation2005; Weng et al. Citation2009).
Iodine speciation in soil (IO3− ↔ I2 ↔ I− conversions) is controlled by mutual and complex relations between soil pH and redox potential (Eh) (Johnson Citation2003; Fuge Citation2013). In aerobic conditions (in upland fields), humic acids may also be involved in the reduction of IO3− to I2. As a result of further transformations, I2 can volatilise from soil or form organo-iodine compounds after interaction with soil organic matter (Yamaguchi et al. Citation2005).
Studies conducted by Kashparov et al. (Citation2005) revealed that after 239-day cultivation of radish, lettuce, bean and wheat in various soil types (podzoluvisol, greyzem, meadow chernozem, typical chernozem), less than 3% of exogenous radioactive 125I isotope was leached into soil layers deeper than 20 cm. A similarly limited range of iodine mobility in the soil after one-time application has been noted by Weng et al. (Citation2008). In an experiment conducted with the use of a PVC column filled with sandy loam topsoil, iodine stability and low migration within a 20-cm layer of soil was observed under oxidised conditions (app. + 650 mV, Ashworth et al. Citation2003). Results of our previous studies (Smoleń et al. Citation2016b) demonstrated that plant fertigation with KI or KIO3 solution is a more efficient method of iodine biofortification of spinach than one-time pre-sowing introduction of these compounds into the soil.
The key to ensuring the fertility of soils is conducting organic fertilisation. At the same time, this indirectly reduces iodine transfer from soil to plants. Often, as a substitute of organic fertilisation, exogenous concentrates of humic and fulvic acids are introduced into the soil. Current research on plant biofortification with iodine has not covered the problem of interaction of the application of such concentrates with iodine fertilisation. However, identification of these issues is crucial due to the here presented involvement of humic and fulvic acids in the process of iodine sorption and/or transformation in the soil environment.
The aim of this research was therefore to determine the interaction between fertigation with KIO3 and the pre-sowing application of exogenous humic and fulvic acid concentrates on the yield, efficacy of iodine biofortification and chemical composition of spinach plants.
Materials and methods
Plant material and treatments
In the spring seasons of 2011 and 2012, we conducted an experiment with spinach cultivation (Spinacia oleracea L.), cv. ‘Olbrzym zimowy’, in a plastic tunnel. The plants were cultivated in open-work containers sized 60 × 40 × 20 cm, which were lined with foil in order to prevent leaching of water and mineral nutrients from the soil. The containers were filled with 45 dm3 silt loam (35% sand, 28% silt and 37% clay) with a mean organic matter content of 2.07% and the following concentrations of the available nutrient forms soluble in 0.03 M acetic acid: N (N–NO3+N–NH4) 159.5 mg, P 61.0 mg, K 59.1 mg, Mg 167.7 mg, Ca 1430.0 mg, S 80.4 mg, Na 28.5 mg, Cl 12.0 mg and I (iodine) 1.74 mg in 1 dm−3 soil. Soil pH(H2O) was 6.66, redox potential (Eh): +309.8 mV, with a soil salinity of (electrical conductivity, EC) 0.44 mS cm−1. In 2011 and 2012, the same soil batch was used, which was obtained in spring 2011 from a field at the Experimental Station of the University of Agriculture in Kraków, Poland (50°07′910 N; 19°84′764 E). The soil to be used in the second year was stored in a heap, protected from precipitation and sunlight. The experiments were set and conducted individually in 2011 and 2012, that is, each year, iodine, humic and fulvic acids were applied to the soil (freshly collected or stored, depending on the year). There was, however, a difference in the total amount of water used for plant irrigation per pot from seed sowing to harvest: 11.5 dm3 in 2011 and 16.0 dm3 in 2012 ().
Table 1. Amounts of water, iodine and chlorides introduced into the soil with irrigation; natural soil iodine contents.
Prior to spinach cultivation, one-time pre-sowing soil fertilisation with Ca(NO3)2, KH2PO4, KNO3 and K2SO4 (all compounds as clean fertilisers for hydroponic cultivation, Yara International) was conducted to adjust soil content of mineral nutrients to the concentrations of 200 mg N, 70 mg P and 200 mg K dm−3 of soil. All fertilisers were applied once before seed sowing. Supplementation with other nutrients was not performed due to their optimal content in the soil.
The studied factors included pre-sowing application of exogenous humic and fulvic acids and soil fertigation with KIO3 solution. The source of humic and fulvic acids was the commercial product Humistar (HU), manufactured by TRADECORP. It is an extract obtained from leonardite, a natural form of humates, and contains 12% w/w of humic acids and 3% w/w of fulvic acids. Iodine was introduced as KIO3 of analytical grade (Sigma-Aldrich, Germany).
The treatments were as follows: (1) control (without HU and iodine application – natural content of iodine in the soil; ); (2) pre-sowing HU application at a dose of 0.0125 cm3 dm−3 of soil [HU 0.0125]; (3) pre-sowing HU application at a dose of 0.05 cm3 dm−3 of soil [HU 0.05]; (4) pre-sowing HU application at a dose of 0.2 cm3 dm−3 of soil [HU 0.2]; (5) fertigation with KIO3 [KIO3]; (6) fertigation with KIO3 and pre-sowing HU application at a dose of 0.0125 cm3 dm−3 of soil [KIO3+HU 0.0125]; (7) fertigation with KIO3 and pre-sowing HU application at a dose of.05 cm3 dm−3 of soil [KIO3+HU 0.05] and (8) fertigation with KIO3 and pre-sowing HU application at a dose of 0.2 cm3 dm−3 of soil [KIO3 + HU 0.2]. The highest HU dose (0.2 cm3 dm−3 of soil) was the same as in the previous study (Smoleń et al. Citation2016b). The experiment presented here is focused on testing three different HU doses that each time increased fourfold.
During cultivation, plants in containers were irrigated with the same amount of tap water (), that is from 500 to 1000 ml per watering. The lower amount of water was applied during germination and early growth stages, the higher amount at the intensive growth stage up to the harvest. Watering was performed every second day. On average, 13.7 dm−3 of water were used per container during cultivation for 38 and 43 days in 2011 and 2012, respectively. Fertigation with KIO3 (treatments no. 5–8) was conducted with each watering, using a 0.0004% iodine solution, the same dose as in the previous studies (Smoleń et al. Citation2016b). The total iodine amount of 1.17 mg I dm−3 of soil () was introduced into the soil during spinach cultivation from seed sowing to harvest. The amount of chlorides introduced into the soil with tap water was also estimated; based on the average water content of 15.0 mg Cl dm−3, it was approximately 4.58 mg Cl dm−3 of soil.
The experiment was carried out according to a randomised method in four replications for each treatment. Each replicate (one container) consisted of 4 rows with 10 plants per row, totalling 40 plants per replication. Seed sowing was performed on 22 and 14 March in the subsequent years, with 20 seeds in a row. After germination, the plants were singled out, leaving 10 seedlings per row (40 plants per container). Spinach was harvested on 29 April 2011 and 26 April 2012. During harvest, the crop yield was determined, expressed as spinach plant mass (above-ground parts – leaves with petioles and shoots). Spinach leaves and leaf petioles were selected for chemical analyses.
Plant analysis
Nitrate (V) and chloride concentrations in fresh spinach leaves were assayed immediately after harvest; NO3− by the FIA technique with the use of an FIA Modula MLE, Germany (PN-EN ISO 13395 Citation2001), and Cl− ions by the nephelometric method. Dry matter content was estimated at 105°C.
After fresh sample preservation in boiling 96% ethanol (using the reflux condenser), the contents of free amino acids, soluble sugars and phenolic compounds were assessed spectrophotometrically. The concentration of free amino acids was determined after a reaction with ninhydrin, soluble sugars after a reaction with anthrone dissolved in concentrated H2SO4 and phenolic compounds after reaction with Folin–Ciocalteu reagent. Analysis of free amino acid content was performed as this parameter provides information on the incorporation of mineral nitrogen in plant tissues. The assayed level of phenolic compounds in plant extracts may be an indicator of plant stress; additionally, they are most common antioxidants positively affecting consumer health.
Leaf samples were dried at 70°C in a laboratory dryer with forced air circulation. Dried samples were ground in a Pulverisette 14 variable speed rotor mill, FRITSCH, using a 0.5-mm sieve. Subsequently, iodine concentration was determined after incubation of 0.5 g dried plant material with 1 ml of 25% TMAH and 10 ml of double-distilled water for 3 h at 90°C in closed falcon tubes (PN-EN 15111 Citation2008). Afterwards, the samples were cooled to room temperature and 20 ml of double-distilled water were added; samples were mixed on a vortex and centrifuged for 15 min at 4500 rpm and the supernatant liquid was subjected to analysis.
Leaf N content was assayed by the Kjeldahl method. The levels of 33 elements (P, K, Ca, Mg, S, Na, B, Cu, Fe, Mn, Mo, Zn, Al, Ba, Ce, Cd, Cr, Li, Ni, Se, Sr, Ti, Ag, Co, Dy, La, Lu, Pb, Sc, Th, Y, Yb and V) in plant samples were analysed after digestion in 65% super pure HNO3 in the microwave system CEM MARS-5 Xpress. Contents of iodine and the 33 elements were determined with the use of the high-dispersion spectrometer ICP-OES Prodigy Teledyne Leeman Labs. The results of macro-, micro- and trace element concentration in spinach leaves are presented in Appendix 1 (Figures S1–S3).
Soil analysis
In soil samples treated with water (1:2 vol/vol, soil: H2O), measurements of pH and redox potential (Eh) were conducted potentiometrically; total soil salinity (EC) was measured using a conductivity meter. For the assessment of available forms of N–NH4, N–NO3, Cl and iodine, soil extraction with 0.03 M acetic acid was employed (Nowosielski Citation1988). Determination of N–NO3 and N–NH4 was conducted by FIA (PN-EN ISO 11732 Citation2005, PN-EN ISO 13395 Citation2001); for I, an ICP-OES spectrometer was used and for chlorides, we chose the nephelometric method. All analyses were conducted on soil samples collected before and after plant cultivation.
Additionally, the contents of P, K, Mg, Ca, S, Na (by ICP-OES) and soil organic matter were assayed (using Tiurin’s method), but only prior to plant cultivation. The results obtained prior to plant cultivation were used for soil characterisation and are presented in the ‘Plant material and treatments’ section.
Data analysis
Based on the iodine content in the soil before cultivation (1.74 I dm−3 of soil) and the amount of iodine introduced into the soil by KIO3 fertigation (), the total amount of iodine present in the soil in each of the tested combinations [ICsoil] was calculated. Using the data of iodine content in spinach [ICplants (dry matter)], the value of the iodine transfer factor [TFleaf] in the soil-to-leaf system was calculated with the formula TFleaf = [ICplants (dry matter)] / [ICsoil].
All data were subjected to analysis of variance using the analysis of variance module of Statistica 10.0 PL. To determine the significance between the means, Tukey’s test was used, employing a significance level of P < .05.
All results are presented as averages from 2011 and 2012, since in both research years, we obtained similar effects of the investigated factors on yield and chemical composition of the plants and the soil.
Biofortification target – consumer safety of iodine-enriched spinach
In individual countries throughout the world, various eating habits prevail. The consumption of leafy vegetables by vegans and vegetarians is higher than by people consuming products of animal origin. According to various sources, daily or one-serving intake of leafy vegetables ranges from 50 g f.w. (Voogt et al. Citation2010) to 200 g f.w. (Ray et al. Citation2013). For that reason, the parameters describing ‘biofortification target’ were calculated separately for one serving and daily intake of spinach in the amount of 50 g f.w. and 200 g f.w., respectively.
The daily iodine requirement for children above 12 years old and adults (with the exception of pregnant and lactating women) is 150 µg I (Andersson et al. Citation2007). Based on the results, daily intake of I (DI) and percentage of the recommended daily allowance of iodine (% RDA-I) from 50 g f.w. or 200 g of spinach were calculated. ‘Risk assessment’ was also conducted by calculating green vegetables hazard quotient (HQgv) – the risk to human health resulting from the intake of iodine through consumption of fresh spinach based on a 70-kg adult. The HQgv was calculated according to the United States Environmental Protection Agency (USEPA) Protocol (Iris Citation2011), using the following equation:where ADD is the average daily dose of iodine (mg I kg−1 body weight·day−1) and RfD represents the recommended dietary tolerable upper intake level of iodine (mg I kg−1 body weight·day−1). The dietary tolerable upper intake level suggested for the general population is 1100 µg I·day−1 (1.1 mg day−1) and 15.72 µg I kg−1 day−1 for a 70-kg adult (Kessler Citation2009).
The ADD for 50 or 200 g portions of spinach was computed as follows: ADD = (MI · CF · DI) / BW. Here, MI is the iodine concentration of the plant (mg kg−1 d.w.), CF is the fresh to dry weight conversion factor for plant samples (calculated as the ratio of dry weight to fresh weight; 0.087 on average), DI is the daily intake of green vegetables (kg, taken as 0.05 or 0.2 kg) and BW is the body weight (kg) of humans, assumed as 70 kg. The presented method of HQgv calculation represents only iodine intake with spinach. The full balance of iodine in the diet, including iodine from table salt, water or any other ford sources, was not considered.
Results
Spinach biomass and efficiency of iodine biofortification of spinach
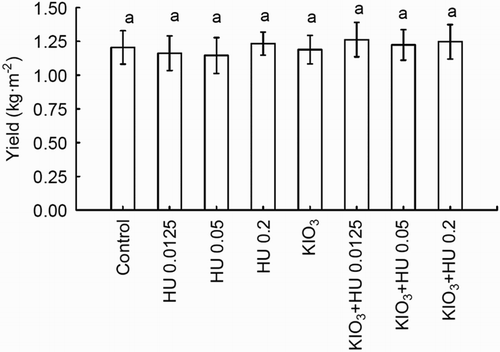
Compared to the control, no significant influence of the investigated factors on spinach yield was observed ().
Figure 1. Effect of Humistar application and soil fertigation with KIO3 on spinach yield. Means of the two-year study followed by the same letters are not significantly different at P < .05; bars indicate standard error (n = 8).
The results of statistical analysis for all tested combinations revealed that iodine content in spinach and iodine TF values significantly depend on the pre-sowing HU introduction and fertigation with KIO3 ((A,B)). Narrowing the analysis to the control and three combinations with pre-sowing application of HU alone revealed that each dose of humic acid concentrate lowered iodine accumulation in spinach as compared to the control ((A)). The content of iodine in control plants was 4.17 mg I kg−1 d.w., while in the combinations with increasing HU doses, it was 2.65, 3.06 and 2.31 mg I kg−1 d.w., respectively (36.5%, 26.6% and 44.7%, respectively, lower than in the control). As a consequence, values of iodine TF for these three combinations with HU were lower than in the control ((B)).
Figure 2. (A) Iodine content and (B) TF value in spinach after Humistar application and soil fertigation with KIO3. Means of the two-year study followed by the same letters are not significantly different at P < .05; bars indicate standard error (n = 8).
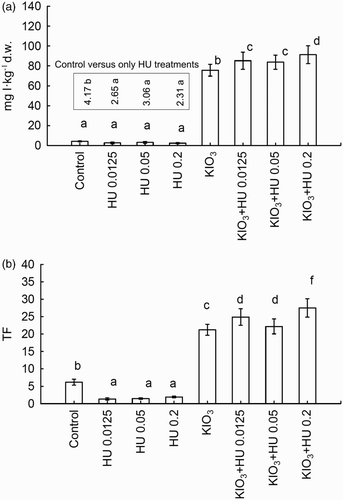
After the introduction of KIO3 into the soil, a significant increase of iodine content and iodine TF in spinach was observed; these values were significantly higher than in the control combination ((A,B)). Fertigation with KIO3 performed in HU-enriched soil (for each of HU doses) caused an additional significant increase of these parameters in relation to the application of KIO3 alone and the control. The highest iodine contents were found in plants cultivated in the soil enriched with the highest dose of HU (0.2 cm3 HU dm−3 of soil) and fertigated with KIO3.
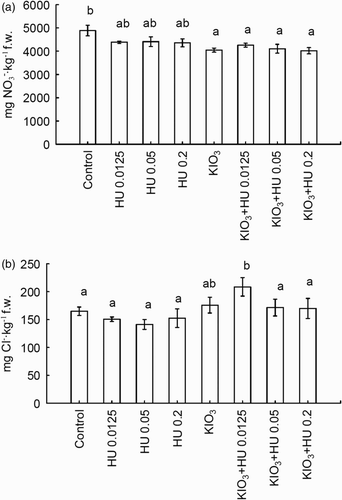
Accumulation of NO3− and Cl− in spinach
Nitrate (V) concentration in spinach was significantly affected by the introduction of HU and fertigation with KIO3 ((A)). The investigated factors caused a substantial reduction of nitrate (V) accumulation in spinach ((A)). This was primarily a result of fertigation with KIO3 separately as well as in combination with pre-sowing application of HU at three different doses. In the case of chlorides, only after fertigation with KIO3 + HU 0.05, a significant increase of Cl− in spinach was observed as compared to the control ((B)).
Dry matter, soluble sugars, phenolic compounds and amino acids
A significant, and importantly, highly varied effect of the application of HU and fertigation with KIO3 was observed with respect to the contents of dry matter, soluble sugars, phenolic compounds and amino acids in spinach (). In comparison to the control, a significant increase of dry matter and soluble sugar concentrations occurred in combinations with the application of KIO3 alone and KIO3 + HU 0.2. In this aspect, lower doses of HU applied separately and in combination with KIO3 fertigation did not affect dry matter and soluble sugar contents in spinach. Referring to the control plants, pre-sowing application of HU 0.05 and HU 0.2 as well as combined fertilisation with KIO3 + HU 0.05 caused a significant reduction of phenolic compounds in spinach. Soil fertigation with KIO3, (irrespective of HU application in all tested doses) considerably decreased amino acid concentrations in spinach, both in relation to the control as well as to the combination with pre-sowing HU application. Amino acid contents in spinach plants were most significantly reduced by KIO3 fertigation without applying HU.
Table 2. Contents of dry matter, soluble sugars, phenolic compounds and amino acids in spinach depending on Humistar application and soil fertigation with KIO3.
Mineral composition of spinach
A significant effect of the applied factors was noted with respect to the contents of the analysed macro-, micro- and trace elements in spinach plants, with the exception of Ba, Ag and Lu; these data are presented in Appendix 1 (Figures S1–S3). Here, we only describe the most significant relations.
In comparison to the control, increased nitrogen accumulation as well as decreased levels of K (Figure S1), Cu, Fe, Mn, Ce, Li, Se (Figure S2), La, Th and Y (Figure S3) were noted in spinach plants from all tested combinations.
The application of KIO3 + HU 0.0125, KIO3 + HU 0.05 and KIO3 + HU 0.2, as compared to the control, but mainly to the introduction of HU alone (in respective doses), contributed to a substantial decrease in the concentration of the following elements: K (Figure S1), Fe, Mn, Zn, Al, Cd, Cr, Li, Ni (Figure S2), Co, La, Pb, Sc, Th, Y, Yb and V (Figure S3). In the case of Ce, this effect was observed only for plants fertilised with KIO3 + HU 0.2, while of Ni, we found this effect for plants from the combinations with KIO3 + HU 0.0125 and KIO3 + HU 0.2 application (Figure S2).
Chemical properties of soil after spinach cultivation
Soil chemical analyses after spinach cultivation revealed a significant influence (in comparison to the control) of the investigated factors on pH, soil salinity (EC), redox potential (Eh) and the contents of iodine, N–NO3, N–NH4 and chlorides, determined after soil extraction with 0.03 mol acetic acid (). It should be noted that in all tested combinations, there was a significant reduction of the EC value and the N–NO3 content as well as an increase of soil pH and iodine content compared to the control.
Table 3. Effects of Humistar application and soil fertigation with KIO3 on the values of pH, EC, Eh and the content of I, N–NH4, N–NO3 and Cl in soil after spinach cultivation.
The greatest increase of soil pH occurred after the application of KIO3 + HU 0.05 and KIO3 + HU 0.2. In these cases, we also observed a significant decrease of soil chloride. The highest iodine and the lowest N–NO3 levels were determined in 0.05 HU-enriched soil fertigated with KIO3 solution. At the same time, with this combination, the greatest EC decrease was noted, accompanied by a significant increase of soil N–NH4.
Consumer safety
In comparison to the control, pre-sowing application of HU at three different doses (without KIO3) similarly decreased the value of percentage of recommended daily allowance for I (RDA-I) and HQ for iodine intake from a 50 and 200-g portion of fresh spinach leaves by adults (). Fertigation with KIO3 (applied alone or together with HU) significantly increased the values of the parameters that describe food safety and provision of RDA-I of consumed iodine-enriched spinach. The highest values were noted for KIO3 + HU 0.2. With the estimated consumption of 200-g spinach, these values were higher than for the 50-g portion.
Table 4. Percentage of recommended daily allowance for I (RDA-I) and hazard quotient (HQ) for intake of iodine through consumption of 50 and 200-g portions of fresh spinach leaves by adult humans (70 kg body weight).
Consumption of both 50 and 200 g portions of spinach from the control and HU combinations does not cover the daily requirement of this micronutrient for adults, that is 150 µg I day−1. On the other hand, iodine-enriched plants would provide iodine amounts substantially exceeding RDA-I. It is worth mentioning that the 50-g portion of biofortified spinach was characterised by a HQgv value lower than 1, which represents a safe dose. The consumption of a 200-g portion would pose a risk of excessive iodine intake for humans and is above the tolerable upper intake level of 1100 µg I day−1.
Discussion
The average single serving of leafy vegetables (including spinach) is about 50 g f.w. (Voogt et al. Citation2010). The recommended daily intake of iodine for older children (aged > 12 years) and adults (with the exception of pregnant and nursing women) is 150 µg I day−1 (Andersson et al. Citation2007). Based on these values, it was calculated that a 50-g serving of fresh leaves of iodine-biofortified spinach would supply between 329.6 and 406.2 µg I 50 g−1 f.w. of leaves, respectively, for the combinations with KIO3 fertigation and KIO3 + HU 0.2. It is, therefore, a value greatly exceeding the recommended daily dose of iodine. For comparison, 50 g of leaves of the control plants contained 17.9 µg I. In practice, spinach biofortification with iodine by fertigation should be performed with considerably lower doses of KIO3 or with a lower frequency of plant watering with the tested solution. Determining the optimum iodine doses for fertigation purposes requires further studies on various types of soils and substrates, in soil and under covers.
The safety of plants subject to the described method of iodine application is substantiated by the lack of any visual symptoms of plants damage. If toxic levels of iodine accumulation were exceeded, leaf chloroses and necroses or dying of whole plants, as observed in other studies (Mackowiak et al. Citation2005; Hong et al. Citation2009) would have been noted. Intensified synthesis and accumulation of phenolic compounds in plants (Michalak Citation2006) is a frequent response to biotic and abiotic stress factors, including the application of excessive iodine doses in plant cultivation (Blasco et al. Citation2008). A similar or lower value of phenolic content, compared to the control, was noted in combinations with plant fertigation with KIO3 (with or without pre-sowing application of HU), indicating that an increased iodine concentration in leaves is not a stress factor for plants. The obtained results also demonstrated (in comparison to the control) a tendency of decreasing levels of phenolic compounds in plants treated only with HU at doses of 0.05 and 0.2 cm3 HU dm−3 soil. This suggests that, when applied prior to sowing, humic acids can improve plant growing conditions to some extent. They moderated the adverse impact of the habitat conditions on the plants.
It needs to be remembered that exogenous humic and fulvic acids, after introduction into the soil, affect its physicochemical properties (Delgado et al. Citation2002). In our research, the application of increasing HU doses combined with KIO3 fertigation (KIO3 + HU at doses of 0.0125, 0.05 and 0.2 cm3 dm−3 of soil) increased pH and Eh values, lowered total soil salinity (EC) and reduced the content of N–NO3 and Cl− in the soil. This may unambiguously indicate that due to the interaction between HU and IO3− ions (with K+ ions present), soil chemical properties change during cultivation, which, as a consequence, impacts the efficacy of plant biofortification with iodine. The simultaneous decrease of soil N–NO3 can be related to improved plant uptake of this macronutrient, reflected by increased N% d.w. accumulation in spinach. In the mentioned combinations, the best level of nitrogen assimilation into organic compounds was also observed as indicated by the lowest level of nitrate ions and increased N-total level in leaves. After HU introduction into the soil, particularly in combination with iodine, a substantial decrease of soil nitrate was noted accordingly. The process nitrate uptake by plants is accompanied by the excretion of OH− and HCO3− ions, which may result in increased soil pH and Eh levels in the combinations with KIO3 and KIO3 + HU.
Taking into consideration processes of iodine sorption with soil organic matter (described in the section ‘Introduction’), it could be expected that application of HU will limit its availability and uptake by spinach plants. After the sole application of HU in each of the doses, no statistically significant changes in iodine content were observed when compared to the control. Yet, iodine content in the spinach from these combinations (in comparison to the control) dropped by 36.5%, 26.6% and 44.7%, respectively, for HU 0.125, HU 0.05 and HU 0.2. The observed relations are further confirmed by significantly lower, compared to the control, values of iodine TF, which proves that pre-sowing HU application reduced the mobility of endogenous iodine in the soil.
The stimulating effects of exogenous humic and fulvic acids on iodine uptake by spinach with daily fertigation with KIO3 solution confirms the results of our earlier research (Smoleń et al. Citation2016b). We assume that IO3− ions introduced into the soil during fertigation could have been reduced to I2 or I− by soil microorganisms; this process has also been observed on root surfaces (Kato et al. Citation2013). These two iodine forms may have reacted with humic and fulvic acids present in HU. Most likely, it was I2 (as it is more reactive than IO3− or I−) that formed covalent bonds with aromatic rings of humic and fulvic acids. As a result, after applying KIO3 fertigation, the complex compounds produced (I + humic acids, I + fulvic acids or I + soil organic matter) could have facilitated iodine uptake by spinach. However, this is only a hypothesis and needs to be verified in further studies conducted on the same or on other species of higher plants. It should be mentioned that the stimulative action of HU on iodine uptake by plants is exerted only with fertigation with the IO3− form, but not with I−; importantly, it does not occur with single pre-sowing application of HU simultaneously with KI and KIO3 (Smoleń et al. Citation2016b).
Other causes of increased iodine accumulation in spinach after the application of KIO3 + HU (positively correlated with the HU dose) may have been: (a) direct stimulation by HU of IO3− uptake by roots and/or (b) reduction of IO3− fixation by HU directly as a result of HU interaction with Cl− ions (present in irrigation water). The reduction of Cl− levels in the soil was noted with increasing HU doses when applied with KIO3 fertigation. It was not, however, directly reflected by chloride contents in spinach plants. The application of the two highest doses of HU with KIO3 fertigation may have led to the leaching of Cl− to the bottom of the containers (impeding plant uptake). The other explanation may include chloride transformation into other speciation forms combining with other elements (compounds) present in the soil. The results of our research do not provide grounds for direct verification of the above assumptions. The possibility of chlorine fixation by soil organic matter has, however, been previously reported (Oberg & Sanden Citation2005).
The influence of humic and fulvic acids on limited solubility (bioavailability in the soil) and absorption of heavy metals by roots is widely recognised (Tyler & McBride Citation1982). So far, the interaction of exogenous humic and fulvic acids with iodine on the mineral composition of plants has not been described. Therefore, it is difficult to discuss the results of decreasing contents of numerous metallic elements (K, Fe, Mn, Zn, Al, Cd, Cr, Ce, Li, Ni, Co, La, Pb, Sc, Th, Y, Yb and V) in spinach plants from the combinations with KIO3 + HU when compared to the sole application of HU in all three doses. Further studies on processes in the soil environment are required and should focus on the application of iodine to soils enriched with humic and fulvic acids.
The substantial level of iodine biofortification of plants by KIO3 fertigation (applied individually and together with HU) was not followed by any negative consequences with respect to biomass yield or analysed characteristics of crop quality, that is, the content of NO3− and soluble sugars. The only exception was a decrease of soluble amino acids in spinach, with the potential causes discussed above. The distinguishingly high level of sugar accumulation in plants with KIO3 fertigation (without HU) may be the evidence of stimulating action of IO3− ions applied daily in low concentrations, which increases biosynthesis or reduces carbohydrate catabolism in plants. In this context, the presumed involvement of nitrate reductase (normally conducting NO3− to NO2− reduction) in the process of IO3− reduction to I− in plants should also be mentioned (Hung et al. Citation2005). Increased supply of IO3− ions for plants can slow down the rate of nitrate (V) reduction, thus increasing accumulation. It seems that the reduced nitrate (V) accumulation in spinach noted in our study was directly related to the lower content of N–NO3 in soil fertigated with KIO3 solution.
Suitability of I-enriched spinach for human consumption
The issue of food safety of iodine-enriched vegetables (including leafy vegetables) has been differentially described and defined (Voogt et al. Citation2010; Lawson et al. Citation2015, Citation2016; Smoleń et. al Citation2016a). The level of iodine in biofortified lettuce was lower (Voogt et al. Citation2010; Lawson et al. Citation2016), oscillated around or exceeded RDA-I (Lawson et al. Citation2016; Smoleń et. al Citation2016a), depending on agro-technical approaches to iodine application. It is worth mentioning that RDA-I was estimated based on the average consumption of 50 g f.w. (Voogt et al. Citation2010; Smoleń et. al Citation2016a) or 80–100 g f.w. of lettuce (Lawson et al. Citation2015, Citation2016).
For instance, Lawson et al. (Citation2016) specified ‘target range’ as ‘a projected I concentration of the harvested produce’ and defined it between 50 and 100 μg 100 g−1 f.w. lettuce. According to these authors, the consumption of 80–100 g f.w. of such enriched crop should ‘fill the dietary I gaps in the German population without running the risk of exceeding the upper tolerable I intake level amounting to 600–1100 mg I day–1’ (EFSA Citation2006). It needs to be underlined that a defined upper tolerable intake level of iodine concerns only mineral forms of this element. No maximum allowance intake of organically bound iodine (as in biofortified plants) has yet been determined. Kopeć et al. (Citation2015) revealed that iodine from enriched lettuce is less excreted and better accumulated in thigh muscles and livers of rats that mineral iodine (KI). It has also been shown that extracts from I-enriched plants may inhibit Caco-2 cancer cell proliferation as opposed to using KI (Koronowicz et al. Citation2016).
In the present study, the obtained level of iodine biofortification of spinach was too high in relation to adult RDA-I. A 50-g portion of fresh spinach poses no risk to consumer health as opposed to a 200-g portion, for which the values of HQgv exceeded 1. It needs to be mentioned, however, that spinach can be consumed fresh or after thermal processing, the latter leading to iodine losses. Properly adjusted temperature, time and method of thermal processing allow to limit iodine losses in vegetables, still maintaining them as an excellent source of iodine (Salau et al. Citation2010; Cerretani et al. Citation2014; Bieżanowska-Kopeć et al. Citation2016).
Conclusions
The stimulating impact of HU (humic and fulvic acids) applied with KIO3 fertigation on iodine uptake by plants was demonstrated. The effect of iodine fortification of spinach was most noticeable when KIO3 fertigation was applied on soil treated with the highest dose of HU (2 cm3 HU dm−3 of soil). Iodine TF values in combinations with KIO3 fertigation were independent of the used HU dose. We also consider it necessary to conduct further research into the processes occurring in the rhizosphere after application of exogenous humic and fulvic acids and iodine fertigation. Recognition of these relations seems particularly important due to the noted decrease in the contents of some nutrients, heavy metals and trace elements (K, Fe, Mn, Zn, Al, Cd, Cr, Ce, Li, Ni, Co, La, Pb, Sc, Th, Y, Yb and V) in spinach grown in HU-enriched soil fertigated with KIO3 as compared to the sole application of HU at all three doses.
A positive finding of this study is that the increased iodine content (along with increased iodine TF value, after the application of HU + KIO3 fertigation) was accompanied by no negative effect on the growth and biomass produced as well as on crop quality with respect to the contents of dry weight, soluble sugars, nitrate (V) and chlorides in the spinach plants. We further confirmed, based on the level of phenolic compound accumulation in plants, that the conducted process of iodine biofortification of spinach plants did not cause plant stress.
The obtained results indicate that a tested approach of combining KIO3 fertigation with pre-sowing application of HU may reduce the costs of iodine biofortification of crop plants. Pre-sowing introduction of HU applied with KIO3 fertigation is far more efficient in terms of increasing iodine contents in plants than sole KIO3 fertigation. It should, however, be aimed at lowering KIO3 concentrations in fertigation solutions or reducing the frequency of its administration so the total amount of iodine introduced into the soil would not exceed 1 mg I dm−3 soil. This would allow to increase iodine content in spinach to the level safe for those people who consume large amounts of fresh vegetables (>200 g f.w.). At the same time, the obtained range of iodine content in spinach should cover a substantial percentage of RDA-I, even after thermal processing.
Figure S1
Download TIFF Image (8.7 MB)Figure S2
Download TIFF Image (18.3 MB)Figure S3
Download TIFF Image (13.4 MB)Acknowledgements
The results of the study were reported to the Polish Patent Office on 30. 12. 2014, Patent application No. P.410807.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Andersson M, de Benoist B, Darnton-Hill I, Delange F. 2007. Iodine deficiency in Europe: a continuing public health problem. Geneva: World Health Organization.
- Ashworth DJ, Shaw G, Butler AP, Ciciani L. 2003. Soil transport and plant uptake of radio-iodine from near-surface groundwater. J Environ Rad. 70, 99–114. doi: 10.1016/S0265-931X(03)00121-8
- Bieżanowska-Kopeć R, Pysz M, Kapusta-Duch J, Kopeć A, Smoleń S, Koronowicz A, Piątkowska E, Rakoczy R, Skoczylas Ł, Leszczyńska T. 2016. The effects of peeling and cooking on the mineral content and antioxidant properties in carrots enriched with potassium iodate and/or selenite (SeIV) and selenite (SeVI). Intern J Food Sci Nut. Online. doi:10.1080/09637486.2016.1205550.
- Blasco B, Rios JJ, Cervilla LM, Sanchez-Rodrigez E, Ruiz JM, Romero L. 2008. Iodine biofortification and antioxidant capacity of lettuce: potential benefits for cultivation and human health. Ann Appl Biol. 152:289–299. doi:10.1111/j.1744–7348.2008.00217.x doi: 10.1111/j.1744-7348.2008.00217.x
- Cerretani L, Comandini P, Fumanelli D, Scazzina F, Chiavaro E. 2014. Evaluation of iodine content and stability in recipes prepared with biofortified potatoes. Intern J Food Sci Nut. 65:797−802. doi: 10.3109/09637486.2014.917155
- Charlton KE, Phil M, Gemming L, Yeatman H, Ma G. 2010. Suboptimal iodine status of Australian pregnant women reflects poor knowledge and practices related to iodine nutrition. Nutrition. 26:963–968. doi: 10.1016/j.nut.2009.08.016
- Delgado A, Madrid A, Kassem S, Andreu L, del Carmen del Campillo M. 2002. Phosphorus fertilizer recovery from calcareous soils amended with humic and fulvic acids. Plant and Soil. 245:277–286. doi: 10.1023/A:1020445710584
- EFSA. 2006. Tolerable upper intake levels for vitamins and minerals. Scientific committee on food – Scientific panel on dietetic products, nutrition and allergies. European Food Safety Authority 2006. [cited 2015 Mar 11]. Available from: http://www.efsa.europa.eu/sites/default/files/efsa_rep/blobserver_assets/ndatolerableuil.pdf
- Fuge R. 2013. Soils and iodine deficiency. In: Selinus O, Alloway B, Centeno JA, Finkelman RB, Fuge R, Lindh U, Smedley P, editors. Essentials of medical geology: revised edition. London: Elsevier Academic Press; p. 417–432.
- Hong CL, Weng HZ, Yan AL, Islam AU. 2009. The fate of exogenous iodine in pot soil cultivated with vegetables. Environ Geochem Health 31:99–108. doi: 10.1007/s10653-008-9169-6
- Hung CC, Wong GTF, Dunstan WM. 2005. Iodate reduction activity in nitrate reductase extracts from marine phytoplankton. Bull Mar Sci. 76:61−72.
- Iris. 2011. Integrated risk information system – database. Washington, DC: United States Environmental Protection Agency.
- Johnson CC. 2003. The geochemistry of iodine and its application to environmental strategies for reducing the risks from iodine deficiency disorders (IDD). British Geol Survey Comm. Report CR/03/057N, p. 48.
- Kashparov V, Colle C, Zvarich S, Yoschenko V, Levchuk S, Lundin S. 2005. Soil-to-plant halogens transfer studiem 1. Root uptake of radioiodine by plants. J Environ Rad. 79:187–204. doi: 10.1016/j.jenvrad.2004.06.005
- Kato S, Wachi T, Yoshihira K, Nakagawa T, Ishikawa A, Takagi D, Tezuka A, Yoshida H, Yoshida S, Sekimoto H, Takahashi M. 2013. Rice (Oryza sativa L.) roots have iodate reduction activity in response to iodine. Front Plant Sci. 4:227. doi: 10.3389/fpls.2013.00227
- Kessler J. 2009. Are there side effects when using supraphysiologic levels of iodine in treatment regimens? Comprehensive Handbook of Iodine – Nutritional, Biochemical, Pathological and Therapeutic Aspects. Red: Preedy V., Burrow G.N., Watson R.R. Academic Press: 107−118.
- Kopeć A, Piątkowska E, Bieżanowska-Kopeć R, Pysz M, Koronowicz A, Kapusta-Duch J, Smoleń S, Rakoczy R, Skoczylas Ł, Leszczyńska T, Ledwożyw-Smoleń I. 2015. Effect of lettuce biofortified with iodine by soil fertilization on iodine concentration in various tissues and selected biochemical parameters in serum of Wistar rats. J Funct Foods. 14:479–486. doi: 10.1016/j.jff.2015.02.027
- Koronowicz AA, Kopeć A, Master A, Smoleń S, Piątkowska E, Bieżanowska-Kopeć R, Ledwożyw-Smoleń I, Skoczylas Ł, Rakoczy R, Leszczyńska T, et al. 2016. Transcriptome profiling of Caco-2 cancer cell line following treatment with extracts from iodine-biofortified lettuce (Lactuca sativa L.). PLoS ONE. 11:e0147336. doi: 10.1371/journal.pone.0147336
- Lawson PG, Daum D, Czauderna R, Vorsatz C. 2016. Factors influencing the efficacy of iodine foliar sprays used for biofortifying butterhead lettuce (Lactuca sativa). J Plant Nut Soil Sci. 179:661–669. doi: 10.1002/jpln.201600213
- Lawson PG, Daum D, Czauderna R, Meuser H, Härtling JW. 2015. Soil versus foliar iodine fertilization as a biofortification strategy for field-grown vegetables. Front Plant Sci. 6. doi: 10.3389/fpls.2015.00450
- Mackowiak CL, Grossl PR, Cook KL. 2005. Iodine toxicity in a plant-solution system with and without humic acid. Plant Soil. 269:141–150. doi: 10.1007/s11104-004-0401-6
- Maillant S, Sheppard MI, Echevarria G, Denys S, Cessac EL. 2005. Transport and retention of aged anthropogenic iodine in a boreal peat bog. Radioprot. Suppl. 40:S237–S243. doi: 10.1051/radiopro:2005s1-037
- Melse-Boonstra A, Jaiswal N. 2010. Iodine deficiency in pregnancy, infancy and childhood and its consequences for brain development. Best Pract Res Clin Endocrin Metab. 24:29–38. doi: 10.1016/j.beem.2009.09.002
- Michalak A. 2006. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Polish J Environ Stud. 15:523−530.
- Muramatsu Y, Uchida S, Sriyotha P, Sriyotha K. 1990. Some considerations on the sorption and desorption phenomena of iodide and iodate on soil. Water Air Soil, Pollut. 49:125−138. doi: 10.1007/BF00279516
- Muramatsu Y, Yoshida S, Uchida S, Hasebe A. 1996. Iodine desorption from rice paddy soil. Water, Air and Soil Poll. 86:359–371. doi: 10.1007/BF00279167
- Nowosielski O. 1988. The rules in development of fertilizing strategies in horticulture. Warsaw: PWRiL Publisher. (In Polish).
- Oberg G, Sanden P. 2005. Retention of chloride in soil and cycling of organic matter-bound chlorine. Hydrol Processes. 19:2123–2136. doi: 10.1002/hyp.5680
- Pless JD, Benjamin Chwirka J, Krumhansl JL. 2007. Iodine sequestration using delafossites and layered hydroxides. Environ Chem Lett. 5:85–89. doi: 10.1007/s10311-006-0084-8
- PN-EN 15111. 2008. Foodstuffs – determination of trace elements – determination of iodine by ICP-MS (inductively coupled plasma mass spectrometry). Warsaw: Polish Committee of Standardization. (In Polish).
- PN-EN ISO 11732. 2005. Water quality. Determination of ammonium nitrogen. Method by flow analysis (CFA and FIA) and spectrometric detection. Warsaw: Polish Committee of Standardization. (In Polish).
- PN-EN ISO 13395. 2001. Water quality – determination of nitrite nitrogen and nitrate and the sum of both by flow analysis (CFA and FIA) and spectrometric detection. Warsaw: Polish Committee of Standardization. (In Polish).
- Ray P, Singhal SK, Datta SP, Rattan RK. 2013. Biofortification of Indian spinach (Beta vulgaris L. var. all green) with zinc application in acid and alkaline soils amended with organics. Agrochim. 57:149–165.
- Salau BA, Ajani EO, Odufuwa KT, Adegbesan BO, Soladoye MO. 2010. Effect of processing on iodine content of some selected plants food. African J Biotechnol. 9:1200–1204. doi: 10.5897/AJB09.1455
- Schlegel ML, Reiller P, Mercier-Bion F, Barré N, Moulin V. 2006. Molecular environment of iodine in naturally iodinated humic substances: Insight from X-ray absorption spectroscopy. Geochim Cosmochim Acta. 70:5536–5551. doi: 10.1016/j.gca.2006.08.026
- Smoleń S, Kowalska I, Czernicka M, Halka M, Kęska K, Sady W. 2016a. Iodine and selenium biofortification with additional application of salicylic acid affects yield, selected molecular parameters and chemical composition of lettuce plants (Lactuca sativa L. var. capitata). Front Plant Sci. 7:1553. doi:10.3389/fpls.2016.01553.
- Smoleń S, Ledwożyw-Smoleń I, Sady W. 2016b. The role of exogenous humic and fulvic acids in iodine biofortification in spinach (Spinacia oleracea L.). Plant Soil. 402:129–143. doi: 10.1007/s11104-015-2785-x
- Tyler LD, McBride MB. 1982. Influence of Ca, pH and humic acid on Cd uptake. Plant Soil. 64:259−262. doi: 10.1007/BF02184258
- Voogt W, Holwerda HT, Khodabaks R. 2010. Biofortification of lettuce (Lactuca sativa L.) with iodine: the effect of iodine form and concentration in the nutrient solution on growth, development and iodine uptake of lettuce grown in water culture. J Sci Food Agric. 90:906–913. doi:10.1002/jsfa.3902.
- Weng HX, Weng JK, Yan AL, Hong CL, Yong WB, Qin YC. 2008. Increment of iodine content in vegetable plants by applying iodized fertilizer and the residual characteristics of iodine in soil. Biol Trace Elem Res. 123:218–28. doi: 10.1007/s12011-008-8094-y
- Weng HX, Yan AL, Hong CL, Qin YC, Pan L, Xie LL. 2009. Biogeochemical transfer and dynamics of iodine in a soil–plant system. Environ Geochem Heath. 31:401–411. doi: 10.1007/s10653-008-9193-6
- White PJ, Broadley MR. 2009. Biofortification of crops with seven mineral elements often lacking in human diets – iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 182:49–84. doi: 10.1111/j.1469-8137.2008.02738.x
- Yamaguchi N, Nakano M, Tanida H. 2005. Transformation of iodine species in soil under upland field and submerged paddy field conditions. SPring-8 Res. Front. [cited 2012 Jun 24]. Available from: http://www.spring8.or.jp/pdf/en/res_fro/05/112-113.pdf
- Yoshida S, Muramatsu Y, Uchida S. 1992. Studies on the sorption of I− (iodide) and IO3− (iodate) onto andosols. Water Air Soil Poll. 63:321–329. doi: 10.1007/BF00475499