ABSTRACT
Flowers of Gold3 kiwifruit (Actinidia chinensis var. chinensis ‘Zesy002’, marketed as Zespri® SunGold Kiwifruit) were hand pollinated with pollen from different A. chinensis var. chinensis and A. chinensis var. deliciosa males. Fruit were evaluated for weight, firmness, dry matter content, soluble solids content, seed weight and seed number. Fourier Transform Infrared (FTIR) spectroscopy was used to estimate titratable acidity and the concentrations of the major fruit sugars and acids. Fruit from pollination by male cultivar M43 weighed significantly less than those from pollination by males M91 and M33. These differences could be explained by variation in total seed number between pollen treatments. However, the choice of pollen donor from among those tested appeared to have little impact on Gold3 fruit composition. Regardless of the pollen donor, larger fruit tended to have higher sugar and lower acid concentrations.
Introduction
It has long been reported in many crops that the choice of pollen donor can affect a range of important fruit traits including fruit set, size, shape, colour, developmental timing and composition (reviewed by Denney Citation1992). Effects on fruit traits can often be attributed largely to variation in seed number (Kahn et al. Citation1994; Mizrahi et al. Citation2004; Pritchard and Edwards Citation2006; Jalikop and Kumar Citation2007). However, there are reports of pollen donor effects that were independent of seed number (Colbert and de Oliveira Citation1990; Keulemans et al. Citation1996) or in single-seeded fruits including peach (Sharma et al. Citation1977), avocado (Degani et al. Citation1990), date (Zirari Citation2010) and mango (Dag et al. Citation1999; Honsho et al. Citation2012).
In New Zealand commercial kiwifruit orchards, a new yellow-fleshed tetraploid kiwifruit cultivar, Gold3 (Actinidia chinensis var. chinensis ‘Zesy002’, marketed as Zespri® SunGold Kiwifruit), has largely replaced the diploid cultivar ‘Hort16A’ (marketed as Zespri® Gold Kiwifruit), which proved to be susceptible to infection by Psa (Pseudomonas syringae pv. actinidiae) (Costa and Ferguson Citation2013). Gold3 growers plant mostly tetraploid A. chinensis var. chinensis male cultivars (eg M91 or M33) for bee-mediated pollination, and use stored pollen of hexaploid A. chinensis var. deliciosa male cultivars (eg ‘Chieftain’ or M56) for artificial pollination (Seal and Jia Citation2014).
There is some evidence that the choice of pollen donor can affect a range of important commercial fruit characteristics in kiwifruit (McNeilage et al. Citation1992; Chen et al. Citation1996; Qi et al. Citation2007; Seal et al. Citation2013a, Citation2013b). A preliminary hand pollination study of Gold3 fruit by Seal and Dunn (Citation2011) suggested that pollination by each of two tetraploid A. chinensis var. chinensis males or a commercial pollen mix from hexaploid A. chinensis var. deliciosa males produced much larger fruit than pollination by diploid A. chinensis var. chinensis males. However, there were no significant differences in fruit weight following the use of pollen from the tetraploid or hexaploid males. This was supported by the results of a larger hand pollination study by Seal and Jia (Citation2014) that showed no significant differences in fruit weight or dry matter (DM) content in Gold3 following pollination using pollen from commercial males of tetraploid A. chinensis var. chinensis or hexaploid A. chinensis var. deliciosa.
The aim of this study was to test the hypothesis that the use of different males for pollination of Gold3 kiwifruit can affect important fruit characteristics. Gold3 fruit produced following hand pollination with pollen from M91, M33 (tetraploid A. chinensis var. chinensis males), M36 or M43 (hexaploid A. chinensis var. deliciosa males) was compared. The comparison was extended beyond fruit weight, DM content and seed traits to include the major sugars and acids. The results are likely to be of interest to growers and pollen companies considering different pollination options and contribute to the wider understanding of the significance of pollen donor effects on fruit characteristics.
Materials and methods
In November 2013, newly opened flowers of A. chinensis Planch. var. chinensis Gold3 at the Te Puke Research Centre, New Zealand, were hand pollinated with pollen from male cultivars of tetraploid A. chinensis Planch. var. chinensis (M91 and M33) and hexaploid A. chinensis var. deliciosa A. Chev. (A. Chev.) (M36 and M43). Pollen of M91 and M33 was prepared from freshly collected flowers, whereas pollen of M36 and M43 (supplied by PollenPlus Ltd) was stored from the previous spring. The stainability of pollen samples (5 × 200 grains) with aniline (cotton) blue in lactophenol (Stanley and Linskens Citation1974) was: M91, 91.1%; M33, 98.6%; M36, 94.8%; and M43, 96.6%, indicating high potential viability of all samples. Four floral shoots were selected on each of 21 canes across three vines of Gold3 grafted in 2011 on A. chinensis var. deliciosa seedling rootstocks. On each cane, shoots of a similar size and structure were selected. Shoot type varied among canes, but most were short and self-terminating. Long shoots were trimmed to enable bagging of the flowers. The vines, trained on a pergola, received standard management, but no budbreak or fruit size enhancers were applied. One shoot on each cane was randomly assigned to each pollination treatment. If necessary, flower buds were thinned to ensure that there were equal numbers (from 4 to 6) of flowers on each shoot within each cane. Each shoot was enclosed in a paper bag before flowering to exclude insect pollinators and wind-blown pollen. For hand pollination, a vial containing pollen was inverted over the pistil of each female flower on a selected shoot to ensure that copious amounts of pollen were deposited. Therefore, the amount of pollen was not a limiting factor to pollination. Shoots were re-bagged after each hand pollination. Paper bags were finally replaced with net bags once all fruit had set. In total, about 100 flowers were pollinated with each pollen type.
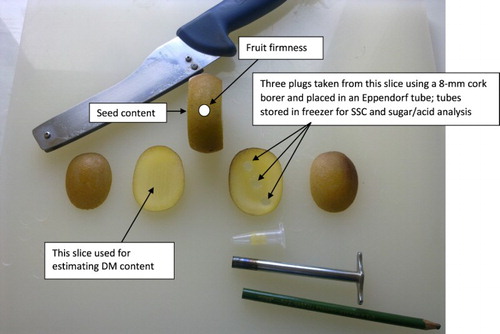
Fruit set was recorded on 20 December 2013. All fruit were harvested on 29 April 2014, when the soluble solids content (SSC) measured using a hand-held refractometer (Atago, Japan) of a sample of fruit from open pollination on the same vines had reached 10%. After the fresh weight (FW) of individual fruit was recorded, fruit were stored for 2 weeks at 0°C, then kept at 20°C until judged by touch to be within the industry-accepted eating firmness range of around 0.4–0.8 kgf. Fruit firmness (FF) of an exposed flesh surface on one side of each fruit was assessed using a GUSS motorised fruit texture analyser with a 7.9-mm diameter probe applied at a speed of 5 mm/s to a depth of 8 mm. Using a double-bladed knife, two longitudinal slices (2-mm thick) were cut through the outer pericarp on each side of the fruit, taking care not to remove any seed from the inner pericarp (). One longitudinal slice was used to determine the DM content by weighing and drying to constant weight after a 24-h period at 65°C. Note that this differs from the standard method for estimating DM content, in which an equatorial slice (including seeds) is used. An 8-mm diameter cork-borer was used to remove three plugs of flesh tissue along a diagonal transect across the second slice. All three plugs were placed into a single 1.5-mL Eppendorf tube. Each tube was labelled and placed in a −80°C freezer until all samples had been collected. The remaining central portion of each fruit (containing all the seeds) was placed in enzyme solution (5% Lafazym® extract) until the flesh had broken down. The seed was then separated from the pulp by filtering through muslin and then dried for 48 h at room temperature. The total seed weight (TSW) was recorded, then 50 randomly selected seed from each fruit were counted and weighed. Total seed number (TSN) was estimated by dividing the TSW by the average seed weight from the 50-seed sample. Individual seed weight (ISW) was estimated by dividing the TSW by the TSN.
Figure 1. Procedure for sampling fruit of Gold3 (Actinidia chinensis var. chinensis ‘Zesy002’) kiwifruit for firmness, dry matter (DM) content, soluble solids content (SSC), seed content and sugar/acid analysis.
Preparation for Fourier Transform Infrared (FTIR) analysis involved thawing tubes 12 at a time and liquefying the contents of each by crushing in situ with a small plastic pestle. Once the tube contents formed a smooth gel with no lumpy solids remaining, the tubes were centrifuged (3 min, 12,100 g) to separate solid tissue and a clear pale-yellow juice. Collection of the FTIR spectrum was performed as described by Clark (Citation2016).
Measurements for titratable acidity (TA) (g citric acid equivalents per 100 g juice (%)) and the concentrations (mg/100 mL juice) of free organic acids (malic, quinic and citric acid) and free sugars (glucose, fructose and sucrose) were determined by their spectral properties using Partial Least Squares (PLS) regression models developed specifically for Gold3. Details of FTIR models for Gold3 and other kiwifruit selections (Clark, unpublished data) will be the subject of a separate publication. However, prediction statistics, the R2 (goodness-of-fit between predicted and actual measurements) and the root mean square error of prediction (RMSEP) for each attribute in Gold3 juices are as follows: TA (98.8%, 0.04%); malic acid (91.0%, 25 mg/100 mL juice); quinic acid (96.6%, 25 mg/100 mL juice); citric acid (97.7%, 63 mg/100 mL juice); glucose (93.3%, 424 mg/100 mL juice); fructose (83.5%, 434 mg/100 mL juice); and sucrose (94.7%, 345 mg/100 mL juice). Full details concerning spectral measurements and model development for analysis of fruit juices by FTIR are described by Clark (Citation2016). The reference chemical characterisation methods underpinning this study were: TA determination by potentiometric titration with sodium hydroxide to pH 8.2 using a Mettler Toledo Autotitrator (dilution of 1 mL of juice to 30 mL water); 0.1 mL of juice was diluted with ethanol (1:5 v/v) for high-pH analysis of individual sugars by high-performance anion exchange chromatography (Dionex Corporation, USA) with pulsed amperometric detection (Rocklin and Pohl Citation1983); and 0.1 mL was taken for analysis of individual acids by stable isotope analysis using liquid chromatography–mass spectrometry (LC-MS; AB SCIEX 5500 QTRAP triple quadruple) based on the method of Ehling and Cole (Citation2011). Although SSC can be measured easily by FTIR, in this instance, the SSC values reported are those measured directly by standard refractometry.
The statistical analyses were carried out using the linear mixed model (LMM) procedure (Proc MIXED) in SAS software, Version 9.4 for Windows 7 (Copyright © 2008 SAS Institute Inc.). The experiment was set out as a randomised block design with canes within vine forming the blocks, and individual shoots within a cane forming the experimental units to which the pollen treatments were allocated. The fitted model included the pollen treatment as a fixed effect and the cane block factor as a random effect. Where necessary, predictor covariates were included in the model to test hypotheses relating to the direct and indirect effect of the pollen treatment. The usual model adequacy checks were performed by examining various plots (histograms, normal probability plots and scatter plots) of the residuals. For each response, the least square mean estimates of treatment effects and their standard errors were tabulated and multiple pairwise comparisons of treatment means were carried out by the Tukey–Kramer method.
Results
Overall, 93% of the flowers that were hand pollinated had retained fruit about 1 month after flowering and 86% retained fruit through to harvest (). Fruit losses appeared to be higher following pollination with pollen from the A. chinensis var. deliciosa males M36 and M43, but the numbers were too small to allow a reliable test of the significance of the differences among treatments. Some of the losses could be attributed to wind or pest damage.
Table 1. Fruit set of Gold3 (Actinidia chinensis var. chinensis ‘Zesy002’) kiwifruit about 1 month after hand pollination with pollen from males M91, M33 (tetraploid A. chinensis var. chinensis), M36 or M43 (hexaploid A. chinensis var. deliciosa).
summarises the physical and compositional data for the fruit of Gold3 following the different pollination treatments. Mean FW among pollen treatments ranged from 117 g (M43) to 128 g (M91). In New Zealand, a tray count of 30 for Class 1 Gold3 fruit includes fruit from 114 to 124 g. The mean firmness of the harvested fruit when sampled for compositional analysis was close to the target of 0.6 kgf with low standard deviations. DM contents ranged from 17.0% to 22.7%, SSC from 14.6% to 19.6% and TA values from 0.92% to 1.61%. The concentration ranges (mg/100 mL juice) for the free acids were 10–335 (malic), 644–1253 (quinic) and 586–1327 (citric), and for the free sugars were 4344–6681 (glucose), 4635–5894 (fructose) and 1216–4956 (sucrose).
Table 2. Summary of physical and compositional data of Gold3 (Actinidia chinensis var. chinensis ‘Zesy002’) kiwifruit pollinated using pollen from males M91, M33 (tetraploid A. chinensis var. chinensis), M36 or M43 (hexaploid A. chinensis var. deliciosa).
shows the significance of the overall pollen treatment effect for the different fruit traits and the results of pairwise comparisons between pollen treatments. The overall pollen treatment effect was significant for FW, quinic acid, TSN, TSW and ISW. While variation in FW among pollen treatments was significant (P ≤ 0.01), when the model was adjusted to take account of TSN or TSW the pollen treatment effect was no longer significant (P = 0.09). In pairwise comparisons, fruit from pollination by M43 weighed significantly less than those from pollination by M91 and M33. Other pairwise comparisons of FW between pollen treatments showed no significant differences. Fruit from pollination by M43 also had a significantly lower TSN, TSW and ISW than fruit from all other pollen treatments. Conversely, fruit from pollination by M91 had a significantly higher TSN and TSW than fruit from all other pollen treatments.
Table 3. P-values for overall pollen treatment effect and adjusted P-values for pairwise comparisons of means (Tukey–Kramer method) for physical and compositional traits between pollen treatments in Gold3 (Actinidia chinensis var. chinensis ‘Zesy002’) kiwifruit.
There were no significant differences between pollen treatments in FF, DM content, SSC, TA, or the concentrations of individual sugars and acids, except that fruit from pollination by M36 had a significantly higher quinic acid concentration than fruit from pollination by M33.
shows the overall correlations among traits. The data for all pollen treatments were combined, as correlations based separately on each pollen treatment (not shown) were similar to the overall correlations. TA was positively correlated with the individual acids, especially citric acid (r = 0.61), as expected, and negatively correlated with the individual sugars, especially glucose (r = −0.6) and sucrose (r = −0.49). TA was also moderately and negatively correlated with FW (r = −0.5). DM content was positively correlated with SSC (r = 0.87) and both were correlated with the individual sugars (DM, r = 0.6–0.72; SSC, r = 0.62–0.87). There was a moderate negative correlation between DM content and TA (r = −0.36). FW was positively correlated with TSW (r = 0.75), TSN (r = 0.68) and sucrose concentration (r = 0.56).
Table 4. Pearson correlation coefficients among traits for fruit of Gold3 (Actinidia chinensis var. chinensis ‘Zesy002’) kiwifruit (combined data for all pollen treatments).
Discussion
Two of the males compared in this study are hexaploid and belong to A. chinensis var. deliciosa. The other two males (and the female cultivar Gold3) are tetraploid and belong to A. chinensis var. chinensis. Pollination by the two A. chinensis var. chinensis males produced larger fruit than pollination by the A. chinensis var. deliciosa males. However, the difference in FW between pollination with M33 (A. chinensis var. chinensis) and M36 (A. chinensis var. deliciosa) was not significant so we cannot conclude that A. chinensis var. chinensis pollen is necessarily better than A. chinensis var. deliciosa pollen for pollinating Gold3. However, pollination with pollen from M43 (A. chinensis var. deliciosa) produced fruit with significantly lower FW, TSN, TSW and ISW than pollination with pollen from either M91 or M33 (A. chinensis var. chinensis). As New Zealand growers are paid more for larger fruit, a reduction of 11 g in mean FW following pollination by M43 rather than M91 could be commercially significant if reproduced consistently in a commercial environment with no compensatory increase in the quantity or quality (DM content) of fruit. As the differences in FW among pollen treatments could be accounted for by differences in TSN, these are not xenia effects (Denney Citation1992), but the results suggest that pollen from M43 may be less compatible with Gold3 than pollen from the A. chinensis var. chinensis males tested. In a similar Gold3 hand pollination study the previous season, Seal and Jia (Citation2014) found no significant differences in the FW of Gold3 fruit from pollination by M91 or the hexaploid A. chinensis var. deliciosa males ‘Chieftain’ and ‘King’, although pollination by M91 again produced the largest fruit. In this study, while pollination with M91 pollen produced fruit that weighed on average 4 g more than those following pollination with M33 pollen, the difference was not statistically significant. However, pollination by M91 resulted in significantly higher TSW and TSN than pollination by M33. This suggests that M91 pollen may be more compatible with Gold3 than M33 pollen.
The dominant sugars and free acids detected here are the same as those reported by Sivakumaran et al. (Citation2016) for Zespri® SunGold Kiwifruit (Gold3), Zespri® Sweet Green Kiwifruit, ‘Hayward’ and ‘Hort16A’, and by Nishiyama et al. (Citation2008) for ‘Hayward’, ‘Hort16A’ and other Actinidia genotypes.
We found almost no evidence that the use of the male donors tested differentially affected the DM content or sugar or acid composition of fruit of Gold3. FW was negatively correlated with TA. Therefore, as there were some significant differences in FW among the pollen treatments, we might have expected these differences to be reflected in differences in acidity measures. However, no significant differences were found, except that pollination by M36 gave a 5% higher quinic acid concentration than pollination by M33. While the relative amounts of acids within fruit pollinated by M36 had a slightly different profile, this had no overall effect on TA itself. This is not surprising since the proton-donating ability of quinic acid (a large molecular weight monoprotic acid) is low relative to those of citric acid, a triprotic acid, and maleic acid, a diprotic acid. The absence of any other significant variation in TA may be because the differences in FW among the pollen treatments were relatively small (about 9%), despite the use of genetically diverse males (different ploidies and varieties). Recent evidence suggests that TA measurements obtained from kiwifruit tissue frozen then thawed (the extraction method used in this study) may not correlate well with acidity as perceived by taste panellists (Ian Hallett et al., unpublished data). However, the method used here does allow valid comparisons of absolute acid concentrations not directly influenced by the firmness of the fruit or the way the juice was expressed.
In this study, pollen donor effects were investigated in only one location and one season. It is possible that environmental variation may affect the extent of pollen donor effects. In cherimoya, total SSC was affected by pollen source in a wet year but not in a dry year, possibly because of the added impact of moisture stress on fruit size and hence sugar concentrations (Jalikop and Kumar Citation2007). Pollen donor effects on fruit characteristics of the date palm (Phoenix dactylifera L.) are also reported to vary from year to year, suggesting an interaction with humidity, temperature or rainfall (Rezazadeh et al. Citation2013). Crop load, inter-fruit and inter-shoot competition may also affect fruit traits when different pollen treatments are applied within the same vine (Lai et al. Citation1990), and results may differ when a single pollination treatment is applied on a whole-vine basis in a commercial situation.
Conclusions
In pairwise comparisons of the four pollen donors tested, only M43 produced significant differences in FW following hand pollinations of Gold3. The mean FW following pollination by M43 was 11% and 6% lower than that following pollination by M91 and M33, respectively. These differences could be accounted for by differences in TSN. There were very few significant differences (one of 54 pairwise comparisons) in fruit composition between pollen treatments, despite differences in the ploidy of the pollen donors. Correlations showed that smaller fruit tended to have more acid, less sugar and a lower DM content, but this could not be linked to the choice of pollen donor. This suggests that future research aimed at improving the sugar/acid balance in Gold3 fruit should focus on reducing the effects of factors contributing to the production of small fruit (eg shading) rather than pollen donor effects. However, this does not negate the need to ensure effective pollination. On the contrary, the positive correlation between TSN and FW confirms the importance of good pollination, whichever pollen donor is used.
Acknowledgements
We thank Zespri Group Ltd for financial support and the Field Research Network and FASTLab staff at the Plant & Food Research Te Puke Research Centre for their technical assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Chen QH, Zhang ZH, Qin ZQ, Jiang YC. 1996. Studies on male species selection and pollen induction of Jinkui kiwifruit (in Chinese). China Fruits. 2:23–24.
- Clark CJ. 2016. Fast determination by Fourier-transform infrared spectroscopy of sugar-acid composition of citrus juices for determination of industry maturity standards. NZ J Crop Hort Sci. 44:69–82. doi: 10.1080/01140671.2015.1131725
- Colbert S, de Oliveira D. 1990. Influence of pollen variety on raspberry (Rubus idaeus L.) development. J Hered. 81:434–437. doi: 10.1093/oxfordjournals.jhered.a111021
- Costa G, Ferguson AR. 2013. Bacterial canker of kiwifruit: response to a threat. Acta Hort. 1095:27–40. DOI:10.17660/ActaHortic.2015.1095.2.
- Dag A, Gazit S, Eisenstein D, El-Batsri R, Degani C. 1999. Effect of the male parent on pericarp and seed weights in several Floridian mango cultivars. Scientia Hort. 82:325–329. doi: 10.1016/S0304-4238(99)00059-X
- Degani C, Goldring A, Adato I, El-Batsri R, Gazit S. 1990. Pollen parent effect on outcrossing rate, yield, and fruit characteristics of ‘Fuerte’ avocado. HortScience. 25:471–473.
- Denney JO. 1992. Xenia includes metaxenia. HortScience. 27:722–728.
- Ehling S, Cole S. 2011. Analysis of organic acids in fruit juices by liquid chromatography – mass spectrometry: an enhanced tool for authenticity testing. J Agric Food Chem. 57:2035–2039.
- Honsho C, Inada M, Yuji K, Tojiki M, Kurogi S, Kanzaki S, Tetsumura T. 2012. Efficiency of hybrid formation by open-pollination of two cultivars in a closed plastic house and the effect of the male parent on fruit characteristics in mango. J Japan Soc Hort Sci. 81:27–34. doi: 10.2503/jjshs1.81.27
- Jalikop SH, Kumar R. 2007. Pseudo-xenic effect of allied Annona spp. pollen in hand pollination of cv. ‘Arka Sahan’ [(A. cherimola × A. squamosa) × A. squamosa]. HortScience. 42:1534–1538.
- Kahn TL, Adams CJ, Arpaia ML. 1994. Paternal and maternal effects on fruit and seed characteristics in cherimoya (Annona cherimola Mill.). Scientia Hort. 59:11–25. doi: 10.1016/0304-4238(94)90087-6
- Keulemans J, Brusselle A, Eyssen R, Vercammen J, van Daele G. 1996. Fruit weight in apple as influenced by seed number and pollinizer. Acta Hort. 423:201–210. doi: 10.17660/ActaHortic.1996.423.26
- Lai R, Woolley DJ, Lawes GS. 1990. The effect of inter-fruit competition, type of fruiting lateral and time of anthesis on the fruit growth of kiwifruit (Actinidia deliciosa). J Hort Sci. 65:87–96.
- McNeilage MA, Seal AG, Steinhagen S, McGowan J. 1992. Evaluation of kiwifruit pollinizers. Acta Hort. 297:277–282. doi: 10.17660/ActaHortic.1992.297.36
- Mizrahi Y, Mouyal J, Nerd A, Sitrit Y. 2004. Metaxenia in the vine cacti Hylocereus polyrhizus and Selenicereus spp. Ann Bot. 93:469–472. doi: 10.1093/aob/mch055
- Nishiyama I, Fukuda T, Shimohashi A, Oota T. 2008. Sugar and organic acid composition in the fruit juice of different Actinidia varieties. Food Sci Technol Res. 14:67–73. doi: 10.3136/fstr.14.67
- Pritchard KD, Edwards W. 2006. Supplementary pollination in the production of custard apple (Annona sp.) – the effect of pollen source. J Hort Sci Biotech. 81:78–83. doi: 10.1080/14620316.2006.11512032
- Qi XJ, Han LX, Li M, Xu SK, Zhu YS, Li WX, Qiao SR. 2007. Studies on pollen xenia of kiwifruit. J Fruit Sci. 24:774–777.
- Rezazadeh R, Hassanzadeh H, Hosseini Y, Karami Y, Williams RR. 2013. Influence of pollen source on fruit production of date palm (Phoenix dactylifera L. cv. Barhi) in humid coastal regions of southern Iran. Scientia Hort. 160:182–188. doi: 10.1016/j.scienta.2013.05.038
- Rocklin RD, Pohl CA. 1983. Determination of carbohydrates by anion exchange chromatography with pulsed amperometric detection. J Liq Chromatogr. 6:1577–1590. doi: 10.1080/01483918308064876
- Seal A, Dunn J. 2011. Selecting the best males for Gold3. NZ Kiwifruit J. 216:53–55.
- Seal A, Jia Y. 2014. Ploidy: what is it and why is it important? NZ Kiwifruit J. 223:17–18.
- Seal AG, Dunn JK, De Silva HN, McGhie TK, Lunken RCM. 2013a. Choice of pollen parent affects red flesh colour in seedlings of diploid Actinidia chinensis (kiwifruit). NZ J Crop Hort Sci. 41:207–218. doi: 10.1080/01140671.2013.803129
- Seal AG, Dunn JK, Jia YL. 2013b. Pollen parent effects on fruit attributes of diploid Actinidia chinensis ‘Hort16A’ kiwifruit. NZ J Crop Hort Sci. 41:219–229. doi: 10.1080/01140671.2013.803130
- Sharma SD, Tripathi SN, Dhuria HS, Chauhan JS. 1977. Note on the effect of pollen parent on physico-chemical properties of some peach varieties. Indian J Agri Sci. 47:632–634.
- Sivakumaran S, Huffman L, Sivakumaran S, Drummond L. 2016. The nutritional composition of Zespri® SunGold Kiwifruit and Zespri® Sweet Green Kiwifruit. Food Chemistry. DOI:10.1016/j.foodchem.2016.08.118.
- Stanley RG, Linskens HF. 1974. Pollen: biology, biochemistry, management. In: Chapter 6, Viability tests. Berlin: Springer Verlag; p. 67–86.
- Zirari A. 2010. Effects of time of pollination and of pollen source on yield and fruit quality of ‘Najda’ date palm cultivar (Phoenix dactylifera L.) under Draa valley conditions in Morocco. Acta Hort. 882:89–94. doi: 10.17660/ActaHortic.2010.882.9
