ABSTRACT
The commercial potato Solanum tuberosum L. 2n = 4x = 48 is a tetrasomic (AAAA) species, with a narrow genetic base. Wild genomic allotetraploid species such as S. stoloniferum (AABB) have great importance in potato breeding, but getting homoploid hybrid by direct crossing between the cultivated potato and wild allotetraploid species is difficult due to genic imbalance in endosperm. In this study chromosome pairing during metaphase I of meiosis in three exceptional homoploid hybrids between S. stoloniferum × S. tuberosum was analysed. The main chromosome configuration at metaphase I was bivalent and univalent with the frequencies of 15.17 and 6.56, respectively. Low frequency of trivalent (1.88) and tetravalent (0.7) was also observed. Further unequal segregation in first and second meiotic division was observed. Results indicated that although one genome of stoloniferum was different from its other genome as well as those of tuberosum, intergenomic pairing occurred between A and B genomes.
Introduction
The commercial potato Solanum tuberosum L. 2n = 4x = 48 is an autotetraploid species or at least had tetrasomic behaviour. Despite having a large number of wild relatives, presumably more than any other major crops, the potato has a narrow genetic base, which limits the progress of new cultivar development (Brown Citation1993b; Ortiz Citation2001; Tome et al. Citation2007). Potatoes (tuber-bearing Solanum spp.) exist in five cytological levels, including 2x, 3x, 4x, 5x and 6x with the basic chromosome number of x = 12. Approximately 15% of wild potato species are tetraploid, but unlike the cultivated ones that are considered as an autotetraploid, most of them are segmental or genomic allotetraploid (Hawkes Citation1992, Citation1994). Species in the series of Longipedicellata are the example of such typical wild allotetraploid potatoes that are distributed from the south states of USA to Mexico. Species in this series have some characteristics that are important for potato breeders, including resistance to late blight, Ervinia, potato virus Y, heat and drought tolerance and lack of enzymatic tuber browning (Hawkes Citation1994; Brown et al. Citation2004). However, in spite of having the same ploidy level with the cultivated potato, getting homoploid hybrids (in this case 4x) from crossing these two groups of potato are very difficult because there is a post zygotic crossing barrier referred as endosperm balance number (EBN) (Ortiz and Ehlenfeldt Citation1992; Hermsen Citation1994; Carputo et al. Citation1999; Jackson and Hanneman Citation1999). Therefore, the incorporation of these species into potato breeding is done using bridge species and ploidy manipulation, which is tedious and time-consuming (Watanabe et al. Citation1994; Ortiz Citation2001; Brown et al. Citation2004).
However, with making numerous controlled pollinations, it is not impossible to find the exceptional homoploid hybrids (in this case 4x) in the crosses involved the parents with same ploidy but with different EBN (Bamberg and Hanneman Citation1990; Janssen et al. Citation1997; Jansky and Hamernik Citation2009). The author in this manner has been obtained few homoploid hybrids between cultivated potato and allotetraploid species in the Longipedicellata series, three of these hybrids belonged to the crosses of S. stoloniferum × S. tuberosum. As mentioned, the S. tuberosum is an autotetraploid with some multivalent in meiosis and genomic formula of AAAA and S. stoloniferum is an allotetraploid with the genomic formula of AABB and regular meiosis with 24 bivalent (Swaminathan Citation1954b; Hawkes Citation1994). Therefore, the aforementioned hybrids would be auto-allotetraploid with the genomic formula of AAAB. Although based on genomic composition, it is expected that the hybrid would be sterile or with very low fertility; interestingly they had sufficient fertility to obtain further progeny in crosses. The production of hybrid from the wide crosses is the first step in using the germplasm of wild relatives in crop breeding. However, the subsequent fertility of hybrids, and the occurrence of recombination between the genomes of wild and cultivated parents in hybrids is an important aspect for getting the desirable genotypes in segregating progeny with the traits of interest for breeders and without the undesirable ones (Brown Citation1993a; Ramanna and Jacobsen Citation2003). The observation of chromosomes’ pairing behaviour in hybrids during meiosis can aid in the understanding of the likelihood of the utility of hybrids for subsequent breeding; for instance, lack of pairing between chromosomes of hybrids and appearance of multiple univalents will indicate sterility of hybrids along with the lack of recombination between the genomes of wild and cultivated parents in the affected chromosomes. Conversely, polyvalent formation, although it will facilitate the recombination between the wild and cultivated parents chromosomes, does not guarantee the equal chromosome segregation and may result in semi sterility.
Although the potatoes have been the subjects of many cytogenetical studies, to the best knowledge of present authors there is just one study (Wangenheim Citation1955) about the chromosome pairing in tetraploid hybrids between the genomic allotetraploid (AABB) and autotetraploid (AAAA) cultivated potatoes. So the aim of this study is to clarify the meiotic chromosome pairing behaviour of A and B genome chromosomes in three S. stoloniferum × S. tuberosum hybrids; this is important for the development of future potato cultivars from these hybrids as well as having the potential to gain further insights into the relationships between A and B genomes in tuber-bearing Solanum.
Material and methods
The three interspecific hybrids used in this study were from the direct crossing between S. stoloniferum × S. tuberosum (Panahandeh et al. Citation2010); hereinafter, they will be referred as stbr1, stbr 2 and stbr 3. The general aspects of meiosis in one of them (stbr1) has been previously studied by the present author and his co-workers (Panahandeh et al. Citation2008), but in that study, the chromosome pairing behaviour during metaphase I of meiosis was not analysed.
The tubers of three hybrids were planted in Khalat-poushan Research Station of Faculty of Agriculture, University of Tabriz. Young flower buds were collected and fixed in 6:3:1 ethanol:chloroform:acetic acid (V/V/V) for two days and then, rinsed with distilled water and transferred to 70% ethanol and kept in the refrigerator until being studied.
For study after washing the flower buds with distilled water, a piece of anther was squashed in one drop of 1% acetoorcein (w/v) and observed under the low magnification; if they were at meiotic division stages, the remaining anthers of the same flower bud were hydrolysed in 1N HCl for 8–10 min at 60°C, then washed thoroughly with distilled water and transferred to 2% aceto-iron-haematoxylin for 12 h. The stained anthers were afterwards rinsed with distilled water for some minutes. The microscopic slides were made by squashing the anthers in one drop of 45% of acetic acid and studied by Nikon 1200 light microscope. The photomicrographs were captured by CCD Nikon digital camera from suitable plates. Finding the suitable metaphase plate for precise detection of chromosomes pairing was difficult and more than 2000 microscopic slides were prepared for this study.
Results
shows the results of analysing the chromosome pairing behaviour during metaphase I in the three auto-allotetraploid interspecific hybrid potatoes. As seen, the predominant pairing configurations were bivalent ( (A,B)) in all three hybrids and the frequency of bivalents was similar in three interspecific hybrids (15.12, 14.4 and 16). Univalents were frequently observed in these hybrids; the highest frequency of the univalents was observed in stbr1 (9.08 with the range of 3–17). stbr2 and stbr3 had similar frequencies of univalent, 5.6 and 5, respectively. Stbr1 and stbr3 had similar frequencies of trivalents (2.08 and 2.7, respectively). In these two hybrids (stbr1 and stbr3), the lowest observed pairing configurations were quadrivalents, but in stbr2, the frequency of tetravalent was slightly higher than that of trivalent (1 and 0.875, respectively).
Figure 1. Chromosome pairing and segregation in interspecific auto-allotetraploid hybrid potato. A, Metaphase I in stbr1 with 1IV, 5III, 11II, 4I. B, Metaphase I in stbr3 with1III, 21II and 3I. C, Late anaphase I and early telophase I with unequal (22–26) distribution of chromosomes in stbr1. D, Telophase II in stbr2 with 23–23/25–25 chromatids segregation.
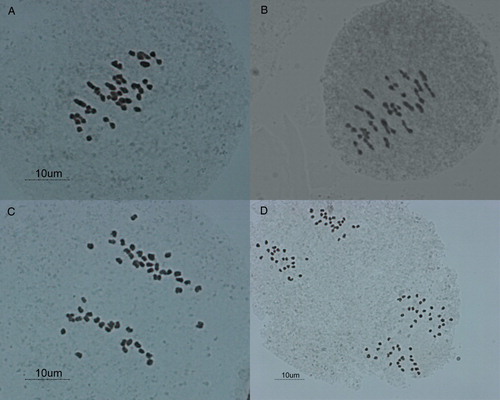
Table 1. Chromosomes pairing configuration in metaphase I of three tetraploid interspecific hybrids between S. stoloniferum × S. tuberosum (stbr).
The other meiotic stages appeared similar in the three hybrids (data were not shown) with laggard chromosomes and chromatids were observed with the relatively high incidence in anaphase and telophase (I and II).
Unequal segregation was observed in all three hybrids ((C,D)), but the precise analysis was carried out just in stbr1 and stbr 3, the results of which are shown in .
Table 2. The percent of different segregation in first meiotic division of two interspecific hybrid potatoes.
Discussion
Chromosome pairing is a key point of meiosis and important from the genetics, plant breeding, crop evolution and genome analysis viewpoints. Although genes that control chromosomes pairing (Sears Citation1976; Singh Citation2003) have been identified, genome affinity also plays an important role in chromosome pairing. Results obtained in this study indicated that some pairing between the two different genomes (A and B) of tuber-bearing Solanums occurred in these hybrids.
The results obtained from the chromosome pairing analysis were in accordance with those of the meiosis in an auto-allotetraploid AAAB genome formula. Results showed that the mean bivalent frequency was more than 12 (the basic chromosome number in Solanum) in three hybrids; on other hand, the presence of the univalent (9.06, 5.6 and 5) in hybrids emphasised the presence of one different genome (B) that was different in some degree from the basic genome (A) of tuber-bearing Solanum species. As demonstrated, the mean frequency of the univalent was less than 12 (the basic chromosome number in potatoes), indicating that allosyndesis or intergenomic pairing (between A and B genomes) had occurred in the stoloniferum× tuberosum hybrids. In addition, the bivalent number of more than 12 and presence of some multivalent (III, IV) are also the future evidence for the allosyndesis between A and B genomes. The presence of two different genomes in S. stoloniferum was reported by potato cytologist based on its regular meiosis and meiotic behaviour of its triploid hybrids by diploid potatoes (Swaminathan Citation1954b; Matsubayashi Citation1991; Hawkes Citation1994). Recently, Pendinen et al. (Citation2008) confirmed the presence of two different genomes in S. stoloniferum by molecular cytogenetic techniques. The occurrence of allosyndesis is important from the plant breeding point of view and provides an opportunity for getting chromosomes that are the mosaic of two genomes in segregating progenies (Ramanna and Jacobsen Citation2003).
Although we did not use parental species (tuberosum and stoloniferum) for the comparison in this study, based on the results in the literature, the meiotic behaviour of the hybrids was quite different from that of the wild parent (stoloniferum), which have regular chromosome pairing during meiosis with nearly 24 bivalents observed (Swaminathan Citation1954b; Hawkes Citation1994, also see Dvorak Citation1983, for the review) and was somewhat closer to the chromosome pairing behaviour in S. tuberosum, in which some multivalents (III and IV) along with a low number of univalents were reported. However, the results reported for tuberosum cultivars are variable and were not constant but the frequency of multivalent reported for tuberosum in most cases was more than the one observed for the hybrids studied here (See Swaminathan Citation1954a, Citation1954b; Watanabe et al. Citation1994). It seems that the most predominant difference in the chromosome pairing of hybrids studied here from that of the cultivated potato was in the frequency of univalent. Swaminathan (Citation1954a, Citation1954b) observed a frequency of univalent in S. tuberosum which did not exceed 2.22, while in these hybrids, it was greater than five.
In the literature, there is very rare information about the chromosomes pairing in tetraploid hybrids between the genomic allotetraploid (AABB) and cultivated autotetraploid potato. This is because of the difficulty of producing such hybrids. Wangenheim (Citation1955) studied meiosis in one tetraploid F1 hybrid from S. tuberosum × S. stoloniferum. The average chromosomes association was 7.62 I, 18.28 II, 0.74 III and 0.40 IV. This is close to our results but the multivalent frequency in our material slightly is higher. Watanabe et al (Citation1994) by embryo rescuing obtained hybrids between S. tuberosum and S. acaule – a wild segmental allotetrploid (AAÁÁ) species (Matsubayashi Citation1991; Camadro et al. Citation1992); they are investigated meiosis in two AAAÁ genome hybrid clones, the frequency of univalent, bivalent, trivalent and quadrivalent in these hybrids were (10.09, 9.8), (17.1, 17), (0.4, 0.3) and (0.7, 0.4) respectively. These results are very close to that of the Wangenheim (Citation1955) in tuberosum × stoloniferum hybrid.
Although with general consideration, the result of these studies is close to each other, but taking in account that S. acaule is a segmental allotetraploid, it is expected that the hybrids of tbr ×acl have more multivalent than sto × tbr; however, the results show that the frequency of multivalent in sto × tbr hybrids studied here is slightly higher than tbr × acl, while the Wangenhaim (Citation1955) results were so close to Watanabe et al. (Citation1994) results. Such difference may be attributed to the difference of genetic background of these exceptional hybrid groups; for instance, the hybrids studied by Watanabe et al. (Citation1994) and also by Wangenheim (Citation1955) had tuberosum cytoplasm, but our hybrid cytoplasm was from wild species (stoloniferum).
The incidence of laggard chromosome and chromatids in first and second anaphase was not unexpected based on the genomic formula as was the prevalence of univalents and multivalents especially trivalents. The occurrence of trivalents and univalents would result in unequal segregation in anaphase I and accordingly anaphase II. Khazanedari and Jones (Citation1997) concluded that univalents are the main cause of meiotic segregation disorders in leek although multivalents also contribute to this phenomenon.
In spite of relatively high meiotic irregularity in the studied hybrids, they had sufficient pollen stainability (more than 30%) as well as female fertility, but backcrosses with the cultivated parent were successful only in one direction and in spite of producing enough open pollinated and selfing fruit and seeds, crossing them as pollen donor with cultivated parents was unsuccessful (Panahandeh et al. Citation2008). Indeed, precise chromosome number analysis in the selfed and BC1 progeny of these hybrids was in agreement with the results of chromosome pairing and unequal segregation observed here and the author finds these hybrids as a good source of aneutetraploid progeny in potato (Panahandeh Citation2013).
Triploid hybrids between the species in Longipedicellata series with diploid species that were closely related to the cultivated potato (AA) had 9–10 univalents and bivalents (see Dvorak Citation1983). Considering that the chromosome complement of sto × tbr (AAAB) and triploids of allotetraploid sp. × diploid species hybrids (AAB) had equal degrees of homology, it seems that tetraploid hybrids demonstrated more affinity for intergenomic pairing. This was the situation that Watanabe et al. (Citation1994) also observed and pointed out that the hybrids between allotetraploid and cultivated tetraploid species, with having the genome of one species as diploid (here tuberosum AA genome) and the other species as haploid (stoloniferum AB) genome provides more opportunity for intergenomic pairing because the presence of two genomes of cultivated potato (AA) conserves some normal chromosome behaviour, while the genomic difference between the two genomes of S. stoloniferum would enhance heterogenetic chromosome pairing with the chromosomes of S. tuberosum.
Relatively normal chromosomal behaviour in these hybrids contributed to the fertility of them and provides an opportunity for producing aneuploid stocks especially with the addition chromosomes of B genome that could be used in the production of alien addition lines (Panahandeh, Citation2013). With the ability to use these hybrids as the female parent with cultivated potato, it is possible to continue the backcross to produce the alloplasmic lines with stoloniferum cytoplasm along with tuberosum nucleus.
It is believed that genome differentiation has not been extensive in tuber-bearing Solanum, so the tuber-bearing Solanum species for maintaining its integrity is developed pre and post zygotic interspecific cross incompatibility (Camadro et al, Citation2004). Nevertheless, Pendinen et al. (Citation2008) used molecular cytogenetic methods and showed the differentiation between A and B genomes of the species in Longipedicellata series and their putative diploid ancestors, but also pointed out some of the differentiation of which may occur after polyploidization. On the other hand, Dvorak (Citation1983) reviewed the literature related to chromosome-pairing studies in Solanum and concluded that there was no structural genomic differentiation on tuber-bearing Solanum, and the regular bivalent formation in polyploid species such as stoloniferum had the genetic control that suppressed the homologous pairing in polyploidy status but not in diploid. Based on the results of this study, it seems that as stated by Hawkes (Citation1994), although in most cases, there is no gross structural genomic differentiation between the genome of tuber-bearing Solanum, but cryptic structural differentiation exists between A and B genomes in Longipedicellata species. However, auto-allotetraploid hybrids such as those studied here facilitate the homologous paring between A and B genomes in the polyploid nature.
Acknowledgments
The author wishes to thank his colleague Dr F. Zare-Nahandi for improving the manuscript English.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Bamberg JB, Hanneman RE. 1990. Allelism of endosperm balance number (EBN) in Mexican tuber-bearing Solanum species. Theor Appl Genet. 80:161–166. doi: 10.1007/BF00224380
- Brown CR. 1993a. Characteristics of 2n plollen producing triploid hybrids between Solanum stoloniferum and cultivated diploid potatoes. Am Potato J. 65:75–84. doi: 10.1007/BF02867455
- Brown CR. 1993b. Origin and history of the potato. Am Potato J. 70:363–373. doi: 10.1007/BF02849117
- Brown CR, Mojtahedi H, Bamberg J. 2004. Evaluation of Solanum fendleri as a source of resistance to Meloidogyne chitwoodi. Amer J Potato Res. 81:415–419. doi: 10.1007/BF02870202
- Camadro EL, Carputo D, Peloquin SJ. 2004. Substitutes for genome differentiation in tuber-bearing Solanum: interspecific pollen-pistil incompatibility, nuclear-cytoplasmic male sterility, and endosperm. Theoretical and Applied Genetics. 109:1369–1376. doi: 10.1007/s00122-004-1753-2
- Camadro EL, Masuelli RW, Cortes MC. 1992. Haploids of the wild tetraploid potato Solanum acaule ssp. acaule: generation, meiotic behaviour, and electrophoretic pattern for the aspartate aminotransferase system. Genome. 35:431–435. doi: 10.1139/g92-064
- Carputo D, Monti L, Werner JE, Frusciante L. 1999. Uses and usefulness of endosperm balance number. Thor Appl Genet. 98:478–484. doi: 10.1007/s001220051095
- Dvorak J. 1983. Chromosome pairing in polyploid species of Solarium, sect. Petota. Can J Genet Cytol. 25:530–539. doi: 10.1139/g83-080
- Hawkes JG. 1992. Biosystematics of the potato. In: Harris P, editor. The potato crop. London: Chapman –Hall; p. 13–64.
- Hawkes JG. 1994. Origins of cultivated potatoes and species relationships. In: Bradshow JE, Mackay GR, editor. Potato genetics. Wallingford: CAB Int.; p. 3–43.
- Hermsen JGT. 1994. Introgression of genes from wild species, including molecular and cellular approaches. In: J. E. Bradshow, G. R. Mackay, editors. Potato genetics. Wallingford: CAB Int.; p. 516–538.
- Jackson SA, Hanneman Jr RE. 1999. Crossability between cultivated and wild tuber-and non-tuber-bearing Solanums. Euphytica. 109:51–67. doi: 10.1023/A:1003710817938
- Jansky SH, Hamernik AJ. 2009. The introgression of 2x 1 EBN solanum species into the cultivated potato using Solanum verrucosum as a bridge. Genetic Resources and Crop Evolution. 56:1107–1115. doi: 10.1007/s10722-009-9433-3
- Janssen GJ, Van Norel A, Verker- Baker B, Janssen R, Hoodgendoorn J. 1997. Introgression of resistance to root – knot nematodes from wild central American Solanum species into S. tuberosum subsp. tuberosum. Theor Appl Genet. 95:490–496. doi: 10.1007/s001220050588
- Khazanehdari KA, Jones GH. 1997. The causes and consequences of meiotic irregularity in the leek (allium ampeloprasum ssp. Porrum) implication for fertility, quality and uniformity. Euphytica. 93:313–319. doi: 10.1023/A:1002914808150
- Matsubayashi M. 1991. Phylogenetic relationships in the potato and its related species. In: Tsuchiya T, Gupta PK, editors. Chromosome engineering in plants: genetics, breeding, evolution, part B. New York: Elsevier; p. 93–118.
- Ortiz R. 2001. The state of the use of potato genetic diversity. In: H. D. Cooper, C. Spillane, T. Hodgkin, editors. Broadening the genetic base of crop production. Oxford: CAB Int.; p. 181–200.
- Ortiz R, Ehlenfeldt MK. 1992. The importance of endosperm balance number potato breeding and evolution of tuber-bearing Solanum species. Euphytica. 60:105–113.
- Panahandeh J. 2013. Aneuploidy in the progenies of inter-specific hybrid (AAAB genome) tetraploid potatoes. Journal of Horticultural Science & Biotechnology. 88(6):710–714. doi: 10.1080/14620316.2013.11513028
- Panahandeh J, Valizadeh M, Khosroshahly M, Rahimzadeh Khoei F. 2010. The investigation of pollen-pistil relationship in crosses between two subspecies of cultivated potato with wild allotetraploid relatives. Agricultural Biotechnology. 9(2):55–63. (in Persian).
- Panahandeh J, Valizadeh M, Khosroshahly M, Yermshin AP, Khoei FR, Mahna N. 2008. Microsporogenesis and crossing behavior of a tetraploid, interspecific inter-EBN hybrid potato. Scientia Horticulturae. 116:348–353. doi: 10.1016/j.scienta.2008.02.006
- Pendinen G, Gavrilenko T, Jiang J, Spooner DM. 2008. Allopolyploid speciation of the Mexican tetraploid potato species solanum stoloniferum and S. hjertingii revealed by genomic in situ hybridization. Genome. 51(9):714–720. doi: 10.1139/G08-052
- Ramanna MS, Jacobsen E. 2003. Relevance of sexual polyploidization for crop improvement – a review. Euphytica. 133:3–8. doi: 10.1023/A:1025600824483
- Sears ER. 1976. Genetic control of chromosome pairing in wheat. Annu Rev Genet. 10:31–51. doi: 10.1146/annurev.ge.10.120176.000335
- Singh RJ. 2003. Plant cytogenetics, 2nd Ed. Boca Raton, FL: CRC Publication.
- Swaminathan MS. 1954a. Microsporogenesis in some commercial potato varities. Journal of Heredity. 45(6):265–272. doi: 10.1093/oxfordjournals.jhered.a106489
- Swaminathan MS. 1954b. Nature of polyploidy in some 48- chromosome species of the genus Solanum, section Tuberarium. Genetics. 39:59–76.
- Tomé LGO, Davide LC, Pinto CABP, Alves AA, Salgado CC. 2007. Pollen viability and meiotic analysis of Solanum commersonii commersonii Dun., Solanum commersonii malmeanum Bitt. and Solanum tuberosum L. Crop Breeding and Applied Biotechnology. 7:387–393. doi: 10.12702/1984-7033.v07n04a09
- Wangenheim KHFV. 1955. Zur ursache der kreuzungsschwierigkeiten zwischen solanum tuberosum L. und S. acaule bitt. bzw. S.stoloniferum schlectd. et bouche. Z Pflanzenz. 34:7–48.
- Watanabe KN, Orrillo M, Vega S, Masuelli R, Ishiki K. 1994. Germ plasm enhancement with disomic tetraploid Solanum acaule. II.assessment of breeding value of tetraploid F, hybrids between tetrasomic tetraploidS. Tuberosum and S. acaule. Theor Appl Genet. 88:135–140. doi: 10.1007/BF00225888
