ABSTRACT
Dendrobium is a kind of popular orchid with long display life of flowers. Since it has a long vegetative period to flowering, it is necessary to develop methods to promote flowering. The effects of thidiazuron (TDZ) on flowering and the morphological characteristics of the Dendrobium ‘Sunya Sunshine’ were investigated. One-year-old and uniform height potted plants were used. Plants were spayed and root irrigated with the same amount of TDZ solution in different concentration treatments. The results showed that TDZ promoted the emergence of new shoots, but it did not affect plant morphology, such as the stem height, stem diameter, leaf size. TDZ application of 30 mg·L−1 increased the floral bud formation rate to 87.1%, increased inflorescence number and stalk thickness, but it also decreased the flower number per inflorescence and flower size, and shortened the flowering duration. This study indicated that TDZ could promote flowering, and the optimum concentration of the TDZ solution was 30 mg·L−1.
Introduction
Orchids are an important group of ornamental plants comprising more than 25,000 species and hybrids (Dressler Citation1993). Dendrobium is the second-largest genus and consists of nearly 2000 natural species (Lavarack et al. Citation2006; Wood Citation2006; Wang et al. Citation2014), many of which are important commercial orchids. They are second among the potted flowering plants sold in the USA and account for around 20% of total orchid sales (US Department of Agriculture Citation2016). Thailand is the major producer and exporter of tropical orchids, but other Asian countries such as Singapore, Indonesia, Malaysia, and the Philippines are also important producers. In addition to the USA and Japan, which are the main consumer countries, China is also an important market for Dendrobium (Ling Citation2002).
Dendrobium orchid hybrids are foremost in the ornamental cut flower industry because of their particular flower shape, colourful flower, and longer flowering and vase life relative to other orchid hybrids, and they are also export commodities of numerous tropical countries (Wu et al. Citation2007). In cultivation, commercially planted Dendrobium fall into two types, nobile-type and phalaenopsis-type, and they are usually sold as cut flowers or potted flowering plants (Li et al. Citation2013). In the Chinese market, They are mainly sold as potted flowering plants in recent years (Wu Citation2017).
Phalaenopsis-type Dendrobium is mostly grown in south-Asia. Therefore, enough sunlight and temperature are necessary for its development and flowering. China, especially Hainan Province which is close to Southeast Asia, is very suitable to Phalaenopsis-type Dendrobium cultivation. In production, longer stem height and inflorescence length are important for cut flowers, while shorter stem height, thicker stem diameter and shorter inflorescence are suitable for display as potted plants. Usually, it takes a long vegetative period, 2–3 or more years, to flowering, and the natural flowering period of Phalaenopsis-type Dendrobium is limited from August to November (Lu Citation2003; Wu et al. Citation2007), which does not coincide with New Year's Day, Spring Festival, and other times of high flower sales in China. Therefore, regulating the flowering of Phalaenopsis-type Dendrobium is particularly important. Until now, there have been very few reports on Dendrobium flowering regulation, except some in vitro flowering research (Hee et al. Citation2007; Tee et al. Citation2008; Deb and Sungkumlong Citation2009; Te-chato et al. Citation2009). Those reports show that cytokinins (CTKs) could induce in vitro flowering in Dendrobium, and several approaches such as manipulating photoperiods (Shimomachi et al. Citation2011) and the application of 6-benzyladenine (6-BA) (Blanchard and Runkle Citation2008; Nambiar et al. Citation2012) have been used to regulate flowering in other orchid species. CTKs, are thought to act as one of the multiple components that function as stimuli to promote floral bud formation (Bernier et al. Citation1993). Previous studies have shown that the activity of phenylurea-type CTK is several times higher than that of purine-type CTK.
Thidiazuron (TDZ) is a kind of artificial phenylurea-type CTK, and its activity is stronger than that of 6-BA (Kostenyuk et al. Citation1999). It leads to a wide array of in vitro and in vivo applications including prevention of leaf yellowing, enhanced photosynthetic activity, breaking of bud dormancy, fruit ripening, as well as proliferation of adventitious shoots, callus production, and induction of somatic embryogenesis (Dinani et al. Citation2018). In low concentrations (≥2.5 μM), TDZ enhance axillary bud formation on cultured shoot tip meristems, while moderate concentrations of TDZ (5–10 μM) can result in somatic embryo formation. At higher concentrations, morphological abnormalities like hyperhydricity have been reported (Lu Citation1993), and prolonged exposure to TDZ may cause abnormal shoot morphology, or problems in rooting (Mithila et al. Citation2003). In flower induction, it induced in vitro flowering of Dendrocalamus strictus Nees by initially supplemented with concentration of 0.5 and 1.0 mg/l (Madhulika et al. Citation2000). TDZ at 3.3–10 μM combined with 1.5 μM Naphthalene Acetic Acid (NAA) were the most effective combinations for achieving flower induction in Cymbidium ensifolium var. misericors in vitro (Chang and Chang Citation2003). Although TDZ use would cause morphological abnormalities, genetic evaluation of TDZ-induced explants using flow cytometry, inter-simple sequence repeat (ISSR), molecular markers, and directed amplification of minisatellite-region DNA (DAMD) has shown uniformity and stability in genome size and consistent ploidy level (Faisal et al. Citation2014).
However, the effect of TDZ on the flowering and growth of Phalaenopsis-type Dendrobium is still unclear. There was also no report on flowering regulation of Dendrobium in cultivation by TDZ. Technical challenges related to flowering regulation have restricted the development of the Dendrobium industry. Therefore, it is necessary to verify the effect of CTK on the flowering of these Phalaenopsis-type orchids. In this study, we examined the effect of TDZ on the morphological and flowering characteristics of Phalaenopsis-type Dendrobium, and aimed to develop a flower regulation technique and enhance the potted flowering plants quality of Dendrobium in China.
Materials and methods
Plant materials
The Dendrobium ‘Sunya Sunshine’, which is a local commercial variety in Hainan that belongs to one of the well-known longer-flowering variety Dendrobium ‘Sonia’ lines, was used in this experiment. Tissue culture plantlets were transplanted and rooted. One-year-old plants of uniform height, 15–16 cm, with an average of six leaves were obtained from the Tropical Crops Genetic Resources Institute of the Chinese Academy of Tropical Agricultural Sciences (Hainan, China). The plants were potted in plastic cups with a top diameter of 10 cm containing sphagna moss and coconut shell. During the experiment, the plant materials were grown in the greenhouse and irrigated with tap water when the substrates dried. During the experimental period, the temperature was 26–35°C and humidity was 70–85%.
Experimental design
The experiment began on 29 March, 2016. Unlike the effect of TDZ on the morphogenesis during the plant growing period in vitro, TDZ content could be reduced mostly by watering in cultivation, and its effect could be greatly weakened by instable environment conditions in vivo. Therefore, we considered applying in this study high enough concentrations of TDZ to induce flowering, so that we can observe not only its effect on flowering, but also unfavourable effects of different doses of TDZ. We used five concentrations of TDZ (0, 30, 60, 90 and 120 mg·L−1). Treatments were performed in the afternoon and repeated every 10 days for a total of three applications, and a total amount of 30 ml solution was applied per plant each time, 5 ml as foliar spray and the rest as root irrigation. In TDZ treatment of 0 mg·L−1 (control), we only applied the same amount of clean water. Before the treatment, watering was suspended for 3–4 days, so that the planting substrate was dry enough to absorb all the solution without leaking. The experiment was repeated three times, with 30 plants per replicate.
Morphological and developmental measurements
When the inflorescence emerged 1 cm long from the top of the plant, it was identified as a floral bud formation plant and appearance of inflorescence in this study. The floral bud formation rate is the ratio of inflorescence emerging plants to the 30 samples per replicate; a higher value represents a higher number of flowering plants and greater uniformity. This parameter was measured every 10 d from the first day of the treatment until no more flower buds were formed during the experimental period. Morphological characteristics such as stem height, stem diameter, leaf length, leaf width and the number of new shoots were measured at the end of study. Additionally, first inflorescence emergence time, first flower bloom time, first flower duration (from bloom to wilt of the first flower) and flowering duration (from the bloom of the first flower bud to the wilt of the last flower) were recorded. Length of inflorescence and scape diameter were measured, and the number of inflorescences, number of flowers per inflorescence and the total number of flowers at the end of flowering were counted. The length of the flower was measured from the top of the dorsal sepals to the two lateral sepals, and the width was measured as the length from one end of the petal to the other end of another petal (Yin et al. Citation2008). Flower colour was measured using a colorimeter (NF333; Nippon Denshoku Industries Co., Ltd., Tokyo Japan), and the L*a*b* colour scale of the Commission Internationale del’Eclairage was adopted (Hong et al. Citation2012), and raw data such as L*, a* and b* were obtained. To better integrate the information of the raw data, the colour index for red grapes (CIRG) was calculated according to CIRG = (180-H)/(L* + C), C = (a*2 + b*2 )0.5, H = arctan (b*/a*) (Carreño et al. Citation1995).
Statistical analysis
All experimental data were collected within 150 days, subjected to analysis of variance (ANOVA) and Duncan’s multiple comparison test at P ≤ 0.05 in SPSS 17.0, and plotted using Excel 2013.
Results
Effect of TDZ on vegetative growth parameters
Plant growth characteristics, such as stem height, stem diameter, leaf length, leaf width and the number of new shoots, can reflect the growth status of a plant. After 60 days of treatment, all of the above characteristics were measured, and except for stem diameter in the TDZ30 treatment, there were no significant differences between the TDZ treatments and the control in stem height and diameter (). Similarly, there were no differences between the treatments and the control in leaf length and width, except for leaf width in the TDZ90 treatment (). Therefore, the results indicated that applying TDZ had no influence on plant morphology. As for the number of new shoots, the application of TDZ30 or TDZ60 promoted the emergence of new shoots, the numbers of which were significantly higher than that of the control. However, higher concentrations of TDZ, such as TDZ90 or TDZ120, did not increase the number of new shoots relative to the control ().
Table 1. Effect of TDZ concentrations on the vegetative growth parameters of Dendrobium ‘Sunya Sunshine’ potted plants.
Effect of TDZ on floral bud formation rate
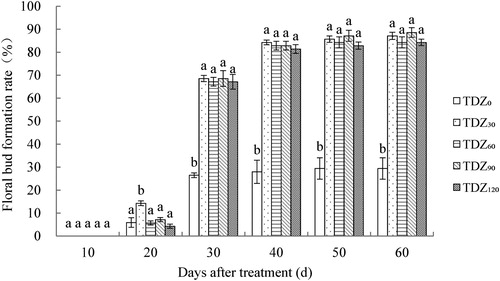
The floral bud formation rate of Dendrobium ‘Sunya Sunshine’ was affected by the different concentrations of TDZ. After TDZ application, the floral bud formation rate was significantly higher than that of the control, indicating much better flowering uniformity than the control. Flower buds could be observed at 10 d after treatment. At 20 d after treatment, the highest floral bud formation rate was 14% in treatment TDZ30 while it was approximately 5% in the other four treatments. As time went on, a large number of flower buds were observed, and the floral bud formation rate of TDZ30 was still the highest (68.6%) at 30 d after treatment. In contrast, that of the control was only 26.5%. After 40 d, the rate slowly increased until 50 d, after which it became highest in the TDZ90 treatment (87.1%) and followed by TDZ30 (85.7%); that of the control was only 29.4% (). There was no difference between the TDZ treatments, indicating that the lowest concentration could also induce high floral bud formation rate in this study.
Figure 1. Effect of TDZ concentrations on the floral bud formation rate of Dendrobium ‘Sunya Sunshine’ potted plants. The data were measured every 10 days from the first day of the treatment performed and represent means of 30 plants per treatment ± standard error. Different letters indicated significant differences at P ≤ 5%.
Effect of TDZ on flowering time and duration parameters
First inflorescence emergence was accelerated by TDZ. The results showed that the first inflorescence under TDZ30 was formed 6 d earlier than the control. Increased TDZ concentration, such as TDZ90, increased the time of the floral bud formation, but it was still significantly earlier than the control (). First flower bloom time in the TDZ30 treatment was a little earlier than the control, but it took more days by increasing the concentration, especially in TDZ90 and TDZ120, which was significantly longer than the control (). Additionally, first flower duration lasted more days in TDZ30 than the control. However, it was significantly shortened by the high concentration of TDZ. Similarly, flower duration was reduced by increasing concentrations of TDZ. But in low concentration treatments TDZ30 and TDZ60, it showed no difference to the control ().
Table 2. Effect of TDZ concentrations on the flowering timing and duration of Dendrobium ‘Sunya Sunshine’ potted plantsa.
Effect of TDZ on inflorescence morphology parameters
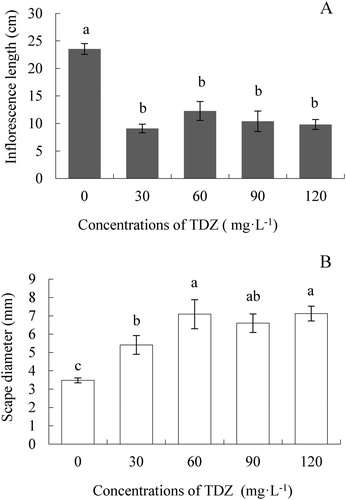
TDZ inhibited inflorescence length, which was significantly shorter in all treatments compared to the control. The inflorescence length was approximately 23 cm in the control, while it was 10 cm, but not significantly different among the TDZ treatments ((A)). Conversely, scape diameter was promoted by TDZ. The highest scape diameter was 7.12 mm in treatment TDZ120 followed by 7.09 mm in treatment TDZ60, while it was 3.48 mm in the control, which was 2 times less than that of the TDZ120 treatment ((B)). This result indicated that the TDZ application could greatly shorten the inflorescence length but increase the scape diameter.
Figure 2. Effect of TDZ concentrations on the inflorescence length (A) and scape diameter (B) of Dendrobium ‘Sunya Sunshine’ potted plants. The data were measured at the time of the first flower bloom and represent means of 30 plants per treatment ± standard error. Different letters indicated significant differences at P ≤ 5%.
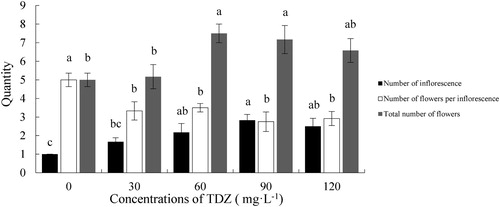
Effect of TDZ on quantity of inflorescences and flowers
Inflorescence number was significantly increased by TDZ treatment, and there was an increasing trend with increasing TDZ concentration. The number of inflorescences in TDZ30 averaged 1.7 per plant, and it was not significantly different than the control with 1.0 per plant. The number in the higher concentration treatments was significantly higher than in the control; the maximum number of inflorescences was 2.8 per plant in TDZ90, which was 2.8 times of the control (). For the number of flowers per inflorescence, the control produced 5 flowers, which was greater than that of all the TDZ treatments (). Although the number of flowers per inflorescence was less, the total number of flowers was higher in the TDZ treatments than in the control; both TDZ60 and TDZ90, which averaged 7.5 and 7.2 flowers per plant, were significantly higher than the control (). These results indicated that TDZ could increase the number of inflorescences and decrease the number of flowers per inflorescence but enhance the total number of flowers.
Effect of TDZ on flower size and colour
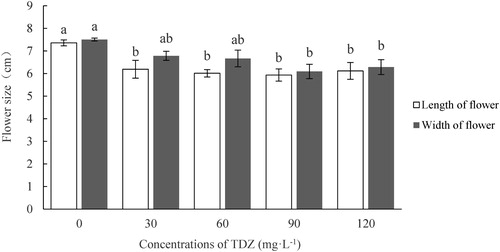
Flower size was influenced by TDZ treatment. The length of the flower was greatest in the control (7.4 cm), whereas those of the other TDZ treatments were approximately 6.0 cm, lower than the control. In terms of flower width, low concentration treatments such as TDZ30 or TDZ60 did not differ from the control (7.5 cm), while the width in the high concentration treatments of TDZ90 or TDZ120 was significantly lower ().
Figure 4. Effect of TDZ concentrations on the flower size of Dendrobium ‘Sunya Sunshine’ potted plants. The data were measured when the petal was fully expanding and represent means of 30 plants per treatment ± standard error. Different letters indicated significant differences at P ≤ 5%.
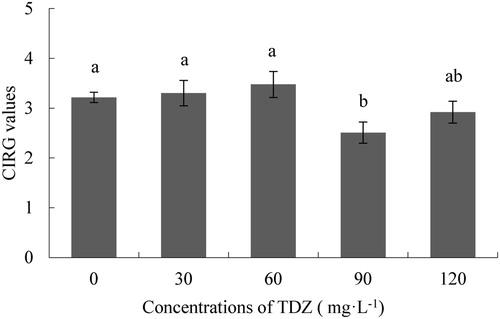
Flower colour is also an important characteristic of Dendrobium. After TDZ application, the CIRG values of TDZ30 and TDZ60 showed a small increase, but they did not differ significantly from the control. At higher concentrations of TDZ, the CIRG value was lower than the control, and TDZ90 exhibited the lowest value. Except for TDZ90, there was no difference between the TDZ treatments and the control (), which indicated that there is no a TDZ effect on the colour of flowers.
Discussion
Cytokinins are common plant growth regulators that promotes cell division, induce bud formation and promote growth; the most commonly used cytokinins are 6-BA and TDZ (Nayak et al. Citation1997). In this study, the effect of TDZ on the flowering and growth of Dendrobium ‘Sunya Sunshine’ was investigated. For flowering and commercial sale, appropriate plant height and thickness are necessary. In our study, the stem height and stem diameter after the application of TDZ exhibited no significant differences from those of the control, except for TDZ30 treatment in stem diameter (). Although leaf length was longer and leaf width was shorter in TDZ90, we still thought that there was no significant effect of TDZ on leaf size, as there was no difference with the other treatments (). These results indicated that applying TDZ did not affect the growth and commodity characteristics of Dendrobium ‘Sunya Sunshine’ in this study.
It was found that new shoots was significantly promoted by applying TDZ (). Since TDZ is largely responsible for its ability to release lateral buds from dormancy or induce bud regeneration in vitro (Mok et al. Citation2005; Singh and Dwivedi Citation2014), we believed that TDZ induced the shoot bud germination at the bottom of pseudobulb of Dendrobium ‘Sunya Sunshine’ in vivo. More new shoots improved the volume of the potted plant, and more stems also improved the value of the potted dendrobium. However, in high concentration of TDZ, the number of new shoots was reduced (). It was concluded that high concentration of TDZ inhibited shoot germination. Similar results were reported, showing that TDZ concentrations greater than 22.7 μM inhibited shoot regeneration in vitro (Montecelli et al. Citation1999).
Flower regulation is very important in Dendrobium cultivation because of its limited flowering season, therefore a high flowering rate would improve flower marketability in other seasons. It has been reported that cytokinins can promote the flowering rate in orchids. In a previous study, the floral bud formation percentage in nodes of Dendrobium wardianum was 84.3% after spraying with 200 mg·L−1 Paclobutrazol (PP333) and 200 mg·L−1 TDZ mixed solution twice in vivo, whereas that of the control was only 54.3% (Wang et al. Citation2008). As shown in our results, the application of TDZ greatly promoted floral bud formation to up to 88.6%. In C. ensifolium, TDZ at 10 μM produced 7.14 inflorescences, while control produced no inflorescence in vitro (Chang and Chang Citation2003). All these reports revealed that TDZ can induce flowering effectively in orchids.
However, in the high concentration treatment, the floral bud formation rate slightly decreased in this study. Similarly, the formation rate was first increased and then decreased by increasing the concentration of 6-BA in Dendrobium ‘Angel White’ in vivo (Nambiar et al. Citation2012). All these results indicated that cytokinins could promote bud but that high concentrations reduced the enhancing effect.
Attempts to regulate the flowering of Dendrobium orchids have been previously reported by Nambiar et al. (Citation2012), who indicated that BA could promote flower bud emergence of almost 77 days earlier than the control, and those flowers could bloom approximately 100 days earlier in vivo. As reported in previous research (Li et al. Citation2011), the application of BA could shorten the first inflorescence emergence time of a nobile-type Dendrobium cultivar by 12 days relative to the control. In this study, it was significantly hastened by 6 days comparing with the control. The above results show that cytokinins can significantly promote Dendrobium flowering, and the different times of the responses in these experiments could be partially attributed to variations in the genetic backgrounds of the varietals, plant size, the kinds of cytokinins and experimental conditions. Therefore, cytokinins could hasten the Dendrobium flowering process, but the effect is cultivar-dependent. However, the first flower duration and flowering duration were shortened in high concentration of TDZ in our study. As Lorteau et al. (Citation2001) reported, cytokinins can stimulate the synthesis of ethylene in plants, which may be one of the reasons that flower senescence was accelerated by applying higher concentrations of TDZ.
Inflorescence length was significantly inhibited in all of the TDZ treatments in this study ((A)). In a previous study, Blanchard and Runkle (Citation2008) found that the inflorescence length of two Phalaenopsis cultivars was 9.8 and 9.0 cm shorter with BA treatment than that of control plants, and the observations seem compatible with the results of our study, in which the inflorescence length was approximately 10 cm with TDZ treatment. In a relevant report, shoot elongation was inhibited in much higher concentrations of TDZ (Kumar and Reddy Citation2012). In this study, the developing inflorescence elongation was similar to the elongation of new shoot. The inhibition of elongation could also affect on inflorescence development. We also could observe a similar inhibition trend in new shoots but not in bigger stem of Dendrobium ‘Sunya Sunshine’ (). It was suggested that the young and tender shoots and developing inflorescence were more sensitive than bigger stems to TDZ. Conversely, the scape diameter was found greatly promoted by TDZ ((B)). This indicated that TDZ had two kinds of effects on inflorescence, inhibiting inflorescence elongation while increasing scape diameter. This may be disadvantageous to Dendrobium as cut-flowers, but it is beneficial for potted-flowering plants. Normally, most of phalaenopsis-type Dendrobium varieties produce a single inflorescence at one pseudobulb in the same time, like in Dendrobium ‘Sunya Sunshine’. However it was observed that this cultivar produced more inflorescences in response to TDZ (). Since TDZ could release lateral buds from dormancy or induce bud regeneration as derailed above. It was indicated that TDZ could stimulate apical meristem development, inducing new shoots at the bottom and inflorescences in the top of pseudobulb.
In terms of the number of flowers, TDZ inhibited elongation of inflorescence and inhibited flower numbers in one inflorescence, but the total flower numbers were increased because of the increased number of inflorescence (). Another study showed a similar result for Hawaiian Dendrobium; the application of cytokinins enhanced the total number of flowers (Sakai et al. Citation2000). This result suggested that the observed decrease in flower number per inflorescence may be caused by competition for nutrition among developing more inflorescences. Furthermore, the flower length and width of the treated plants were shorter than in the control in our study (), and similar results were shown in the experiments by Blanchard and Runkle (Citation2008) and Nambiar et al. (Citation2012), in which the application of BA decreased flower size. The result may also be explained by nutrition competition within the plant because of more flowers produced in response to TDZ.
Conclusions
The application of TDZ clearly induced Dendrobium ‘Sunya Sunshine’ flowering. Our results indicated that the application of TDZ increased the floral bud formation rate, induced earlier flowering and promoted the growth of new shoots. Overall, the flower regulation effect was depended on the concentration applied, and TDZ solution of 30 mg·L−1 appeared to have the optimum effect on Dendrobium ‘Sunya Sunshine’ flowering in our study. After applying TDZ solution, Dendrobium ‘Sunya Sunshine’ flowered uniformly within 2 months. Thus, applying the appropriate TDZ concentration is a potential way to regulate flowering and increase the inflorescence number in Dendrobium cultivation, which was much suitable for potted Dendrobium production.
Acknowledgements
The author thanks laboratory of Crop Gene Resources and Germplasm Enhancement in Southern China, Ministry of Agriculture, China for research.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bernier G, Havelange A, Houssa C, Petitjean A, Lejeune P. 1993. Physiological signals that induce flowering. The Plant Cell. 5:1147–1155. doi: 10.2307/3869768
- Blanchard MG, Runkle ES. 2008. Benzyladenine promotes flowering in Doritaenopsis and Phalaenopsis orchids. Journal of Plant Growth Regulation. 27(2):141–150. doi: 10.1007/s00344-008-9040-0
- Carreño J, Martínez A, Almela L, Fernández-Lopez JA. 1995. Proposal of an index for the objective evaluation of the color of red table grapes. Food Research. 28:373–377. doi: 10.1016/0963-9969(95)00008-A
- Chang C, Chang WC. 2003. Cytokinins promotion of flowering in Cymbidium ensifolium var. misericos in vitro. Plant Growth Regulation. 39:217–221. doi: 10.1023/A:1022892402536
- Deb CR, Sungkumlong T. 2009. Rapid multiplication and induction of early in vitro flowering in Dendrobium primulinum Lindl. Journal of Plant Biochemistry and Biotechnology. 18:241–244. doi: 10.1007/BF03263328
- Dinani ET, Shukla MR, Turi CE, Sullivan JA, Saxena PK. 2018. Thidiazuron: modulator of morphogenesis. In: Vitro. Thidiazuron: from urea derivative to plant growth regulator. Singapore: Springer Press; p. 1–36.
- Dressler RL. 1993. Phylogeny and classification of the orchid family. Cambridge: Cambridge University Press; p. 8.
- Faisal M, Alatar AA, Hegazy AK, Alharbi SA, Sheikh ME, Okla MK. 2014. Thidiazuron induced in vitro multiplication of Mentha arvensis and evaluation of genetic stability by flow cytometry and molecular markers. Industrial Crops and Products. 62:100–106. doi: 10.1016/j.indcrop.2014.08.019
- Hee KH, Loh CS, Yeoh HH. 2007. Early in vitro flowering and seed production in culture in Dendrobium Chao Praya Smile (Orchidaceae). Plant Cell Reports. 26:2055–2062. doi: 10.1007/s00299-007-0421-9
- Hong Y, Bai XX, Sun W, Jia FW, Dai SL. 2012. The numerical classification of chrysanthemum flower color phenotype. Acta Horticulturae Sinica. 39:1330–1340.
- Kostenyuk I, Oh BJ, So IS. 1999. Induction of early flowering in Cymbidium niveo-marginatum Mak in vitro. Plant Cell Reports. 19:1–5. doi: 10.1007/s002990050701
- Kumar N, Reddy MP. 2012. Thidiazuron (TDZ) induced plant regeneration from cotyledonary petiole explants of elite genotypes of Jatropha curcas: a candidate biodiesel plant. Industrial Crops and Products. 39:62–68. doi: 10.1016/j.indcrop.2012.02.011
- Lavarack B, Harris W, Stocker G. 2006. Dendrobium and its relatives. East Roseville (NSW): Kangaroo Press; p. 1–10.
- Li CH, Ren Y, Huang SR, Huang SH, Yang GS. 2013. Floral colors of Phalaenopsis type Dendrobium and their flavonoid composition. Acta Horticulturae Sinica. 40:107–116.
- Li WS, Ke HL, Deng XG, Liu YW, Ling XB. 2011. Effects of 6-BA and GA3 on Dendrobium nobile-type cultivar. Chinese Journal of Tropical Crops. 6:1016–1019.
- Ling XB. 2002. 热带兰在海南产业化发展中的前景与问题 [Prospect and problems of tropical orchid in the industrialization of Hainan]. Tropical Forestry. 30:31–33. Chinese.
- Lorteau MA, James BJ, Catherine F. 2001. Effects of cytokinin on ethylene production and nodulation in pea (Pisum sativum) cv. Sparkle. Physiologia Plantarum. 112:421–428. doi: 10.1034/j.1399-3054.2001.1120316.x
- Lu C. 1993. The use of thidiazuron in tissue culture. In Vitro Cell Dev Biol-Plant. 29(2):92–96. doi: 10.1007/BF02632259
- Lu SC. 2003. 热带洋兰秋石斛 [Tropical orchid Dendrobium]. China Flower & Penjing. 9:4–5. Chinese.
- Madhulika S, Uma J, Jaiswal VS. 2000. Thidiazuron–induced in vitro flowering in Dendrocalamus strictus Nees. Current Science 79:1529–1530.
- Mithila J, Hall JC, Victor JM, Saxena PK. et al. 2003.Thidiazuron induces shoot organogenesis at low concentrations and somatic embryogenesis at high concentrations on leaf and petiole explants of African violet (Saintpaulia ionantha Wendl). Plant Cell Reports. 21(5):408–414. doi: 10.1007/s00299-002-0544-y
- Mok MC, Martin RC, Dobrev PI, Vanková R, Ho PS, Sakakibara KY, Sakakibara H, Mok DWS. 2005. Topolins and hydroxylated thidiazuron derivatives are substrates of cytokinin O-glucosyltransferase with position specificity related to receptor recognition. Plant Physiology. 137(3):1057–1066. doi: 10.1104/pp.104.057174
- Montecelli S, Gentile A, Damiano C. 1999. In vitro shoot regeneration of apple cultivar gala. In: International symposium on methods and markers for quality assurance in micropropagation. Acta Horticulturae. 530:219–224.
- Nambiar N, Siang TC, Mahmood M. 2012. Effect of 6-benzylaminopurine on flowering of a Dendrobium orchid. Australian Journal of Crop Science. 6:225–231.
- Nayak NR, Patnaik S, Rath SP. 1997. Direct shoot regeneration from foliar explants of epiphytic orchid Acampe praemorsa (Roxb.) Blatter and McCann. Plant Cell Reports. 16:583–586.
- Sakai WS, Adams C, Braun G. 2000. Pseudobulb injected growth regulators as aids for year around production of Hawaiian Dendrobium orchid cut flowers. Acta Horticulture. 541:215–220. doi: 10.17660/ActaHortic.2000.541.30
- Shimomachi T, Mitsumizo T, Matsuzono R. 2011. Effect of night break treatment on growth and flowering in Dendrobium Phalaenopsis. Acta Horticulture. 907:303–307. doi: 10.17660/ActaHortic.2011.907.49
- Singh P, Dwivedi P. 2014. Two-stage culture procedure using thidiazuron for efficient micropropagation of Stevia rebaudiana, an anti-diabetic medicinal herb. 3 Biotechnology. 4(4):431–437.
- Te-chato S, Nujeen P, Muangsorn S. 2009. Paclobutrazol enhance bud break and flowering of Friederick's Dendrobium orchid in vitro. Journal of Agricultural Technology. 5:157–165.
- Tee CS, Maziah M, Tan CS. 2008. Induction of in vitro flowering in the orchid Dendrobium Sonia 17. Biologia Plantarum. 52:723–726. doi: 10.1007/s10535-008-0139-8
- U.S. Department of Agriculture, Floriculture crops. 2015 summary, 2016 Washington (DC): Agricultural Statistics Board.
- Wang Y, Zhou JC, Zheng BQ, Chen ZH, Huang ZH. 2014. 石斛兰 [Dendrobium]. Beijing: China Forestry Publishing House. Chinese.
- Wang ZH, Zhu GF, OU MC, Wang BQ. 2008. Induction of plant growth regulators on early in vivo flowering of nobile-type Dendrobium. Guangdong Agricultural Sciences. 10:37–39.
- Wood HP, 2006. The dendrobiums. Portland (OR): Timber Press; p. 1–10.
- Wu RH, Li ZJ, Wang Y, 2007. 秋石斛品种及其温室栽培 [Cultivation of Vitro Dendrobium Varieties in Greenhouse]. Agricultural Engineering Technology (Greenhouse & Horticulture). 1:34–35. Chinese.
- Wu XJ, 2017. 盆栽秋石斛市场前景广阔 [Potted Dendrobium has a broad market prospect]. China Flower and Gardening News, November 28th, (A01). Chinese.
- Yin JM, Ren Y, Yang GS. 2008. 石斛兰种质资源描述规范和数据质量控制规范 [Descriptors and data quality control for Dendrobium (Dendrobium Sw.)]. Beijing: China Agriculture Press. Chinese.