ABSTRACT
Four terroirs where cv. Malvasia Istriana is grown under equal viticultural practices and climatic conditions, but on different soil types, were studied. Grape juice samples were taken during ripening at three grapevine growth stages (S35 – berries begin to colour and enlarge; S36 – berries with intermediate Brix values; and S38 – berries harvest ripe). Malvasia Istriana grape juice showed a specific free amino acids profile during all three grapevine growth stages, where arginine was the most abundant free amino acid, followed by alanine, threonine, tyrosine, aspartic acid, serine, histidine and glutamic acid. Leucine was the least abundant free amino acid on all studied terroirs at all grapevine growth stages. Total free amino acids decreased at S38, compared to both S35 and S36, on all terroirs, except Fiorini terroir where total free amino acids concentration at S38 was significantly lower compared to S36, but significantly higher compared to S35.
Introduction
Free primary amino acids, which include all amino acids with the exception of proline and hydroxyproline, are important as nitrogen sources for yeast and lactic acid bacteria fermentation, as well as precursors in flavour compounds synthesis such as higher alcohols and biogenic amines (Bordiga et al. Citation2016). In vineyards with general nitrogen deficiency caused by soil and grapevine, low N status could lead to slow and sluggish must fermentation (Janjanin et al. Citation2016). The minimum amount of assimilable nitrogen required to complete fermentation is 150 mg L−1 and should be in free amino acid form (Ünal et al. Citation2015). Henschke and Jiranek (Citation1993) indicated that the amino acids identified as the best yeast nitrogen sources include: glutamic acid, glutamine, aspartic acid, asparagine, threonine, histidine, alanine, arginine, serine and tyrosine, while proline and hydroxyproline are much less assimilated.
Grape juice free amino acids profile is affected by numerous factors including: rootstock, vineyard nitrogen status, grapevine water status, berry maturity and environmental conditions (Bell and Henschke Citation2005), as well as by grapevine variety (Soufleros et al. Citation2003). Above mentioned factors affecting grape juice amino acids profile also create the concept of terroir (Van Leeuwen and Seguin Citation2006).
The terroir concept is a complex system in which grapevine, environmental conditions and grape grower/winemaker interact (Van Leeuwen and Seguin Citation2006). As a part of the terroir concept, soil type affects grapevine water and mineral uptake, root-zone temperature, and thus influences grapevine growth and physiology, berry composition and finally, wine quality (Coipel et al. Citation2006). So, it can be assumed that vineyard soil type as a terroir component will affect grape juice free amino acids profile.
Several Mediterranean countries produce wines from the Malvasia group of varieties, including: Spain, Italy, Greece and Croatia (Lacombe et al. Citation2007). Malvasia Istriana (Vitis vinifera L.) is a local white grapevine variety in Croatia. It is the most widespread in the region of Istria, where it covers about 60% of total vineyards and it is the second most widespread variety in Croatia (Bubola et al. Citation2012).
Garde-Cerdán et al. (Citation2009) studied free amino acids dynamics during ripening of different red grapevine varieties and reported that the qualitative compositions of the studied varieties were similar when looking at the majority free amino acids, which could indicate an adaptation of plants to the growing area. Kovačević Ganić et al. (Citation2005) studied free amino acids concentration of Malvasia Istriana musts and wines grown at different rootstock, bud number, and harvest time. Arginine, alanine, glutamic acid, aspartic acid, and histidine were the most abundant free amino acids in all samples and harvest time significantly influenced free amino acid concentration. Raspor et al. (Citation2009) studied the differences in free amino acids composition of Malvasia Istriana musts and wines fermented with local and commercial starter yeasts. The average concentration of total free amino acids in must prior to fermentation was 1061 mg L−1, whereas arginine, alanine, threonine, serine, and glutamic acid were the most abundant free amino acids.
Roullier-Gall et al. (Citation2014a) researched the terroir impact on four distinct vineyards located 30 km apart to differentiate grapes and corresponding wines according to complex chemical fingerprints. The same strategy was successfully implemented to discriminate grapes and related wines from two distinct terroirs in Burgundy separated by less than 2 km and managed by the same grape grower/winemaker (Roullier-Gall et al. Citation2014b).
Since the research on differentiation of terroirs using free amino acids profile is rather rare, the aim of the present work was to study the soil type impact on Malvasia Istriana grape juice free amino acids profile during ripening, due to the importance of free amino acids for fermentation and wine quality. The research was performed in four distinct vineyards located less than 4 km apart.
Materials and methods
Vineyard site and plant material
The experiment was set up in four vineyards on different terroirs in the north-western part of the Istrian peninsula, in the winegrowing area of Brtonigla during 2013 growing season. All four vineyards could be considered homogenous in terms of their climatic and topographic (temperatures, sunshine hours, rainfall, altitude, slope), as well as viticultural (plant density, rootstock, vine training, pruning) parameters. The selected terroirs were: Fiorini (lat. 45°22′05″ N; long. 13°35′19″ E; 68 m a.s.l.; rows NW-SE), Skrinjari (lat. 45°22′04 ″ N; long. 13°37′55 ″ E; 124 m a.s.l.; rows NW-SE), Zmergo (lat. 45°22′06″ N; long. 13°38′45″ E; 130 m a.s.l.; rows NE-SW) and Skrline (lat. 45°22′27″ N; long. 13°38′57″ E; 121 m a.s.l.; rows NE-SW). Soil type on Fiorini terroir is a typical deep red soil (terra rossa); on Skrinjari terroir is a brown soil on marl; while on Zmergo and Skrline terroirs soils are two rendzinas on marl with elevate active lime amounts (data not shown) ().
Chemical soil properties of the selected terroirs were analysed according to JDPZ (Citation1966) and are shown in . Multiple individual soil samples were taken systematically from the whole vineyard area and homogenised into average soil samples.
Table 1. Soil chemical properties of the selected terroirs in the winegrowing area of Brtonigla.
According to pH values measured in KCl (), terroirs are classified as follows: Fiorini soil is acid, Skrinjari close to neutral, while Zmergo and Skrline are alkaline. Terroirs showed different organic matter and total nitrogen percentage (). Organic matter ranged from 0.36% on Skrline to 1.10% on Fiorini terroir, while total nitrogen ranged from 0.04% on Skrline to 0.10% on Skrinjari terroir.
Climatic data were recorded by a meteorological station (Agra CDA), placed near the experimental plots (lat. 45°21′57″ N; long. 13°37′13″ W; altitude 113 m a.s.l.). The average temperature for the year of research was 14.3 °C; while during grapevine growing cycle (April to October) the average temperature was 18.1°C. The accumulated precipitation during the year was 1026.3 mm, while during grapevine growing cycle (April to October), precipitation was 523.1 mm.
Malvasia Istriana vines used in this trial were grafted onto SO4 rootstock and no irrigation was applied. Double Guyot was applied and pruned to double cane with eight buds on each cane, and two replacement two-bud spurs, trained onto VSP trellis system. The row spacing was 2.2 m and vine spacing was 0.8 m, resulting in 5680 vines per hectare. In each vineyard three plots with 45 vines per plot were selected (experimental unit). All cultivation practices were manual, equal and usual for this winegrowing area.
Grape juice sampling
Grape juice samples were collected at three grapevine growth stages during 2013 growing season: stage 35 (S35) – berries begin to colour and enlarge; stage 36 (S36) – berries with intermediate Brix values; and stage 38 (S38) – berries harvest-ripe, according to modified Eichhorn and Lorenz system (Coombe Citation1995). For each plot, 10 berries were picked from the upper, middle and lower third of 10 randomly sampled clusters (total of 100 berries). Berries were crushed by hand and separate triplicate juice samples were filtered through a plastic sieve to remove skins and seeds.
Grape juice free amino acid analysis
Free amino acids in grape juice were determined according to Pripis-Nicolau et al. (Citation2001) with some modifications. An Agilent 1100 binary gradient HPLC, equipped with an automatic sampling system, and 1200 Agilent fluorescence detector were used. The excitation and emission wavelengths were 356 and 445 nm, respectively. Separation of amino acids derivates was carried out using Lichrosher (Merck) RP 18 (125 × 4 × 5 μm). Amino acid derivatisation by o-phtaldialdehyde (OPA) and iodoacetic acid (IDA) was performed automatically by the high-performance liquid chromatography auto sampler right before injection.
Total free amino acids
Total free amino acids concentration was calculated as the sum of all free amino acids measured. These free amino acids represent the assimilable portion of the total free amino acid component (total amino acids minus secondary amino acids – proline and hydroxyproline) that is of oenological significance (Bell and Henschke Citation2005). Very little proline is taken up by yeast under any conditions, thus proline, hydroxyproline, larger molecular weight peptides and protein, are referred to as yeast-non-assimilable nitrogen (YNAN) (Bell and Henschke Citation2005).
Statistical analysis
All variables were examined using one-way analysis of variance (ANOVA) using studied terroirs as factors. Data were examined separately by grapevine growth stage. Means were compared using Tukey’s HSD test to establish whether there were significant differences among terroirs (p ≤ 0.05). Partial Least Square regression analysis (PLS) was also used. The multivariate method employed is based on PLS regression combined with categorical classification. The purpose of the PLS analysis was to test the hypothesis that different terroirs and sampling times will affect grape juice amino acid content of Malvasia Istriana. Moreover, the PLS VIP values were used to identify which parameters account for the highest amount of variance. All statistical analyses were performed using Statistica software (Statistica 13.3, Tibco, Inc).
Results
Grape juice free amino acids concentration from the studied terroirs at growth stage S35 is shown in . The significantly highest concentrations of the majority of analysed free amino acids were determined on Skrinjari terroir, with the exception of tyrosine and valine, whose highest concentrations were measured on Skrline terroir.
Table 2. Grape juice free amino acid concentration of cv. Malvasia Istriana from selected terroirs at grapevine growth stage 35 – berries begin to colour and enlarge.
shows free amino acids concentration in grape juice from the studied terroirs at growth stage S36. The highest concentrations of arginine, cysteine, glycine, histidine, leucine, lysine, methionine, and threonine were determined on Skrinjari terroir, while the significantly highest concentrations of aspartic acid, glutamic acid, and tyrosine were determined on Fiorini terroir. Alanine and serine concentrations showed no difference between Fiorini and Skrinjari terroir, but were significantly higher in comparison to the other terroirs. Valine concentrations showed no significant difference among terroirs.
Table 3. Grape juice free amino acid concentration of cv. Malvasia Istriana from selected terroirs at grapevine growth stage 36 – berries with intermediate Brix values.
Free amino acids concentration of Malvasia Istriana grape juice from selected terroirs at growth stage S38 is shown in . Alanine, aspartic acid, isoleucine, and tyrosine concentrations were the significantly highest on Fiorini terroir. The significantly highest concentrations of arginine, cysteine, glutamic acid, histidine, leucine, lysine, and threonine were determined on Skrinjari terroir. Methionine concentrations were the significantly highest on Zmergo terroir. Glycine and serine concentrations showed no difference between Fiorini and Skrinjari terroirs, but were significantly higher compared to the other terroirs. Valine concentration was significantly lower on Skrline terroir and showed no difference among the other terroirs.
Table 4. Grape juice free amino acid concentration of cv. Malvasia Istriana from researched terroirs at grapevine growth stage 38 – berries harvest-ripe.
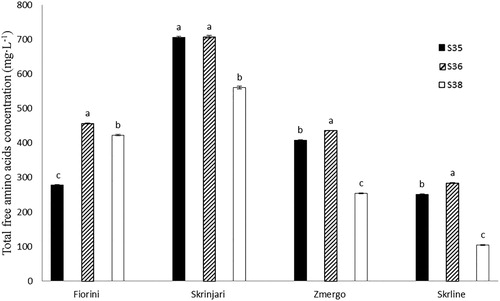
Grape juice total free amino acids concentration without proline and hydroxyproline (TAA) is shown in . Total free amino acids concentration on Fiorini terroir was the highest at S36 and the lowest at S35. On Skrline terroir, TAA concentration showed no difference at S35 and S36, but TAA concentration at S38 was significantly lower. Total free amino acids concentration followed similar dynamics on Skrline and Zmergo terroirs, showing the highest concentration at S36, while the lowest concentration was at S38, and S35 TAA was significantly lower compared to S36 TAA.
Figure 2. Total free amino acids (TAA) concentration of cv. Malvasia Istriana grape juice from the selected terroirs at grapevine growth stages 35 (berries begin to colour and enlarge), S36 (berries with intermediate Brix values) and S38 (berries harvest-ripe).
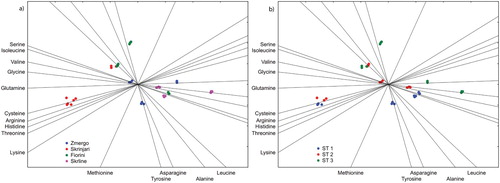
Based on PLS analysis, valine had the highest predictive power to separate the researched terroirs/soil types (A). Even though with less predictive power, cysteine and serine were also able to separate the terroir effect. The second most powerful predictive power was noted for asparagine. Asparagine, along with tyrosine, was able to separate the researched growth stages (B). Histidine and glycine had the lowest predictive power in the PLS analysis.
Discussion and conclusions
Previous research of the soil type impact on Malvasia Istriana showed that soil type has a strong influence on grape and wine quality (Radeka et al. Citation2005). In the present research soil type impact on free amino acids concentration and profile of grape juice during different ripening stages was determined. Arginine, cysteine, histidine, leucine, lysine, and threonine concentrations were significantly highest on Skrinjari terroir at all three studied grapevine growth stages. Skrinjari terroir has the highest total nitrogen content and pH value close to neutral (). It suggests that soil pH value and soil total nitrogen content may affect grape juice free amino acids concentration.
Hernández-Orte et al. (Citation2002) found that arginine, proline, histidine, glutamine, and asparagine were the most abundant free amino acids in Tempranillo grape juice. Martínez-Gil et al. (Citation2012) noted that in all studied varieties arginine, alanine, serine and threonine were the most abundant free amino acids, except for Petite Verdot, which had more glutamic acid and threonine. In three Turkish white grapes the most abundant free amino acids were arginine, histidine and alanine (Ünal et al. Citation2015). The analysed Malvasia Istriana grape juice free amino acids profile during the studied grapevine growth stages, regardless the soil type impact, showed a specific variety ‘fingerprint’ which could differentiate Malvasia Istriana from other grapevine varieties. Arginine was the predominant free amino acid in Malvasia Istriana grape juice, followed by alanine, threonine, tyrosine, aspartic acid, serine, histidine and glutamic acid. The sum of the above mentioned free amino acids (including arginine) corresponded to almost 89% of TAA (data not shown). Raspor et al. (Citation2009) reported that the most abundant free amino acids in Malvasia Istriana must prior to alcoholic fermentation were: alanine, arginine, histidine, lysine and tyrosine. It was partially confirmed by results obtained in our study with the exception of lysine, because lysine was not detected in concentrations above 37.19 mg L−1. Specific concentrations of free amino acids reported by the authors were different, probably due to a different terroir where grapes were grown and different vine nitrogen status. Treeby et al. (Citation1998) noted that, excluding proline, the most abundant amino acids in Chardonnay grape juice were arginine, glutamine, alanine, serine, glutamic acid, threonine and histidine. Reported results are very similar to the present research, suggesting that white grapevine varieties may have similar free amino acids profiles. Sommer et al. (Citation2015) noted that, in all studied grapevine varieties, grape juice free amino acids profiles did not differ from one year to another. So, it can be assumed that reported free amino acids profile of Malvasia Istriana from four terroirs analysed during 2013 is consistent.
Due to the importance of grape nitrogen composition and nitrogen deficiency frequently found in some vineyards, it is very important to provide sufficient concentration of total free amino acids in grape juice. Total free amino acids at harvest time (S38) ranged from 104.19 on Skrline terroir to 707.00 mg L−1 on Skrinjari terroir (). Reported results are in agreement with results by Puhelek et al. (Citation2015) where TAA in Kraljevina, another white grapevine variety from Croatia. TAA in Kraljevina grape juice ranged from 492.70–952.04 mg L−1, depending on the studied clone. Obtained TAA results at S38 were lower than previously reported results in grape juice sampled at the same grapevine growth stage by Raspor et al. (Citation2009), probably due to a different terroir where grapes were grown and different grapevine nitrogen status. On Skrinjari, Zmergo and Skrline terroirs, TAA concentrations were the lowest at harvest time (S38). It confirmed results reported by Schaller et al. (Citation1989), who noted a decrease in TAA concentration from veraison to harvest time. Generally, TAA decreased during the studied ripening period on all terroirs (if S35 and S38 are compared). Fiorini terroir was the exception, where TAA concentration at S38 was lower compared to S36, but significantly higher compared to S35. Skrinjari terroir TAA concentration showed no significant difference at S36 compared to S35. On Zmergo and Skrline terroirs (both alkaline) TAA concentration was higher at S36 compared to S35, showing an increase from veraison to the stage when berries have intermediate Brix values. After that, a decrease till harvest time was reported. Regardless sampling time, TAA concentration was the highest on Skrinjari terroir and the lowest on Skrline terroir (). Since soil total nitrogen content on Skrinjari terroir was the highest, while on Skline terroir the lowest, it can suggest that grape juice TAA concentration and soil total N content are connected. Furthermore, soil colour probably also affected TAA, proving that darker soils (black on Skrinjari and red on Fiorini terroir) could heat faster and accumulate more nitrogenous compounds (Bowers and Hanks Citation1965).
Large (Citation1986) reported that arginine is quantitatively the most utilisable amino acid by Saccharomyces yeasts, due to its catabolism by arginase to form L-ornithine and urea, which can form glutamate and ammonium. It is of practical importance for Malvasia Istriana fermentation, since it was the most abundant free amino acid on all studied terroirs. Kliewer (Citation1968) observed that arginine concentration usually increased during the early ripening, but then often decreased as the fruits became ripe to overripe. That was confirmed on Fiorini and Skrinjari terroirs, but not on Zmergo and Skrline, suggesting that soil type may affect arginine dynamics in grape juice during ripening.
Leucine was the least abundant free amino acid on all terroirs included in our research. Hernández-Orte et al. (Citation1999) reported that glycine, isoleucine, leucine, phenylalanine, and lysine were the amino acids with the lowest concentrations at harvest (below 25 mg L−1), which was confirmed in our research.
Martínez-Gil et al. (Citation2012) suggested that winemakers, based on free amino acids grape juice concentration, could define the most adequate winemaking process that will ensure the maximum production of fermentative volatile compounds. It is especially important for neutral varieties such as Chardonnay or Malvasia Istriana. Their volatile compounds are primarily produced during alcoholic fermentation, so higher alcohols, fatty acids and esters are important compounds for wine aroma (Moreno-Arribas and Polo Citation2005). In this sense, free amino acids concentration is crucial, since it can affect many aspects of yeast metabolism, including the above mentioned formation of volatile, as well as non-volatile compounds that are important for the organoleptic quality of wine (Bell and Henschke Citation2005). Lukić et al. (Citation2008) reported that aromatic compounds found in concentrations that significantly surpass their relative odour perception thresholds in Malvasia Istriana samples were all generated during alcoholic fermentation. It gives even more importance to Malvasia Istriana grape juice free amino acids profile. The main compounds detected in their study were etyl octanoate, etyl hexanoate, isoamyl acetate, octanoic and hexanoic acid, isoamyl alcohol and acetaldehyde. All these compounds correlated with serine and threonine grape juice concentration (Hernández-Orte et al. Citation2002). In our research, serine and threonine were among the most abundant free amino acids, so it suggests that our results confirmed the previously described Malvasia Istriana aromatic profile.
Furthermore, as amino acids are associated with taste, the amino acids unassimilated by yeast during fermentation are thought to affect wine taste, also (Wang et al. Citation2014). Despite all terroir components affecting grape juice free amino profile, the use of free amino acids profiles from local varieties in different winegrowing regions would allow to characterise wines with their own personality and different from the rest on international market (Bouzas-Cid et al. Citation2015).
For the first time, free amino acids profile and dynamics in Malvasia Istriana grape juice from different terroirs during ripening were studied. Grape juice free amino acids profiles were determined on four terroirs with different soil types, but under homogenous climatic and viticultural practices. Our research showed a specific Malvasia Istriana grape juice free amino acids concentration ‘fingerprint’, where arginine was the predominant amino acid during all grapevine growth stages and on all terroirs. It was followed by alanine, threonine, tyrosine, aspartic acid, serine, histidine and glutamic acid, while leucine was the least present free amino acid. As shown by PLS analysis, grape juice from four terroirs with different soil types had distinct free amino acids profiles regardless of the researched growth stages. Moreover, PLS revealed the dynamics of grape juice free amino acids content at different grapevine growth stages. The present research showed that the soil type/terroir effect had a significant influence on the free amino acids profile during ripening, but the effect diminished when the berries were at the harvest stage (S38). PLS analysis showed that some free amino acids (valine, cysteine) are able to differentiate terroirs/soil types, while other (asparagine, tyrosine) are able to differentitate growth stage/sampling time. It can be concluded that the specific soil type, primarily through soil total nitrogen content, affects grape juice free amino acids metabolism.
Acknowledgements
The authors would like to thank the Municipality of Brtonigla for support during the experiment.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Igor Palčić http://orcid.org/0000-0003-2935-9319
Ana-Marija Jagatić Korenika http://orcid.org/0000-0002-4539-2605
Igor Pasković http://orcid.org/0000-0002-3937-3926
Marko Petek http://orcid.org/0000-0002-2356-6440
References
- Bell SJ, Henschke PA. 2005. Implications of nitrogen nutrition for grapes, fermentation and wine. Austral J Grape Wine Res. 11:242–295. doi: 10.1111/j.1755-0238.2005.tb00028.x
- Bordiga M, Lorenzo C, Pardo F, Salinas MR, Travaglia F, Arlorio M, Coïsson JD, Garde-Cerdán T. 2016. Factors influencing the formation of Histaminol, Hydroxytyrosol, Tyrosol, and Tryptophol in wine: temperature, alcoholic degree, and amino acids concentration. Food Chem. 197:1038–1045. doi: 10.1016/j.foodchem.2015.11.112
- Bouzas-Cid Y, Falque E, Orriols I, Trigo-Cordoba E, Diaz-Losada E, Fornos-Rivas D, Miras-Avalos JM. 2015. Amino acids profile of two Galician white grapevine cultivars (Godello and Treixadura). Cienc Tec Vitivinic. 30:84–93.
- Bowers SA, Hanks RJ. 1965. Reflection of radiant energy from soils. Soil Sci. 2:130–138. doi: 10.1097/00010694-196508000-00009
- Bubola M, Peršurić D, Kovačević Ganić K, Karoglan M, Kozina B. 2012. Effects of fruit zone leaf removal on the concentrations of phenolic and organic acids in Malvasia Istriana grape juice and wine. Food Tech Biotech. 50:159–166.
- Coipel J, Rodriguez Lovelle B, Sipp C, Van Leeuwen C. 2006. «Terroir» effect, as a result of environmental stress, depends more on soil depth than on soil type (Vitis Vinifera L. cv. Grenache Noir, Côtes Du Rhône, France, 2000). J Int Sci Vigne Vin. 40:177–185.
- Coombe BG. 1995. Growth stages of the grapevine: adoption of a system for identifying grapevine growth stages. Aust J Grape Wine R. 1:104–110. doi: 10.1111/j.1755-0238.1995.tb00086.x
- Garde-Cerdán T, Lorenzo C, Lara JF, Pardo F, Ancín-Azpilicueta C, Rosario Salinas M. 2009. Study of the evolution of nitrogen compounds during grape ripening. application to differentiate grape varieties and cultivated systems. J Agric Food Chem. 57:2410–2419. doi: 10.1021/jf8037049
- Henschke PA, Jiranek V. 1993. Yeasts – metabolism of nitrogen compounds. In: G.H. Fleet, editor. Wine microbiology and biotechnology. Chur, Switzerland: Harwood Academic; p. 77–164.
- Hernández-Orte P, Cacho JF, Ferreira V. 2002. Relationship between varietal amino acid profile of grapes and wine aromatic composition. Experiments with model solutions and chemometric study. J Agric Food Chem. 50:2891–2899. doi: 10.1021/jf011395o
- Hernández-Orte P, Guitart A, Cacho JF. 1999. Changes in the concentration of amino acids during the ripening of Vitis Vinifera Tempranillo variety from the denomination d’Origine Somontano (Spain). Am J Enol Vitic. 50:144–154.
- Janjanin D, Karoglan M, Herak Ćustić M, Bubola M, Osrečak M, Palčić I. 2016. Response of ‘Italian Riesling’ leaf nitrogen status and fruit composition (Vitis vinifera L.) to Foliar nitrogen fertilization. HortScience. 51(3):262–267. doi: 10.21273/HORTSCI.51.3.262
- JDPZ. 1966. Manual of soil analysis: Book I – Chemical methods. Belgrade, Serbia: JDPZ.
- Kliewer WM. 1968. Changes in the concentration of free amino acids in grape berries during ripening. Am J Enol Vitic. 19:166–174.
- Kovačević Ganić K, Peršurić D, Gluhić D, Banović M, Komes D, Gracin L, Lovrić T. 2005. Aroma precursors of Malvasia Istriana wine. Riv Vitic Enol. 58:99–108.
- Lacombe T, Boursiquot JM, Laucou V, Dechesne F, Varès D, This P. 2007. Relationships and genetic diversity within the accessions related to Malvasia held in the Domaine de Vassal Grape Germplasm Repository. Am J Enol Vitic. 58:124–131.
- Large PJ. 1986. Degradation of organic nitrogen compounds by yeasts. Yeast. 2:1–34. doi: 10.1002/yea.320020102
- Lukić I, Plavša T, Sladonja B, Radeka S, Peršurić D. 2008. Aroma compounds as markers of wine quality in the case of Malvazija Istarska young wine. J Food Qual. 31:717–735. doi: 10.1111/j.1745-4557.2008.00230.x
- Martínez-Gil AM, Garde-Cerdán T, Lorenzo C, Lara JF, Pardo F, Rosario Salinas M. 2012. Volatile compounds formation in alcoholic fermentation from grapes collected at 2 ripening stages: influence of nitrogen compounds and grape variety. J Food Sci. 77:C71–C79. doi: 10.1111/j.1750-3841.2011.02441.x
- Moreno-Arribas MV, Polo MC. 2005. Winemaking biochemistry and microbiology: current knowledge and future trends. Crit Rev Food Sci Nutr. 45:265–286. doi: 10.1080/10408690490478118
- Pripis-Nicolau L, De Revel G, Marchand S, Beloqui AA, Bertrand A. 2001. Automated HPLC method for the measurement of free amino acids including cysteine in musts and wines; first applications. J Sci Food Agric. 81:731–738. doi: 10.1002/jsfa.871
- Puhelek I, Jagatić Korenika AM, Mihaljević Žulj M, Maslov L, Preiner D, Jeromel A. 2015. Aminoacids profile of cv. Kraljevina (Vitis vinifera L.) clones candidates. Agric Cons Sci. 80:109–112.
- Radeka S, Peršurić D, Lukić I, Bubola M, Gluhić D, Plavša T. 2005. ‘Terroir’ and quality of grape and wine of Malvasia Istriana. Riv Vitic Enol. 58:141–153.
- Raspor PI, Zagorc TI, Jemec KRP, Čadež NJ. 2009. Yeast biodiversity in Slovenian wine regions: case amino acids in spontaneous and induced fermentations of Malvasia. In: Matica Srpska, editor. Matica Srpska proceedings for natural sciences. Belgrade, Serbia: Matica Srpska, p. 97–110.
- Roullier-Gall C, Boutegrabet L, Gougeon RD, Schmitt-Kopplin P. 2014a. A grape and wine chemodiversity comparison of different appellations in Burgundy: Vintage vs terroir effects. Food Chem. 152:100–107. doi: 10.1016/j.foodchem.2013.11.056
- Roullier-Gall C, Lucio M, Noret L, Schmitt-Kopplin P, Gougeon RD. 2014b. How subtle is the “terroir” effect? Chemistry-related signatures of two “climats de Bourgogne". Plos One. 9(5):e97615. doi: 10.1371/journal.pone.0097615
- Schaller K, Löhnertz O, Geiben R, Breit N. 1989. N-Stoffwechsel von Reben. 1. Mitteilung: N-und Arginindynamik im Holzkörper der Sorte Müller-Thurgau im Verlaufe einer Vegetationsperiode. Vitic Enol Sci. 44:91–101.
- Sommer S, Wegmann-Herr P, Wacker M, Fischer U. 2015. Rationale for a stronger disposition of Chardonnay wines for stuck and sluggish fermentation. S Afr J Enol Vitic. 36:180–190.
- Soufleros EH, Bouloumpasi E, Tsarchopoulos C, Biliaderis CG. 2003. Primary amino acid profiles of Greek white wines and their use in classification according to variety, origin and vintage. Food Chem. 80:261–273. doi: 10.1016/S0308-8146(02)00271-6
- Treeby MT, Holzapfel BP, Walker RR, Nicholas PR. 1998. Profiles of free amino acids in grapes of grafted Chardonnay grapevines. Aust J Grape Wine R. 4:121–126. doi: 10.1111/j.1755-0238.1998.tb00140.x
- Ünal MÜ, Şener A, Şen K, Yilmaztekin M. 2015. Seasonal variation in amino acid and phenolic compound profiles of three Turkish white wine grapes. Turk J Agric For. 39:984–991. doi: 10.3906/tar-1412-82
- Van Leeuwen C, Seguin G. 2006. The concept of terroir in Viticulture. J Wine Res. 17:1–10. doi: 10.1080/09571260600633135
- Wang L, Harada J, Endo Y, Hisamoto M, Saito F, Okuda T. 2014. Diurnal changes in amino acid concentrations in Riesling and Chardonnay grape juices and a possible role of sunlight. Am J Enol Vitic. 65:43. doi: 10.5344/ajev.2014.13144