ABSTRACT
The perception of Leptospermum scoparium (mānuka) in New Zealand has changed from a weed to a valued shrub over the last half century due to the economic benefits of mānuka honey. The scale insect Acanthococcus orariensis (Hoy) (Hemiptera: Eriococcidae), an accidental control agent of L. scoparium that caused the mānuka blight mass dieback in the 1940s, initially was common, but declined once the entomogenous fungus Angatia thwaitesii (Petch) Arx (Fungi: Myriangiaceae) appeared. Subsequently, Acanthococcus campbelli (Hoy) and Acanthococcus leptospermi (Maskell) appear to have replaced Ac. orariensis in the last few decades, but there is limited knowledge on their current distribution. The distribution of Acanthococcus species on L. scoparium was determined at 28 sites throughout New Zealand. The most widespread species was Ac. leptospermi, which was found at 23 sites, whereas Ac. campbelli was found at 13 sites, and Ac. orariensis at just two sites. It appears that Ac. leptospermi is now the most common Acanthococcus species on New Zealand Leptospermum, and the blight caused by Ac. orariensis has not returned.
Introduction
New Zealand harbours approximately 370 scale insect species (Hemiptera: Coccomorpha) (García Morales et al. Citation2016), with approximately 270 species native to New Zealand (Henderson Citation2009). They are classified into ten families, with the Eriococcidae, commonly known as felt scale insects, being the largest family (ca 100 species) in New Zealand (Hoy Citation1954, Citation1958, Citation1959, Citation1962; Hodgson Citation1994; Hodgson and Henderson Citation1996; Henderson Citation2006, Citation2007a, Citation2007b; Henderson et al. Citation2010). This family is diverse in the Southern Hemisphere and is usually hosted by species of the family Myrtaceae (Gullan et al. Citation2004). The family Eriococcidae is characterised by a high degree of generic endemism in New Zealand (66%) (Henderson Citation2009). However, despite this high level of endemism, there are also species such as Acanthococcus orariensis Hoy (formerly in Eriococcus Targioni Tozzetti; Miller and Gimpel (Citation1996)) that arrived accidentally from Australia (Hoy Citation1954). This eriococcid species is hosted by Leptospermum scoparium J.R. Forst et G. Forst (mānuka; Myrtaceae) (Hoy Citation1954, Citation1961), which is indigenous to New Zealand and Australia (Thompson Citation1989).
Leptospermum scoparium has had a controversial place in New Zealand ecology (Essien et al. Citation2019). For farmers it reduced the agricultural land value by overtopping pasture (Gardiner Citation1953), but for conservation, this species played a significant role as a nurse species for other native trees as well as reducing erosion risk in hill country (Hoy Citation1949; Sewell Citation1949; Campbell Citation1953). An opportunity for controlling L. scoparium appeared in 1937 in Orari Gorge (Canterbury), where Ac. orariensis was first found and initially it remained restricted to that part of Canterbury, before it was deliberately spread onto North and South Island farms by landowners in the 1950s in an attempt to control the plant (Hoy Citation1954, Citation1961). Further investigations revealed the species to be native to Australia where it occurs on Leptospermum species, including L. scoparium (Hoy Citation1959), and had presumably arrived into New Zealand unaided prior to 1937 (van Epenhuijsen et al. Citation2000). In New Zealand, Ac. orariensis is associated with the sooty mould Capnodium walteri Sacc. (Fungi: Capnodiaceae), which grows prolifically on the honeydew produced by Ac. orariensis (Mulcock Citation1954). Both agents were together recognised as the cause of mānuka blight in New Zealand that had caused widespread localised death of many mānuka stands throughout the country at the time following its deliberate spread (Hoy Citation1961).
The efficiency of Ac. orariensis as control agent for L. scoparium was suggested to be due to the release from its natural enemies once in New Zealand and the density of the infestations on mānuka (Hoy Citation1961). The subsequent recovery of L. scoparium in 1954 in Te Reinga (Gisborne) prompted investigation as to why and led to the discovery there in 1957 of the host-specific entomogenous fungus Angatia (formerly Myriangium) thwaitesii (Petch) Arx (Fungi: Myriangiaceae) parasitising Ac. orariensis (Hoy Citation1961).
The prevalence of Ac. orariensis appeared to decrease on L. scoparium with the colonisation of An. thwaitesii (Hoy Citation1961). This coincided with the increase of a second Australian scale insect Acanthococcus leptospermi (Maskell), which had been first reported in 1949 (Hoy Citation1961; van Epenhuijsen et al. Citation2000). This species does not appear to be affected by An. thwaitesii (van Epenhuijsen et al. Citation2000), which may help to explain its increase in abundance in New Zealand. A third species of Australian Acanthococcus, Ac. campbelli (Hoy), was recently reported on L. scoparium in New Zealand (Gardner-Gee and Beggs Citation2009; Henderson et al. Citation2010). Whereas Ac. leptospermi seems to be relatively harmless to L. scoparium (van Epenhuijsen et al. Citation2000), the damage caused by Ac. campbelli was suggested to be more significant than that done by Ac. orariensis in an Australian survey (Hoy Citation1959). Hoy (Citation1961) considered Ac. campbelli as a potential future control agent against L. scoparium in New Zealand, so its recent discovery in New Zealand is significant.
The distribution of Ac. leptospermi has not been updated since 1959 (Hoy Citation1961) and is unknown for Ac. campbelli. Given Hoy’s prognosis on the damage likely from Ac. campbelli, and the current interest in mānuka farming due to the antibacterial and antioxidant properties of its honey (Allen et al. Citation1991; Speer et al. Citation2015; McPherson Citation2016; Ministry for Primary Industries Citation2016) there is a need for further research determining the diversity and distribution of eriococcid species on L. scoparium in New Zealand. In this study, we undertook a survey of eriococcid species present on L. scoparium throughout New Zealand to update and illustrate the distribution of eriococcids in 28 locations throughout the country.
Material and methods
Sites with L. scoparium (n = 28) were visited between March 2015 and January 2017 (). The sites all contained accessible stands of L. scoparium and were chosen haphazardly across a wide area throughout New Zealand, but the survey did not cover the entire range of the species. Stem samples infested with sooty mould and scale insects () were collected and placed in zip-lock plastic bags until further examination. In the laboratory, stems were assessed under a dissecting microscope. Fine forceps were used to uncover the waxy sacs of immature males and adult females present on stems; specimens at different life stages were preserved in 70% ethanol. Adult females were slide-mounted for identification following the protocol of Gullan (Citation1984) and specimens identified using taxonomic keys (Hoy Citation1959; Hoy Citation1962; Morales Citation1991; Henderson Citation2011). Specimen slides provided by the New Zealand Arthropod Collection (NZAC) were examined to corroborate identification of different species made from the keys.
Figure 1. Sooty mould and scale insects on Leptospermum scoparium. A, Stand of L. scoparium infested with sooty mould at Lake Wilkie (Southland, South Island). B, Crawlers (first-instar nymphs) emerging from adult female sacs and nymphs settled on a fork of L. scoparium. C, Typical black ‘mantle’ that coats leaves and stems on L. scoparium due to sooty mould and scale insect infestation. D, Plant of L. scoparium infested with sooty mould. E, Dead adult female enclosed in felted cotton sac.

Table 1. Locations sampled between March 2015 and January 2017; includes location number assigned in , NZ official place names, number of slide-mounted specimens per location, and latitude and longitude.
Results
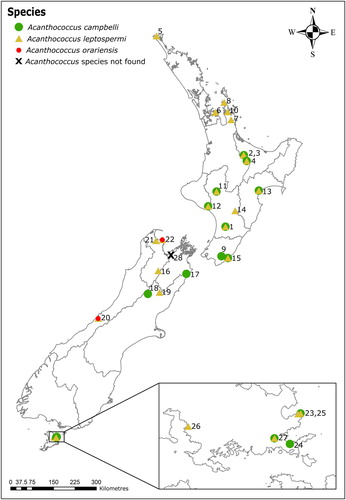
During the survey, 185 adult female specimens of Acanthococcus were slide-mounted and identified. Only three eriococcid species were found: Acanthococcus campbelli, Ac. leptospermi and Ac. orariensis. Acanthococcus leptospermi was the most widespread being present at all but five locations (Lewis Pass Road, Peterson Hill Road, Ruakokoputuna, Waima Road, and United Creek) (). Acanthococcus campbelli was the second most abundant scale species and was found in the southern half of North Island, a third of the South Island and on Stewart Island. Acanthococcus campbelli was found at two sites (Lewis Pass Road and Waima Road) where Ac. leptospermi was not found. Acanthococcus orariensis was found at only two sites, both in South Island (West Coast and Totaranui). United Creek (Nelson) was the only site where no Acanthococcus species were found. During the survey, no plant or branch death was apparent from the infestations, despite scale insects often being frequent.
Figure 2. The distribution of Acanthococcus species present on samples of L. scoparium collected between March 2015 and January 2017. Numbers refer to locations outlined in Table1; 1–15: North Island locations, 16–22: South Island locations, 23–27: Stewart Island locations.
In addition to Acanthococcus species, 36 specimens from other scale insect families were found. Of these, 25 specimens were identified as belonging to the Coelostomidiidae, nine as Pseudococcidae and two as Diaspididae.
Slide specimens of all scale insect have been deposited in Te Papa Tongarewa Museum of New Zealand, Wellington, New Zealand (NMNZ).
Discussion
This study shows that Ac. campbelli and Ac. leptospermi are widespread throughout New Zealand. In contrast, the previously widespread Ac. orariensis was found at only two of 28 sites. These results suggest that the three Acanthococcus species occurred on L. scoparium in New Zealand in distinct stages of replacement. The early stage was characterised by the incursion of Ac. orariensis, which fatally damaged many of its host plants in the 1940s and 1950s (Hoy Citation1961). Following the control of Ac. orariensis by An. thwaitesii, a transitional stage resulted in the replacement of Ac. orariensis by Ac. leptospermi, which became the most abundant species in the 1990s (van Epenhuijsen et al. Citation2000). The current stage appears to be characterised by the arrival and spread of Ac. campbelli, which, together with Ac. leptospermi, is now widely distributed around New Zealand (). Acanthococcus campbelli was reported first on L. scoparium in the Auckland region in 2009 (Gardner-Gee and Beggs Citation2009; Henderson et al. Citation2010), but its wider distribution in New Zealand was unknown until the present study.
The spread of Ac. campbelli and Ac. leptospermi, and the disappearance of Ac. orariensis could be due to the apparent immunity of the former two species to the entomogenous fungus An. thwaitesii, which attacks Ac. orariensis (Hoy Citation1961) and is thought to have significantly reduced the latter’s population. This replacement was predicted by Hoy (Citation1961), who suggested Ac. leptospermi would become the dominant species in the future. Thought not apparently yet present in New Zealand, Hoy also suggested Ac. campbelli would be a potential future control agent of L. scoparium and other Leptospermum species (Hoy Citation1959) and he suggested research on the interaction between Ac. campbelli and An. thwaitesii be conducted (Hoy Citation1961), but this was never carried out. The present study shows that Ac. campbelli is now widespread in New Zealand, but its potential susceptibility to An. thwaitesii is still unknown.
Acanthococcus orariensis is also preyed on in New Zealand by the Australian ladybird Rhyzobius ventralis Erichson (Coccinellidae) (Zondag Citation1977), although R. ventralis is mainly associated with the gum tree scale Ac. coriaceus (Miller & Gimpel) (Kirk Citation1908). So, it might be that, along with An. thwaitesii, the absence of R. ventralis facilitates the establishment of new colonies of Ac. campbelli and Ac. leptospermi, as this predator has not been previously recorded on either species.
There are likely to be other natural enemies of these scale insects in Australia. There have been no recorded scale outbreaks on Leptospermum in Australia and scales are apparently kept are low numbers due to the large number of predators and parasitoids species (Gough Citation1975). However, as Ac. campbelli and Ac. leptospermi are introduced in New Zealand, the lack of predators and parasitoids, may explain their dispersion and success on L. scoparium in New Zealand.
Acanthococcus campbelli and Ac. leptospermi are now widespread on L. scoparium in New Zealand, although there are still regions where they have not been recorded. This suggests a need to continue survey work to help identify the ongoing spread of Ac. campbelli and its potential influence on the productivity of L. scoparium. Research to evaluate the influence of Ac. campbelli on L. scoparium, and the susceptibility of Ac. campbelli to An. thwaitesii is important and would be of interest to both ecologists and mānuka honey producers.
Acknowledgements
We would like to acknowledge Mānuka Farming NZ and the Primary Growth Partnership (New Zealand Ministry for Primary Industries, Manatū Ahu Matua) awarded to Manuka Research Partnership (NZ) Limited, and Comvita Limited for funding and advice. The authors would like also to thank Massey University for the Doctoral Research Dissemination Grant, landowners for permission to sample and the New Zealand Arthropod Collection for the loan of material. The suggestions and corrections made by the two anonymous reviewers are also appreciated.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Julia Bohórquez http://orcid.org/0000-0002-0871-3561
Alastair W. Robertson http://orcid.org/0000-0001-6894-2158
Additional information
Funding
References
- Allen KL, Molan PC, Reid GM. 1991. A survey of the antibacterial activity of some New Zealand honeys. Journal of Pharmacy and Pharmacology. 43:817–822. doi: 10.1111/j.2042-7158.1991.tb03186.x
- Campbell DA. 1953. Manuka blight - soil conservator’s view. Proceedings of the Sixth New Zealand weed and Pest control Conference; 1953 Aug 18–20; Palmerston North (New Zealand): Massey Agricultural College.
- Essien SO, Baroutian S, Dell K, Young B. 2019. Value-added potential of New Zealand mānuka and kānuka products: a review. Industrial Crops and Products. 130:198–207. doi: 10.1016/j.indcrop.2018.12.083
- García Morales M, Denno B, Miller D, Miller G, Ben-Dov Y, Hardy N. 2016. ScaleNet: A literature-based model of scale insect biology and systematics. [Internet]. [accessed 2018 Feb 15]. http://scalenet.info/.
- Gardiner. 1953. Manuka blight - farmer’s view. Proceedings of the Sixth New Zealand weed and Pest control Conference; 1953 Aug 18–20; Palmerston North (New Zealand): Massey Agricultural College.
- Gardner-Gee R, Beggs LR. 2009. Distribution and abundance of endemic coelostomidiid scale insects (Hemiptera: Coelostomidiidae) in Auckland forests, New Zealand. New Zealand Journal of Ecology. 33:138–146.
- Gough, N. 1975. The ecology of gum tree scale (Eriococcus coriaceus Maskell), and its natural enemies [PhD thesis]. South Australia: Waite Agricultural Research Institute: University of Adelaide.
- Gullan PJ. 1984. A revision of the gall-farming coccoid genus Apiomorpha Rübsaamen (Homoptera: Eriococcidae: Apiomorphinae). Australian Journal of Zoology Supplementary Series. 32:1–203. doi: 10.1071/AJZS097
- Gullan PJ, Miller DR, Cook LG. 2004. Gall-inducing scale insects (Hemiptera: Sternorrhyncha: Coccoidea). In: Raman A, Schaefer CW, Whithers TM, editor. Biology, ecology, and evolution of gall inducing arthropods. New Delhi: Oxford & IBH Publishing Co. Pvt, Ltd; p. 159–229.
- Henderson RC. 2006. Four new species and a new monotypic genus Hoheriococcus (Hemiptera: Coccoidea: Eriococcidae) associated with plant galls in New Zealand. New Zealand Entomologist. 29:37–57. doi: 10.1080/00779962.2006.9722139
- Henderson RC. 2007a. A new genus and species of felt scale (Hemiptera: Coccoidea: Eriococcidae) from epiphyte communities of northern rata (Metrosideros robusta Cunn.: Myrtaceae) canopy in New Zealand. New Zealand Entomologist. 30:25–33. doi: 10.1080/00779962.2007.9722148
- Henderson RC. 2007b. Three new genera and six new species of felt scales (Hemiptera: Coccoidea: Eriococcidae) from mountain habitats in New Zealand. Zootaxa. 1449:1–29. doi: 10.11646/zootaxa.1449.1.1
- Henderson RC. 2009. Extinctions and radiations in the New Zealand scale insect fauna. Proceedings XI International Symposium of scale insect Studies; 2007 Sept 24–27; Lisbon, Oeiras (Portugal).
- Henderson RC. 2011. Diaspididae (Insecta: Hemiptera: Coccoidea). Fauna of New Zealand 66. Lincoln: Manaaki Whenua Press.
- Henderson RC, Sultan A, Robertson AW. 2010. Scale insect fauna (Hemiptera: Sternorrhyncha: Coccoidea) of New Zealand’s pygmy mistletoes (Korthalsella: Viscaceae) with description of three new species: Leucaspis albotecta, L. trilobata (Diaspididae) and Eriococcus korthalsellae (Eriococcidae). Zootaxa. 2644:1–24. doi: 10.11646/zootaxa.2644.1.1
- Hodgson CJ. 1994. Eriochiton and a new genus of the scale insect family Eriococcidae (Homoptera: Coccoidea). Journal of the Royal Society of New Zealand. 24:171–208. doi: 10.1080/03014223.1994.9517464
- Hodgson CJ, Henderson RC. 1996. A review of the Eriochiton spinosus (Maskell) species-complex (Eriococcidae: Coccoidea), including a phylogenetic analysis of its relationships. Journal of the Royal Society of New Zealand. 26:143–204. doi: 10.1080/03014223.1996.9517509
- Hoy JM. 1949. Control of manuka by blight. New Zealand Journal of Agriculture. 79:321–324.
- Hoy JM. 1954. A new species of Eriococcus Targ. (Hemiptera, Coccidae) attacking Leptospermum in New Zealand. Transactions of the Royal Society of New Zealand. 82:465–474.
- Hoy JM. 1958. Coccids associated with rata and kamahi in New Zealand. New Zealand Journal of Science. 1:179–200.
- Hoy JM. 1959. Species of Eriococcus Targ. (Homoptera, Coccidae) associated with the genus Leptospermum Forst. South-East Australia and Tasmania. New Zealand Journal of Science. 2:1–34.
- Hoy JM. 1961. Eriococcus orariensis Hoy and other Coccoidea (Homoptera) associated with Leptospermum Forst. species in New Zealand. Palmerston North: Department of Scientific and Industrial Research Bulletin 141.
- Hoy JM. 1962. Eriococcidae (Homoptera: Coccoidea) of New Zealand. Wellington: New Zealand Department of Scientific and Industrial Research Bulletin 146.
- Kirk TW. 1908. Gum tree blight and the natural enemy. Report of the New Zealand Department of Agriculture. 16:117–122.
- McPherson AJ. 2016. Mānuka –a viable alternative land use for New Zealand’s hill country? New Zealand Journal of Forestry. 61:11–19.
- Miller DR, Gimpel ME. 1996. Nomenclatural changes in the Eriococcidae (Homoptera: Coccoidea). Proceedings of the Entomological Society of Washington. 98:597–606.
- Ministry for Primary Industries. 2016. Ministry for Primary Industries 2016 apiculture monitoring programme report. Wellington, New Zealand: Ministry for Primary Industries.
- Morales CF. 1991. Margarodidae (Insecta: Hemiptera). Fauna of New Zealand 21. DSIR Plant Protection, Auckland.
- Mulcock AP. 1954. A disease of manuka Leptospermum scoparium Forst. New Zealand Journal of Agriculture. 82:115–118.
- Sewell TG. 1949. Manuka blight survey. New Zealand Journal of Agriculture. 79:101–104.
- Speer SL, Schreyack GE, Bowlin GL. 2015. Manuka honey: a tissue engineering essential ingredient. Journal of Tissue Science & Engineering. 6:e130. doi: 10.4172/2157-7552.1000e130
- Thompson J. 1989. A revision of the genus Leptospermum (Myrtaceae). Telopea. 3:301–449. doi: 10.7751/telopea19894902
- van Epenhuijsen CWK, Henderson RC, Carpenter A, Burge GK. 2000. The rise and fall of manuka blight scale: a review of the distribution of Eriococcus orariensis (Hemiptera: Eriococcidae) in New Zealand. New Zealand Entomologist. 23:67–70. doi: 10.1080/00779962.2000.9722069
- Zondag R. 1977. Eriococcus orariensis Hoy (Hemiptera: Coccoidea: Eriococcidae), causal agent of manuka blight. New Zealand Forest Service. 23:1–7.
