Abstract
A laboratory bioassay is described for measuring the resistance of kiwifruit vines to armoured scale insects (Hemiberlesia lataniae Signoret and Hemiberlesia rapax Comstock) using excised canes. The method measures the area of the scale cap using a template and compares relative cap sizes between genotypes. The method was tested for two species of kiwifruit (Actinidia deliciosa [A. Chev.] C.F. Liang et A.R. Ferguson and Actinidia chinensis Planch.) using a range of experimental genotypes and two commercial varieties. Large differences in the susceptibility of the genotypes to H. lataniae were observed, but much smaller differences were observed for H. rapax. The bioassay method was tested by comparing results obtained with observations of armoured scale insects on kiwifruit vines in the field. Sampling of simulated scale populations showed that differences in population mean scale cap areas of 0.3–0.5 mm2 could be detected with sample sizes of 50–200. A bioassay of H. lataniae growth on an experimental genotype, comparing it with the commercial kiwifruit cultivars ‘Hayward’ and ‘Hort16A’ and using a template to categorize scale cap size into 0.2 mm2 size categories, showed that the technique was quick, reliable and able to discriminate between kiwifruit varieties. This method could be easily adapted to test the susceptibility of germplasm from a variety of different horticultural plants to a wide range of armoured scale insect pests.
Introduction
New Zealand produces and exports 20% of the world's commercial kiwifruit crop from two varieties; one green-fleshed (Actinidia deliciosa var ‘Hayward’ [A. Chev.] C.F. Liang et A.R. Ferguson) and the other yellow-fleshed (Actinidia chinensis var ‘Hort16A’ Planch.) (Anon Citation2007). The green-fleshed variety was bred in the 1930s and is now grown commercially throughout the world (Anon Citation2007). The yellow-fleshed variety, ‘Hort16A’, was commercialized in 1997 (Ferguson Citation2004; Webby Citation2004).
The principal pests of kiwifruit in New Zealand are two species of armoured scale insect, greedy scale (Hemiberlesia rapax Comstock) and latania scale (H. lataniae Signoret). Both species exist as uniparental, parthenogenetic populations, having two generations per year. The second, summer generation infests fruit from January onwards to harvest (April to June) (Blank et al. Citation1996; Mauchline & Hill Citation2005). The insects are ovoviviparous; the mobile, dispersal (crawler) stage that emerges from the egg beneath the adult's scale cap can survive for up to one day before it settles permanently, spinning a protective cap over itself which is firmly attached to the plant host and grows as the insect increases in size. The insect completes two moults before becoming an adult and may live for up to nine months (Hill et al. Citation2009). Both scale insect species are resident on the wood of kiwifruit vines throughout the year, but also settle on leaves and fruit during the summer (Edwards et al. Citation2008). H. rapax was first recorded in New Zealand in 1879 (Charles & Henderson Citation2002) and has been the main armoured scale pest of kiwifruit since its commercialization until very recently (Steven Citation1990; Edwards et al. Citation2008). H. lataniae was found in New Zealand in 1979 (Morales Citation1988) and has slowly spread throughout the North Island over the past 25 years, displacing H. rapax as the dominant armoured scale pest on ‘Hayward’ but not ‘Hort16A’ kiwifruit (Berry et al. Citation1989; Blank et al. Citation2000; Edwards et al. Citation2008). Both species have worldwide adventive distributions and are highly polyphagous, having been recorded from hundreds of host plants (Davidson & Miller Citation1990; Miller & Davidson Citation1990; CABI Citation2007).
Kiwifruit is a minor crop worldwide and agrochemical companies do not give priority to registering new insecticide products for use on the crop. In addition, importing countries are slow to set insecticide maximum residue levels. Most previously registered organophosphate insecticides can no longer be used on kiwifruit because of pesticide maximum residue level restrictions. As a result, there is a dearth of registered insecticides for controlling pests on kiwifruit that can be used for all export markets.
Since 1997, the New Zealand kiwifruit industry has used an integrated pest management programme (KiwiGreen®) which ensures that insecticides for scale control are applied only in response to demonstrated need, based on packhouse records of pest incidence and crop monitoring (Anon Citation2001). This has been adopted throughout the industry and has resulted in substantial reductions in insecticide use (Suckling et al. Citation2003). However, the industry is still reliant on the use of pre- and post-flowering insecticide applications, applied in response to observed scale insect incidence, and must in the future find alternative strategies to reduce further the reliance on insecticides for armoured scale insect control.
In 2002, a programme to investigate resistance to armoured scale insects in kiwifruit germplasm was initiated at the HortResearch Te Puke Research Centre, Bay of Plenty, which houses the New Zealand Actinidia (kiwifruit) germplasm collection. Preliminary field studies on 25 experimental families of A. chinensis showed large differences in the level of armoured scale insect infestation on leaves and fruit that was shown to be heritable. The levels of H. lataniae varied independently of H. rapax, and H. lataniae densities were the main determinant of total armoured scale insect infestation levels (Hill et al. Citation2007).
Following the discovery of variation in the susceptibility of A. chinensis cultivars, there was a need to develop a reliable and quick bioassay to investigate the nature of the observed putative resistance and to screen kiwifruit breeding lines. As a first step, a quick and accurate method for measuring the size and growth rate of an armoured scale insect's cap (the hard waxy covering over the body) was developed and validated (Hill et al. Citation2005). A previous study of California red scale cap growth using analysis of digital photographs showed that scale cap size can be used as an indicator of host susceptibility, and that California red scale cap size and insect body size are highly correlated (Hare et al. Citation1990). A detailed field experiment of armoured scale insect mortality factors on experimental and commercial kiwifruit genotypes showed that the life history factors most sensitive to differences between host genotypes were crawler settlement survival and subsequent scale insect growth (Hill et al. Citation2009).
Most of the fruit are produced from flower buds on one-year-old kiwifruit canes which can grow to several metres in length. In the dormant period, the vines are pruned to leave about 30 canes per vine. The canes are cut to a standard length of 2–3 m and tied down to supporting structures usually in a pergola system about 1.8 m above the ground. These one-year-old canes have a basal diameter of 10–20 mm. Preliminary trials showed that if a length of cane is removed from a vine during winter, cut to a length of about 40 cm and maintained with one end in water in a constant environment room, it can sustain the growth of armoured scale insects on its bark for three months or more, allowing the insects to grow to adulthood and reproduce.
Based on these preliminary observations, an experimental laboratory bioassay was developed to measure the relative suitability of cane bark as a substrate supporting scale insect growth, and hence as a test of the relative resistance of kiwifruit genotypes to armoured scale insects. In the first experiment, the relative size of armoured scale insects reared on excised canes in the experimental laboratory bioassay was compared with the relative size of scale insects on canes growing on vines in the field. A second experiment was conducted to determine a suitable scale insect bioassay sampling regime by measuring the within- and between-vine and between-cane variation in scale insect size. In a third experiment, the bioassay technique was tested on an experimental kiwifruit variety and two commercial cultivars, ‘Hayward’ and ‘Hort16A’ with H. lataniae.
This paper reports the results of these experiments and the development of a prototype bioassay for measuring the relative resistance of kiwifruit canes to armoured scale insect growth.
Materials and methods
Armoured scale insect cultures
H. lataniae and H. rapax were reared separately as pure cultures on both Red Désirée potatoes and butternut squash mixed in the same container, and maintained within constant temperature incubators (21±2°C and 70±5% RH, and a natural light regime) at the Te Puke Research Centre. Squash, which are a better substrate for the scale insects than potatoes, are available only over the summer months and into autumn, but can last for up to six to eight months in culture. Potatoes, which can last six to eight months, were used when squash were not available. Laboratory cultures are supplemented every year by the addition of field-collected insects.
Experiment 1: Testing excised canes as a bioassay substrate
Twenty A. chinensis vines, each with different parentage, were selected from a single block of experimental kiwifruit vines at the Te Puke Research Orchard (Cheng et al. Citation2004). The vines were selected on the basis of observed scale insect infestation recorded on fruit at harvest in 2003 (Hill et al. Citation2007), to provide a range of suspected susceptibility to scale insects, and thereby to provide a suitable test of the methodology. Vines of the two commercial cultivars (A. chinensis ‘Hort16A’ and A. deliciosa ‘Hayward’) from a different kiwifruit block were used as standards. One-year-old canes (approximately 1.5–2.5 m long and 1–2 cm in diameter) were harvested from the 20 experimental vines from 5–9 July 2004 and from the ‘Hayward’ and ‘Hort16A’ vines on 15 September 2004. The canes were wrapped in black plastic and held in a cool store at 1°C.
The number of adult H. lataniae and adult H. rapax had been recorded on fruit from 18 of the 20 experimental vines at harvest in May 2003 (Hill et al. 2007). Only adults of these two scale insect species can be determined to species using morphological characters, although a DNA probe has been developed since this work was done (Edwards et al. Citation2008). The mean number of fruit per vine was 76 (range 33–111).
The laboratory experiment was set up from 3–5 November 2004 in a controlled environment room at the HortResearch Mt Albert Research Centre, Auckland. The environmental conditions were 20°C and 80% RH for the first three days, 20°C and 70% RH thereafter; with a 16 h light:8 h dark photoperiod regime. Lighting in the rooms was provided by ceiling-mounted fluorescent tubes (F58W/840 Sylvania Luxline®).
The kiwifruit canes were removed from the cold store and ten 40 cm cane lengths of each genotype were cut. Each cane length was labelled, the buds cut off and all wounds (with the single exception of the basal end of the cane—the end nearest the leader) sealed with grafting wax (Smith's Grafting Wax Levin, NZ). Knitting wool yarn was wrapped five times around one inter-bud area on the upper third of the cane to provide a settlement site for the scale insect crawlers. On 4 November 2004, approximately 15 newly emerged H. rapax or H. lataniae crawlers were individually placed on to each cane between the strands of wool, using a fine camel-hair brush.
After inoculation with crawlers, the canes were placed into 42 L plastic bins, with one cane from each of the 22 genotypes in each of 10 bins. Canes were assigned randomly to positions within bins. Each cane was placed into a separate 250 ml plastic cup (external diameter 8 cm, height 6 cm) glued into the bin. Water was poured into each cup to a depth of approximately 2 cm. Water levels in the plastic cups were checked and adjusted weekly, and sprouting leaves and flowers were removed from the canes at the same time. The top of each bin was covered with plastic-coated 3 cm mesh chicken wire to provide support for the canes. The wool was removed from the canes on 12 November 2004.
On 14 December 2004 (six weeks after setup), the number of scale insects on each cane was reduced to a maximum of five, although some canes had fewer than five surviving scale insects. Canes with no live scale insects were discarded. The remaining live scale insects were marked by placing a small dot of non-toxic orange fabric paint close to the scale, making it easy to locate and identify in future cane examinations. The paint was Pebeo™ relief effect paint, conforming to ASTM D-4236 toxicity standard (www.astm.org; Walker & Wineriter Citation1981).
On 18 and 19 January 2005, six one-year-old canes were selected and labelled on each of the 22 experimental vines in the field. Twenty H. rapax or H. lataniae crawlers were established as described above on to three randomly chosen cane settlement sites per vine. All settlement sites were shaded by leaves, or khaki shade cloth material (90% knitted shade cloth, Comshade™, Polyfab, Moorabbin, Australia) tied into the canopy.
The wool was removed a week after crawler settlement. Scale insect numbers at each site were reduced to a maximum of five, and the bark next to the scale was marked with a small dot of Pebeo™ orange fabric paint. Nylon gauze was wrapped around the area of settled crawlers to limit predation and parasitism. To further limit predatory insect (earwigs and coccinellids) incursions (Hill et al. Citation2005), all-weather sticky tape™ (Danco 24 mm wide, Attwoods, NZ) was placed at each end of the scale insect inoculation sites on 22–24 February 2005 and a 1 cm strip of Insect Arrest Gel™ (Chemsol Ltd, Mt Maunganui, NZ) was applied to each band.
Scale insects from the laboratory experiment were photographed on 20 December 2004 (46 days after settlement) using a digital camera (Nikon Coolpix 995). Insects on apparently susceptible canes were in the early stages of the third (adult) instar. A photograph was taken of the largest scale insect on each cane, with the insect aligned in the centre of the image. A calibration frame was attached to the front of the camera to permit the size of objects in the photograph to be measured accurately (Hill et al. Citation2005). Repeat photographs were taken on 11 January 2005 (68 days after settlement).
Photographs of the field-established insects were taken on 22–24 February 2005 (35 days after scale settlement), when it was judged by visual inspection that the scale insects on the most susceptible vines were reaching late second and early third instar. A second series of photographs was taken in the field on 16 March 2005 (57 days after scale settlement). Analysis of the images to determine scale cap area was carried out using ImageJ (version 1.32j; http://rsb.info.nih.gov/ij/) and a digitizer drawing tablet (Dick Smith, NZ model XH1913; Hill et al. Citation2005).
Experiment 2: Sources of variation in scale cap size
The growth of H. rapax and H. lataniae was measured in the laboratory on canes from two experimental genotypes, one apparently resistant (C5) and one moderately susceptible (B6) to scale insects based on the previous experiment, and the two commercial varieties, ‘Hayward’ and ‘Hort16A’. The experiment measured the sizes of scale insect caps on six replicate vines per genotype, four canes per vine and the adjacent (near to the leader) and distant (far from the leader) ends of each cane. On 4 August 2006, one-year-old canes were cut from each of six vines from each genotype (vines were full sib females for genotypes 5C and 6B) or cultivar (vines were clonal for the two commercial cultivars) and stored at 0°C. Four canes per vine were removed from the cool store on 12 September 2006 and each was cut to make two 40 cm lengths of experimental cane that were either adjacent to or distant from the leader, as follows. The first 5–10 cm of the cane from the end of the cane adjacent to the vine leader was removed and discarded to ensure that the end of the experimental cane portion was fresh. The next 40 cm length of cane was cut off to make the adjacent test cane. The next 50 cm of the cane was removed and discarded. Finally, the next 40 cm of cane became the distant test cane. The remainder of the cane was discarded.
The 40 cm cane sections were prepared for scale insect crawler settlement in the same way as for Experiment 1. The canes were inoculated with scale crawlers in mid September 2006 and placed into bins. H. rapax and H. lataniae treated canes were kept separate and 24 canes were placed in each bin, with a total of eight bins per scale species. The bins containing the inoculated canes were held in a temperature and humidity controlled room at the Te Puke Research Centre, at a mean temperature of 21±2°C and 70±5% RH. The mean RH was held at 75–80% for the first four days to facilitate crawler settlement. Water reservoirs were topped up and sprouting leaves removed every three or four days.
On 16 and 17 November 2006 (62 days after settlement), five scale insects on each cane were selected at random and photographs taken using the same procedure described for Experiment 1(Hill et al. Citation2005). The areas of these scale caps were measured using the same method as in the first experiment (Hill et al. Citation2005).
Experiment 3: Testing the bioassay
The method developed in the first two experiments was tested on an experimental kiwifruit genotype ‘Hort22D’. Growth of H. lataniae on this genotype was compared with growth on the two commercial varieties ‘Hayward’ and ‘Hort16A’. Canes were harvested from vines on 24 July 2007, wrapped in polythene for protection, and stored at 0°C. On 19 September 2007, the canes were removed from cool storage and prepared for the bioassay. For each genotype / cultivar, 40 cm lengths of cane were cut from the leader end of 10 canes after first removing and discarding an initial 10 cm. The 10 canes from each of the three varieties were placed in separate bins which were maintained in a constant environment room at 21±2°C and 70±5% RH at the Te Puke Research Centre. On 19–20 September 2007, 60 H. lataniae crawlers were seeded individually on to each cane. New growth sprouting from the excised buds was removed at weekly intervals. The wool was removed from the canes 14 days after H. lataniae crawler settlement.
On 18 December 2007, 13 weeks after crawler settlement, the size of all the scale insects on each cane was measured using a template consisting of circles with areas ranging from 0.4–1.4 mm2 and increasing in size by 0.2 mm2 increments, drawn with a laser printer on to a transparent acetate sheet. Because the scale caps are more or less circular, their area can be easily estimated by this method. Photographs were taken of 10 randomly selected H. lataniae insects from each cane using the calibrated frame attached to the front of the camera. The image of one scale from each cane was chosen at random and its area measured using the image processing software, ImageJ (version 1.37v) (Hill et al. Citation2005). The mean volume of the H. lataniae insects from each variety was estimated from the mean scale cap areas, assuming that the insects were the same area as the cap (in reality it will be somewhat smaller), and were conical in shape, with the dorsal and ventral surfaces meeting at an angle of 30o. Based on these assumptions, the formula for the volume becomes:
The number of developing ‘embryos’ within the body of the female scale insect is an indicator of its fecundity. This was measured for randomly chosen scale insects from each treatment (n = 26 from ‘Hayward’ and 28 from ‘Hort22D’) by mounting the scale insect in water on a microscope slide and counting the maturing eggs within the body of each insect using a compound microscope (25× magnification).
Statistical analysis
Experiment 1
One-way analysis of variance assuming random treatment effects was carried out on scale cap area data for each scale species from the field and laboratory experiments separately on each time occasion (R version 2.1.1 [R-Development-Core-Team Citation2007]). Kendall's rank correlation (τ) was used to test for an association between ranks, comparing the size of scale insect caps in the laboratory and field experiments, the size of the two scale insect species, and for comparing scale insect cap size with numbers of scale on fruit at harvest in 2003.
Experiment 2
A linear mixed-effects model was fitted to the cap area measurements to determine the relative effects of genotype and distance of the cane sample from the vine leader (adjacent vs. distant) on cap size using the nlme package within the R environment (Pinheiro et al. Citation2007; R-Development-Core-Team Citation2007). The model assumes that genotype and distance of cane sample from the vine leader (adjacent to or distant from the leader) were fixed effects, whereas vines and canes within vines were random effects.
Variance components were calculated from similar linear mixed models for each combination of species and genotype to ascertain the relative sizes of the variation due to vines, canes and cane part (adjacent and distant).
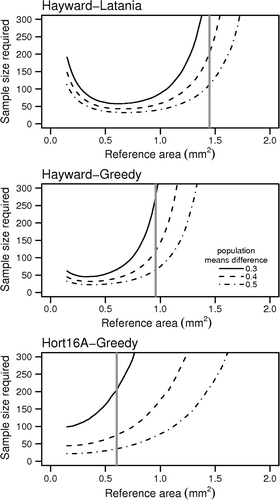
Following this preliminary analysis, populations each of size 100,000 were simulated to represent three genotype–insect species combinations (H. rapax on ‘Hayward’ and ‘Hort16A’ and H. lataniae on ‘Hayward’). The populations were sampled to provide estimates of the proportion of individuals larger than an arbitrary reference area. The variance of the cap areas was quite constant across all the genotypes and it was assumed that any genotype to be assessed would have a similar variance.
For each of the simulated scale insect populations, populations whose scale cap size means were on average 0.3–0.5 mm2 smaller were also simulated. By comparing the proportions of the simulated populations which were greater than an arbitrary reference area, the sample size needed to detect differences at α = 0.05 and β = 0.8 was determined. A large range of reference areas was used and the corresponding sample sizes calculated and plotted. No statistical analysis was necessary on the results of experiment 3 as the outcome was clear-cut.
Results
Preliminary analysis of the data from the first and second experiments showed that although the data were somewhat skewed, the residuals were approximately normal and transformation was unnecessary for the statistical analysis. However, for the simulated distributions, square-root transformation was necessary before population parameters (mean and variance) were calculated.
Experiment 1: Testing excised canes as a bioassay substrate
In the laboratory experiment after 68 days (second assessment occasion), 13% (26) of canes with H. lataniae and 6% (11) of canes with H. rapax were dead or dying. Scale insect caps from these canes were not used in the analysis.
H. lataniae
The ANOVA model explained 81% of the variance in the laboratory experiment and 61% in the field. There were large differences in mean scale cap area between kiwifruit genotypes in both the laboratory and field experiments at both sampling occasions (). A strong correlation was evident between mean H. lataniae cap area for each genotype in the laboratory and the field when comparing both the first (Kendall τ = 0.706; P < 0.0001; df = 21) and second (Kendall τ = 0.576; P < 0.001; df = 21) sampling assessment (). The H. lataniae insects on those canes that supported growth were mature, reproducing adults by the end of the experiment. By contrast, the scale insects on the canes that did not support scale growth were mostly dead or very small first or second instar insects.
Fig. 1 The relationship between mean H. lataniae cap size in the laboratory after 68 days and in the field after 57 days. Kendall τ = 0.576; P < 0.001; df = 21. The data fall into two equal sized groups, with a putative resistant group (open circles) showing little growth compared with the putative susceptible group (solid circles)
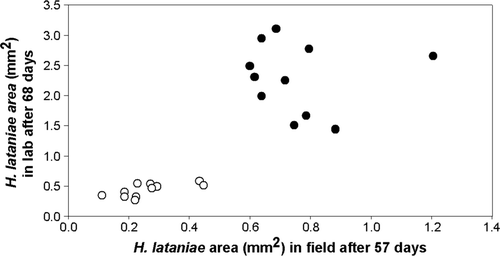
Table 1 Results of analysis of variance tests of the difference between mean cap size of H. lataniae and H. rapax in a laboratory bioassay and field experiments, reared on canes of 20 experimental genotypes and two commercial kiwifruit cultivars after two time periods. Also shown are the range (smallest and largest) of the mean armoured scale insect cap sizes and the sample sizes of data for scale size for genotypes within each experiment
The data in appear to divide the kiwifruit genotypes into two equal sized groups on the basis of mean H. lataniae cap size. This difference is most apparent when growth of individual scale insects between the first and second sampling occasions (days 46 and 68, respectively, in the laboratory bioassay) is calculated. The increase in the mean H. lataniae cap area between days 46 and 68 for the putative resistant group was 0.125±0.013 (SE) mm2, compared with 1.53±0.17 mm2 for the susceptible group.
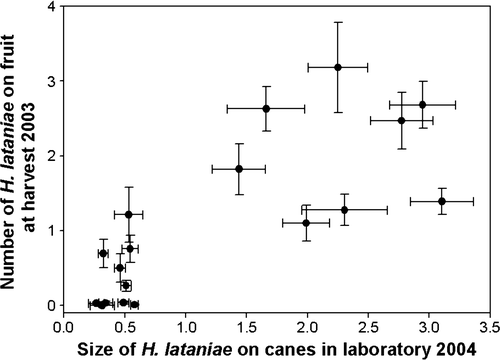
There was a significant correlation between the mean size of H. lataniae caps in the laboratory bioassay (68 days old) with the number of adult H. lataniae on fruit from 18 different genotypes sampled at harvest in 2003 () (Kendall τ = 0.74; P < 0.0001; df = 17).
Fig. 2 The relationship between H. lataniae size in the laboratory after 68 days in 2004 and the number of adult H. lataniae on fruit at harvest in the field on the same vines in 2003. Bars are standard errors of means. Average fruit sample per vine = 76 (range 33–111). Kendall τ = 0.74, P < 0.0001, df = 21.
H. rapax
The field data of one genotype were discarded due to poor crawler settlement. The H. rapax data were more variable than the H. lataniae data. The ANOVA model explained 33% of the data variability in the field, but only 19% in the laboratory data. There were small but significant differences between kiwifruit genotypes in H. rapax mean cap size at both the 46-day and 68-day assessments in the laboratory bioassay (). In the field, there were no significant differences between genotypes in the mean area of H. rapax caps after 35 days, but there were significant differences by day 57 (). There was no correlation between H. rapax cap size between laboratory and field measurements comparing either 46 day-old laboratory caps with 35 day-old field caps (Kendall τ = − 0.248; P = 0.116; df = 20) or comparing 68 day-old laboratory caps with 58 day-old field caps (Kendall τ = 0.066; P = 0.698; df = 20).
There was much less variation in mean H. rapax cap size between genotypes compared with H. lataniae and unlike H. lataniae, the genotypes did not separate into apparently resistant and susceptible groups when comparing mean cap sizes after 46 and 68 days. The average growth in mean H. rapax cap size between 46 and 68 day-old H. rapax based on the H. lataniae genotype groupings was 0.95±0.08 (SE) for the putative H. lataniae-susceptible genotypes and 1.03±0.08 (SE) for the putative H. lataniae resistant genotypes. There was no correlation between H. rapax size in the laboratory and adult H. rapax on fruit at harvest in 2003 (Kendall τ = 0.124; P = 0.5).
Comparison of H. rapax and H. lataniae growth
There was no correlation between H. lataniae and H. rapax mean cap area at either assessment occasion in the laboratory or the field ().
Table 2 Results of rank correlation tests of association (Kendall's τ) between H. lataniae and H. rapax cap size on different kiwifruit genotypes, comparing laboratory and field collected data. Correlations were carried out on √-transformed mean cap sizes
In the laboratory, the mean H. lataniae cap area from the 11 putative resistant genotypes was 0.43 mm2 (range 0.27–0.58 mm2, n = 11) compared with a mean cap area of 2.28 mm2 for the putative susceptible group of genotypes (range 1.44–3.11 mm2; n = 11). The H. rapax cap area measurements showed no groupings of genotypes and an overall mean H. rapax cap size in the laboratory at the end of the experiment of 1.8 mm2 (range 1.36–2.29 mm2, n = 21).
Experiment 2: Sources of variation in scale cap size
At the end of the experiment, after 62 days, three canes from a total of 192 were dead. Scale caps from these canes were not included in the analysis. The results of an analysis of variance of scale cap area for each armoured scale species found significant differences between the genotypes but no significant difference between cane parts (adjacent to or distant from the leader) nor any significant interaction between cane part and genotype (). There was considerable variability in scale cap size between genotypes and, for some genotypes, between vines (). H. lataniae failed to grow on any of the ‘Hort16A’ canes whereas one of the vines from experimental genotype C5 also prohibited H. lataniae growth. A summary of the variance components () shows that, overall, most of the variability in scale cap area occurs between scale insects within a cane. Five of the eight genotype-by- armoured scale species combinations in had residual variance proportions of more than 50%, with the remaining three genotypes over 20%. However, sources of variability of scale insect cap sizes vary considerably among genotypes and scale species. Three of the four genotypes showed very little variation between vines (). This may have been expected of the clonal commercial varieties ‘Hayward’ and ‘Hort16A’ (maximum 3.3%), but was also apparent in B6 (maximum 12.4%); however, C5 showed high between-vine variability (30% and 58%). While cane part showed no significant treatment effect in the ANOVA, it was a major source of scale cap variation in ‘Hort16A’ (41.6% and 33.1%) and for H. lataniae cap size on ‘Hayward’ (38%).
Fig. 3 Box plots showing median, 10th, 25th, 75th and 90th percentiles and outliers of H. lataniae and H. rapax size after growing on canes in a laboratory at 20°C for 62 days for each of the six vines from two experimental genotypes (5C and 6B [vines are full sibs]) and two commercial kiwifruit cultivars (‘Hort16A’ and ‘Hayward’ [vines are clonal])
![Fig. 3 Box plots showing median, 10th, 25th, 75th and 90th percentiles and outliers of H. lataniae and H. rapax size after growing on canes in a laboratory at 20°C for 62 days for each of the six vines from two experimental genotypes (5C and 6B [vines are full sibs]) and two commercial kiwifruit cultivars (‘Hort16A’ and ‘Hayward’ [vines are clonal])](/cms/asset/02f8782c-71ee-41e2-894d-3240d250215f/tnzc_a_477319_o_f0003g.gif)
Table 3 Results of ANOVAs testing for significant differences in cap size of H. lataniae and H. rapax reared on different cane parts (adjacent to or distant from the vine leader) of four kiwifruit genotypes (two experimental and two commercial), and their interaction
Table 4 Components of variance in scale cap area attributable to vines, canes and cane part (adjacent to or distant from the vine leader) for H. rapax and H. lataniae growing on two experimental genotypes, C5 and B6 (vine replicates are full sibs) and two commercial cultivars ‘Hort16A’ and ‘Hayward’ (vine replicates are clonal)
Comparing the mean scale cap areas of both armoured scale species between the two commercial clonal genotypes ‘Hayward’ and ‘Hort16A’, both H. lataniae and H. rapax were significantly smaller on ‘Hort16A’ compared with ‘Hayward’. H. lataniae died while still very small on ‘Hort16A’, whereas H. rapax continued to grow; although there was considerable variability in the size of H. rapax scale caps on ‘Hort16A’ canes (). On ‘Hayward’ fruit, H. lataniae grew to be larger than H. rapax ().
Table 5 Mean cap area for H. lataniae and H. rapax grown in the laboratory at 20°C for 62 days on canes of two commercial kiwifruit cultivars
Simulations of optimal sample sizes
The results of optimal experimental sample size calculations (α = 0.5, β = 0.8) to detect differences in mean H. lataniae and H. rapax cap areas () showed that sample sizes of around 50 are adequate for detecting large differences in cap size (0.5 mm2), but the required sample size rises to 100–200 scale for detecting differences of 0.3 mm2.
Experiment 3: Testing the bioassay
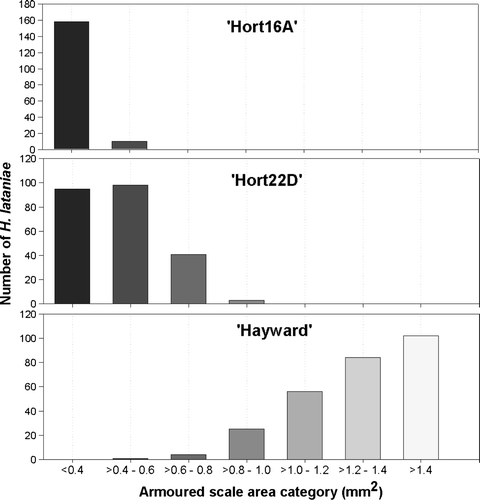
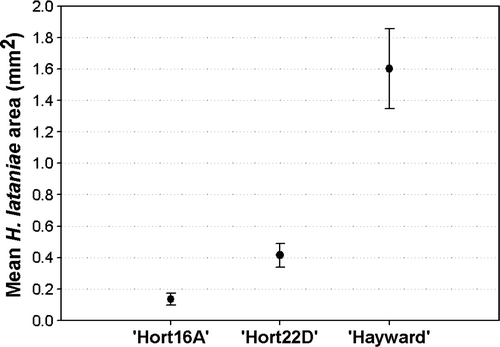
There were considerable differences in the size distribution of the insects between each of the three varieties (). Modal size for ‘Hort16A’ was <0.4mm2, 0.4–0.6 mm2 for ‘Hort22D’ and >1.4mm2 for ‘Hayward’. The mean estimated H. lataniae volume after 13 weeks on ‘Hayward’ canes (0.22 mm3) was 7.6 times larger than on ‘Hort22D’ canes (0.029 mm3) and 41 times larger than on ‘Hort16A’ canes (0.0054 mm3). All scale insects established on ‘Hort16A’ were dead after 13 weeks. The estimated mean H. lataniae volume after 13 weeks on ‘Hort22D’ canes was 5.4 times larger than on ‘Hort16A’. The insects established on ‘Hort22D’ and ‘Hayward’ were alive and crawler release had commenced on both species. The average number of embryos observed within adults developed on ‘Hort22D’ canes was 3.5 (SE = 2.8; n = 28), compared with 33.8 (SE = 2.2; n = 26) on ‘Hayward’ canes.
Discussion
We have demonstrated that lengths of excised kiwifruit cane, kept alive in a constant environment room for two to three months, will support the growth of armoured scale insects. The size of armoured scale insect caps on these canes can be used as a relative measure of the susceptibility of kiwifruit germplasm to armoured scale insects. This method shows promise as the basis of a bioassay for screening kiwifruit germplasm for resistance to armoured scale insects, and could be easily adapted to test germplasm from other perennial crop species against a range of diaspidid pests. However, the use of circular transparent templates for quickly estimating scale area can only be used for diaspidids that have circular caps.
Fig. 6 Mean (±95% CL) H. lataniae insect scale cap size after 13 weeks developing on canes from three kiwifruit varieties at 21°C and 70% RH
The significant correlations between the mean area of H. lataniae caps in the laboratory and field, and between H. lataniae cap area in the laboratory and H. lataniae on fruit at harvest in a previous season, suggest that a cut-cane bioassay measuring scale cap area may be used as a measure of kiwifruit genotype susceptibility to scale insects.
The lack of a correlation between H. rapax cap area in the laboratory and field is probably a reflection of the lack of a strong resistance response to H. rapax across the genotypes tested, reflected in a smaller variation in observed cap size between genotypes, but also to the relatively large within-cane variation in armoured scale cap size. However, the utility of this technique for measuring the resistance of kiwifruit genotypes to H. rapax requires further investigation on a wider range of germplasm, as does the cause of the large variation in H. rapax cap sizes measured within canes.
Analysis of the sources of variation in scale cap area has shown that individual canes are a primary source of variation between scale sizes. The reason for this is not known, but may be related to the settlement site. Armoured scale insects feed by emptying the contents of parenchyma and collenchyma cells (Washington & Walker Citation1990). Crawlers do not appear to sample the substrate prior to settling and spinning a white cap (Foldi Citation1990; Hill & Holmes Citation2009) and we have observed that crawlers of both species in this study prefer to settle beneath wool tied around the canes or adjacent to cracks in the bark, lenticels or other areas of bark roughness. Thus, if the substrate is chosen on the basis of surface features, it is possible that the quality of the food beneath may vary. Highly localized, induced defence responses of kiwifruit bark have been observed in some genotypes in response to H. lataniae feeding (N.A. Mauchline & M.G. Hill, unpublished data) and it is possible that the strength of those responses may vary between scale insect settlement sites. In addition, the insect must reinsert its new mouthparts back into the plant after each moult, and it is possible that success in relocating suitable cells on which to feed may vary. In spite of this inherent variability in scale cap area within a cane, the bioassay results comparing ‘Hort22D’ with the two commercial cultivars demonstrated that the technique is adequate, even when using categorical cap sizes, for assessing the relatively large differences in armoured scale insect size expected of comparisons between resistant and susceptible genotypes.
The optimal duration for the bioassay needs to be based on an understanding of scale growth. Differences between genotypes were well established at the first sampling occasion, 46 days after set up, showing that a bioassay need take no longer than six weeks. During the development of the photographic scale size measurement technique, observations of scale size over time were shown to fit a logistic growth equation, and differences in scale size between genotypes became apparent when the insects were midway through their development and persisted thereafter (Hill et al. Citation2005). Based on known relationships between temperature and development on potatoes under laboratory conditions, for H. rapax (Greaves et al. Citation1994) and H. lataniae (unpublished data), we calculated that at a constant temperature of 21°C in the laboratory, H. lataniae and H. rapax should complete their second moult 35 and 39 days, respectively, after settlement and should begin to lay eggs 78 and 81 days after settlement. Thus, the sampling times of 46 and 68 days were known to correspond with a period of significant logistic growth and this was confirmed by the observed change in mean scale cap size between the 46- and 68-day sampling times. More work is needed to confirm optimal sampling intervals, but the results here show that good results can be obtained with intervals between settlement and scale cap measurement of six to nine weeks with minimal cane death.
The results of the bioassay trial of ‘Hort22D’ showed that it is substantially resistant to H. lataniae, retarding growth but not killing the insect. However, unlike ‘Hort16A’, the insects were able to survive and commence reproduction. Given the small size of the insects and the 10-fold reduction in the number of embryos that the insect can mature at once, it is highly likely that the reproductive potential of H. lataniae on ‘Hort22D’ will be substantially reduced. However, this requires confirmation. This effect of partial resistance leading to very small adult scale insects which retain the capacity to reproduce, has not previously been observed on kiwifruit in New Zealand, but has been recorded for white peach scale on tea varieties in Japan (Mizuta Citation2003).
The technique of using a template in the ‘Hort22D’ experiment to categorize cap size worked well and has shown itself capable of discriminating fairly small differences in mean scale insect scale cap area which is rapid, robust and easy to use. This opens the way for future susceptibility testing of elite kiwifruit selections, parental germplasm and male pollinizers against scale insects.
The differences in H. lataniae and H. rapax cap size in the commercial kiwifruit genotypes ‘Hayward’ and ‘Hort16A’ correlates well with the findings of a recent survey of armoured scale species on leaves and fruit of both genotypes of commercial kiwifruit at harvest throughout New Zealand in 2007 (Edwards et al. Citation2008). On ‘Hayward’, H. lataniae comprised 72% of identifications on leaves and 69% on fruit; while on ‘Hort16A’, H. rapax comprised 70% of identifications on leaves and 99.5% on fruit.
Although this study has demonstrated the potential of using armoured scale insect growth on excised live canes as a screening tool for kiwifruit germplasm resistance to armoured scale insects, several additional factors should be considered when developing this method into a workable bioassay. These include the effects of cane age and cane storage on scale growth, and the possible interaction between storage conditions and genotype. Cane death was a problem during the first experiment, but was insignificant in the second experiment. This may be because the canes were kept in slightly better conditions and water reservoirs were topped up more regularly in the second experiment. However, subsequent trials should check that cane death is not related to genotype. The length of time that canes are stored in a cold store prior to commencement of the experiment and the temperature under which the test is carried out may both influence cane longevity.
Scale insect quality remains an unknown factor and a potential obstacle to developing reliable resistance bioassays of this nature. The two armoured scale insect species used in this study are slow to develop in culture and often exhibit poor settlement. Quality assurance in laboratory-reared insects is a well-researched topic but previous work on armoured scale insect mass rearing (Flanders Citation1951; DeBach & White Citation1960; Tashiro Citation1966) relates mainly to natural enemy production for biological control, where quality is assessed by easily measurable characters of the natural enemies. We have no measures of the quality for our uniparental armoured scale insect cultures and, while we have no evidence to suggest that the quality of our insects is variable, it remains a potential source of variability and inaccuracy, given previous studies showing the potential for deme development and local adaptation within this group of sedentary insects (Edmunds & Alstad Citation1978; Hanks & Denno Citation1994; Alstad Citation2002).
Further work is needed to investigate how armoured scale cap growth and cap area relates to the biology of the insect on a range of genotypes. In the case of H. lataniae on ‘Hort16A’, it is clear that the insect cannot develop on the bark of this cultivar. However, for other genotypes, on which limited but significant scale cap growth occurs, for example ‘Hort22D’, there may be a need to relate bioassay measures of scale cap area to the biology of the insect, in particular survival and fecundity (Hill et al. Citation2009). The results support the conclusion that scale cap size is a useful and practical measure of kiwifruit cane susceptibility to armoured scale insects. Measurement of armoured scale insect survival after crawler settlement would also contribute to a resistance bioassay, particularly where the focus is on finding highly resistant genotypes (e.g., ‘Hort16A’ in this study), however this would not capture more subtle resistance effects such as those observed with ‘Hort22D’ and H. lataniae and recently on tea varieties showing susceptibility to white peach scale, Pseudaulacaspis pentagona (Targioni) (Mizuta Citation2003).
There is a modest published literature on the field-screening of woody perennially crop cultivars for armoured scale insect resistance by assessing insect population levels (e.g., Masoodi et al. Citation1996; Eliason & McCullough Citation1997; Jalaluddin Citation1999; Yanovskii Citation2001), but there are few studies that have investigated the detailed response of the armoured scale insect to the plant (Hare et al. Citation1990; Mizuta Citation2003; Hill et al. Citation2009). None have sought to develop a quick bioassay for mass screening of germplasm. Given the ample evidence in the literature for the differential resistance of crop genotypes to armoured scale insects, such techniques would appear to have a wide application.
H. rapax and H. lataniae are indistinguishable morphologically except in the adult stage. Both species are highly polyphagous, having been recorded feeding on hundreds of different host genera from scores of plant families (Davidson & Miller Citation1990), and yet they respond quite differently when feeding on different genotypes of A. chinensis. The reasons for this are unknown, but are likely to be genetically based (Hill et al. Citation2007). Future research will focus on refining the prototype bioassay and using it as an aid to understand the basis for resistance of kiwifruit germplasm to armoured scale insects, and in the development of future kiwifruit cultivars with enhanced levels of resistance to armoured scale.
Acknowledgements
We thank Doug Allan for assistance with the first experiment. Cathy McKenna and Dave Rogers made valuable comments on an earlier draft. The work was funded by the New Zealand Foundation for Research Science and Technology (project CO6X0301, Insecticide Risk Reduction) and ZESPRI Group Ltd.
References
- Alstad , DN . 2002 . “ Population structure and the conundrum of local adaptation ” . In Mechanisms and deployment of resistance in trees to insects , Edited by: Wagner , MR , Clancy , KM , Lieutier , F and Paine , TD . 3 – 21 . Dordrecht : Kluwer .
- Anon 2001 . Zespri Kiwi Green Manual . Tauranga, , New Zealand ZESPRI Group Ltd .
- Anon 2007 . FreshFacts—New Zealand Horticulture 2006, HortResearch . Auckland, , New Zealand , Plant and Food Research Ltd .
- Berry JA , Morales CF , Hill MG , Lofroth BJ , Allan DJ 1989 . The incidence of three diaspid scales on kiwifruit in New Zealand Proceedings of the 42nd New Zealand Weed and Pest Control Conference . New Plymouth : 8–10 August 1989 . 182 185 .
- Blank , RH , Gill , GSC and Upsdell , MP . 1996 . Greedy scale, Hemiberlesia rapax (Hemiptera: Diaspididae), phenology on kiwifruit leaves and wood . New Zealand Journal of Crop and Horticultural Science , 24 ( 3 ) : 239 – 248 .
- Blank , RH , Gill , GSC and Stannard , K . 2000 . Armoured scale infestation of fruit of Hort16A Kiwifruit . New Zealand Plant Protection , 53 : 205 – 210 .
- CABI 2007 . Crop Protection Compendium . http://www.cabicompendium.org/cpc/home.asp (accessed 14 August 2008) .
- Charles , JG and Henderson , RC . 2002 . Catalogue of the exotic armoured scale insects (Hemiptera: Coccoidea: Diaspididae) in New Zealand . Journal of the Royal Society of New Zealand , 32 : 587 – 615 .
- Cheng , CH , Seal , A , Boldingh , H , Marsh , K , MacRae , EA , Murphy , S and Ferguson , AR . 2004 . Inheritance of taste characters and fruit size and number in a diploid Actinidia chinensis (kiwifruit) population . Euphytica , 138 : 185 – 195 .
- Davidson , JA and Miller , D . 1990 . “ Ornamental plants ” . In Armored scale insects, their biology, natural enemies and control Vol 4B , Edited by: Rosen , D . 603 – 632 . Amsterdam, , Netherlands : Elsevier .
- DeBach , P and White , EB . 1960 . Commercial mass culture of California red scale parasite Aphytis lingnanensis . California Agricultural Experiment Station Bulletin , 770 : 1 – 58 .
- Edmunds , GF and Alstad , DN . 1978 . Coevolution of insect herbivores and conifers . Science , 199 : 941 – 945 .
- Edwards , R , Carraher , C , Todd , JH , Dobson , S , Mauchline , N , Hill , G , McKenna , C and Newcomb , R . 2008 . DNA diagnostics of three armored scale species on kiwifruit in New Zealand . Journal of Economic Entomology , 101 ( 6 ) : 1944 – 1949 .
- Eliason , EA and McCullough , DG . 1997 . Survival and fecundity of three insects reared on four varieties of Scotch pine Christmas trees . Journal of Economic Entomology , 90 ( 6 ) : 1598 – 1608 .
- Ferguson , AR . 2004 . 1904—the year that kiwifruit (Actinidia deliciosa) came to New Zealand . New Zealand Journal of Crop and Horticultural Science , 32 : 3 – 27 .
- Flanders , SE . 1951 . Mass culture of California red scale and its golder Chalcid parasites . Hilgardia , 21 : 1 – 41 .
- Foldi , I . 1990 . “ The scale cover ” . In Armoured scale insects, their biology, natural enemies and control , Edited by: Rosen , D . 43 – 54 . Amsterdam : Elsevier .
- Greaves , AJ , Davys , JW , Dow , BW , Tomkins , AR , Thomson , C and Wilson , DJ . 1994 . Seasonal temperatures and the phenology of greedy scale populations (Homoptera: Diaspididae) on kiwifruit vines in New Zealand . New Zealand Journal of Crop and Horticultural Science , 22 ( 1 ) : 7 – 16 .
- Hanks , LM and Denno , RF . 1994 . Local adaptation in the armored scale insect Pseudaulacaspis pentagona (Homoptera: Diaspididae) . Ecology , 75 ( 8 ) : 2301 – 2310 .
- Hare , JD , Yu , DS and Luck , RF . 1990 . Variation in life history parameters of California red scale on different citrus cultivars . Ecology , 71 ( 4 ) : 1451 – 1460 .
- Hill , MG , Mauchline , N , Cate , LR and Connolly , PG . 2005 . A technique for measuring the growth rate of armoured scale insects . New Zealand Plant Protection , 58 : 288 – 293 .
- Hill , MG , Mauchline , N , Cheng , CH and Connolly , PG . 2007 . Measuring the resistance of Actinidia chinensis to armoured scale insects . Acta Horticulturae , 753 : 685 – 692 .
- Hill , MG , Mauchline , NA , Hall , AJ and Stannard , KA . 2009 . Life table parameters for two armoured scale insect species on resistant and susceptible kiwifruit (Actinidia sp.) germplasm . New Zealand Journal of Crop and Horticultural Science , 37 ( 4 ) : 335 – 343 .
- Hill , MG and Holmes , T . 2009 . An analysis of latania scale (Hemiberlesia lataniae) crawler settlement behaviour on kiwifruit leaves and bark . New Zealand Plant Protection , 62 : 56 – 62 .
- Jalaluddin , SM . 1999 . Preliminary screening of betelvine cultivars against scale insect . Insect Environment , 4 ( 4 ) : 150
- Masoodi , MA , Sofi , AR , Bhagat , AR and Koul , VK . 1996 . Relative susceptibility of apple cultivars to San Jose scale, Quadraspidiotus perniciosus (Comstock) . Pest Management and Economic Zoology , 4 ( 1–2 ) : 119 – 121 .
- Mauchline , NA and Hill , MG . 2005 . Settlement of armoured scale insects on fruit of commercial Actinidia spp . New Zealand Plant Protection , 58 : 294 – 298 .
- Miller , D and Davidson , JA . 1990 . “ A list of the armoured scale insect pests ” . In Armored scale insects, their biology, natural enemies and control , Edited by: Rosen , D . 299 – 306 . Amsterdam : Elsevier .
- Mizuta , T . 2003 . Differences in development and reproduction of the mulberry scale, Pseudaulacaspis pentagona Targioni (Hemiptera: Diaspididae), on resistant and susceptible varieties of tea plant . Japanese Journal of Applied Entomology and Zoology , 47 ( 3 ) : 91 – 95 .
- Morales , CF . 1988 . The occurrence of latania scale, Hemiberlesia lataniae (Signoret) (Hemiptera: Diaspididae), in New Zealand . New Zealand Journal of Experimental Agriculture , 16 ( 1 ) : 77 – 82 .
- Pinheiro J , Bates D , Saikal D , Deepayan S 2007 . nlme: linear and non-linear mixed effects models. R Package, version 3 1 86 .
- R-Development-Core-Team 2007 . R: A language and environment for statistical computing . Vienna, , Austria , R Foundation for Statistical Computing . http://www.R-project.org
- Steven , D . 1990 . “ Entomology and kiwifruit ” . In Kiwifruit science and management , Edited by: Warrington , IJ and Weston , GC . 363 – 412 . Palmerston North, , New Zealand : New Zealand Society for Horticultural Science .
- Suckling , DM , McKenna , C and Walker , JTS . 2003 . “ IPM in New Zealand horticulture ” . In Global perspectives in integrated pest management , Edited by: Maredia , KM , Dakouo , D and Mota-Sanchez , D . 385 – 396 . London : CABI .
- Tashiro , H . 1966 . Improved laboratory techniques for rearing California red scale on lemons . Journal of Economic Entomology , 59 : 604 – 608 .
- Walker , TJ and Wineriter , SA . 1981 . Marking techniques for recognizing individual insects . The Florida Entomologist , 64 ( 1 ) : 18 – 29 .
- Washington , JR and Walker , GP . 1990 . Histological studies of California red scale (Homoptera: Diaspididae) feeding on citrus . Annals of the Entomological Society of America , 83 ( 5 ) : 939 – 948 .
- Webby , JE . 2004 . Celebrating 100 Years—The New Zealand Kiwifruit Industry , Tauranga, , New Zealand : Zespri International Ltd .
- Yanovskii , YP . 2001 . The resistance of apple to San Jose scale . Sadovodstvo i Vinogradarstvo , 1 : 13 – 14 .