Abstract
Toxic honey is produced in New Zealand when honey bees (Apis mellifera) forage on honeydew containing the phytotoxin tutin that is produced by the passion vine hopper (Scolypopa australis) when feeding on the poisonous plant tutu (Coriaria arborea). Observational studies suggest that there are a set of conditions required for the production of toxic honey, but these factors have not been well studied. This research aims to investigate the importance of altitude and weather conditions for the production of toxic honey in New Zealand and makes some recommendations for a review of the New Zealand Food Safety Authority (NZFSA) Food (tutin in honey) Standard 2008 that was introduced after a toxic honey outbreak in 2008. Transects were established in parts of the North Island during the summer of 2009 and were visited weekly for 6 consecutive weeks. Variables recorded were elevation, S. australis density, honeydew abundance, honey bee and wasp (Vespula spp.) presence and temperature and rainfall data. Weather conditions present during the 2008 outbreak were compared with those from 1999 to 2009. S. australis were not present at altitudes of 375 m and above; with more research, some higher areas of New Zealand may be able to be reclassified as ‘low risk’. Honeydew was available in a wide range of temperature and rainfall conditions. Rainfall appeared to wash honeydew from tutu bushes; however, it quickly reappeared in densely populated sites. Honey bee and wasp presence was positively affected by the presence of honeydew, nevertheless 30–50% of the time they were observed foraging in the absence of honeydew, indicating they may feed on small amounts of dew. As the absence of honeydew is an option given by the NZFSA to demonstrate honey safety, this requires investigation. Weather conditions leading up to the 2008 toxic honey outbreak were warm and dry but were not significantly different from other years. Therefore, inadequate management practices, rather than more favourable environmental conditions were probably responsible for the 2008 toxic honey outbreak. Future research should focus on determining the conditions required for toxic levels of honeydew to be incorporated into hives, and regions that have not produced toxic honey historically should be closely studied to determine whether any fit the low-risk criteria.
Introduction
Human toxic honey poisonings have occurred throughout the world, in places such as the USA (Gunduz et al. Citation2008), Turkey (Sutlupmar et al. Citation1993; Koca & Koca Citation2007; Gunduz et al. Citation2008), Japan (Saisho et al. Citation1994) and New Zealand (Howes Citation1949; Love Citation1990). Toxic honey is produced in New Zealand when honey bees (Apis mellifera) collect honeydew produced by passion vine hopper bugs (Scolypopa australis, Ricaniidae) that feed on the sap of the native tutu bush (Coriaria arborea, Coriaceae). Tutu contains the natural phytotoxin tutin, which is harmless to insects but highly toxic to mammals, including humans (Palmer-Jones Citation1947). The symptoms of tutin poisoning in humans include vomiting, dizziness, delirium, increased excitability, coma and violent convulsions. Deaths have occurred as well (Love Citation1990; NZFSA Citation2008a).
Toxic honey poisonings in New Zealand have occurred sporadically and infrequently over the past 120 years (Love Citation1990; NZFSA Citation2008a). Most cases have been centred in the Eastern Bay of Plenty and the Coromandel Peninsula in the North Island and Marlborough Sounds in the South Island (NZFSA Citation2003, Citation2008a). The most recent toxic honey outbreak occurred in March 2008 in Whangamata, on the Coromandel Peninsula from comb honey collected in January 2008. There were 22 reported cases of human poisonings which included severe illness and hospitalisation (NZFSA Citation2008a).
Following this high profile outbreak, the New Zealand Food Safety Authority (NZFSA) developed a new Food Standard (Food [tutin in honey] Standard 2008) in order to better regulate the safety of honey produced in New Zealand. Prior to development of the Food Standard, beekeepers had a responsibility under the Animal Products Act 1999 and the Food Act 1981 to ensure that their honey was safe for human consumption. However, there were no specific guidelines in place for beekeepers to follow. The new Food Standard, which came into operation on 25 January 2009, requires beekeepers to demonstrate compliance for all honey that is harvested between 1 January and 30 June with one of the following options: the honey is tested and contains less than the set maximum level of tutin (<2 mg/kg for extracted honey, <0.1 mg/kg for comb honey); an approved risk management programme (RMP) or food safety programme (FSP) is implemented; the honey is collected from an area with an absence of ‘significant’ amounts of tutu; the honey is collected from an area where honeydew is absent from tutu bushes; or the honey is collected from a specified low-risk location.
Public submissions made to the draft Food Standard voiced concern that there may be insufficient research to validate the need for the new requirements. Several submissions were made in support of research into the exact conditions under which toxic honey is likely to occur and support was shown for more specific high- and low-risk zoning throughout the country (NZFSA Citation2008b). Currently, the entire North Island and the Marlborough region of the South Island are classified as high-risk regions, whereas the only low-risk region is the remainder of the South Island. The NZFSA does acknowledge the significant gaps in data and allows for changes to the Food Standard to be made if future research supports this.
Current knowledge of toxic honey production is largely based on observational studies that suggest there are a number of conditions required for the production of toxic honey. These are high densities of tutu; high densities of S. australis; hot, dry weather conditions; shortage of preferred nectar supplies; and the presence of managed beehives (Love Citation1990; NZFSA Citation2003). Elevation is also thought to restrict the distribution of S. australis populations in New Zealand (Cumber Citation1966). While these potential risk factors are commonly assumed to play important roles in the production of toxic honey in New Zealand and are sometimes used as management tools, hitherto they have received little experimental attention. Although the 2008 toxic honey outbreak had many adverse effects, it offers a unique opportunity to explore the role of weather conditions in the production of toxic honey.
The primary purpose of this study was to investigate the importance of altitude and weather conditions as factors that may influence the production of toxic honey in New Zealand. Additional aims were to make some informed recommendations for consideration in the upcoming review of the NZFSA Food (tutin in honey) Standard and to provide direction for future research. These aims were addressed with the following objectives:
-
To investigate the distribution and density of S. australis populations in relation to elevation.
-
To investigate the relationships between weather (temperature and rainfall) conditions, S. australis population densities, honeydew presence and prevalence, and honey bee and wasp foraging activity.
-
To detect any significant differences in the weather conditions that were present during the 2008 toxic honey episode and other years.
Materials and methods
Elevation and relationships between weather conditions, S. australis population densities, honeydew presence and prevalence, and honey bee and wasp foraging activity
Data collection
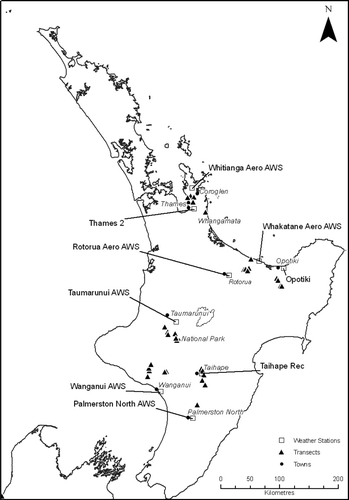
From mid-January to the end of February 2009, 40 transects were set up at various regions of the North Island (). Transects were chosen to be located at sites with high densities of tutu in close proximity (within a 3 km radius) to managed beehives. They were also chosen to represent a wide range of altitudes (18–548 m above sea level). Transects ranged in length from 5–30 m, depending on the amount of accessible tutu present, whether it be as dense and extensive vegetation or as isolated clumps. The location and elevation of each transect was recorded using a handheld GPS (Garmin GPS 60™).
Fig. 1 Locations of transects used to study Scolypopa australis densities and the prevalence and intensity of honeydew on tutu (Coriaria arborea) in January to February 2009 (closed triangles). The locations of the National Institute of Water and Atmospheric Research (NIWA) weather stations from which the weather data were obtained are also shown (open squares).
Each transect was visited once a week for 6 consecutive weeks, covering the period from 13 January to 25 February 2009. The variables recorded were: the density of S. australis nymphs and adults present on tutu along the transect; the presence of honey bees or wasps foraging on honeydew; and the approximate proportion of leaves with honeydew on them (0–100%).
The density of S. australis was estimated using a 1 metre square frame and this was divided into 10×10 centimetre squares to facilitate counting when there were high densities of S. australis.
Weather data
Daily rainfall and daily maximum and minimum temperature data were obtained from the National Institute of Water and Atmospheric Research's (NIWA) online CliFlo database (NIWA Citation2009). The NIWA weather stations used in this study were selected for relevance and quantity of data and proximity to transects (). The weather data was organized so that rainfall and temperature data were obtained for: the day of sampling; the 2 days prior to sampling; and the 7 days prior to sampling.
For the 2- and 7-day data, rainfall was cumulative and temperatures were averages. These categories were chosen to investigate the importance of weather variables over different time periods.
Statistical analysis
A simple regression (PROC REG) was performed to investigate the relationship between S. australis density and elevation. Weather variables were correlated with percent honeydew cover using the CORR procedure. Linear models (PROC GLM), which included the fixed effects of individual transects and each of the weather variables were also used to detect differences in honeydew abundance and honey bee presence. The GLM procedure, including transect and honeydew presence as fixed effects was run to assess whether the presence of honey bees and wasps at tutu bushes was affected by the presence of honeydew. Sites where no S. australis were found were excluded from the linear models. All data were analysed using the Statistical Analysis System (SAS version 9.1 ©2003, Cary, NC, USA).
2008 toxic honey outbreak
Temperature and rainfall data were acquired for Whangamata Township (37°216S, 175°877E) from a local resident. These data were used to examine the specific weather conditions present in Whangamata during the 2008 toxic honey outbreak. Weather conditions for November to January in the 2007–08 season were compared with weather conditions for the same period over the past 10 years using one-way ANOVA. For this analysis, temperature and rainfall data were obtained from NIWA's Whitianga Aero AWS (automatic weather station) (36 834S, 175 677E). This station was chosen because of its extensive records and relative proximity (ca. 43 km N) to Whangamata. The total number of days without rain was also compared between years for the month of January. This was an important comparison because an industry assumption had been that it was safe for bees to forage after heavy rainfall that had been thought to wash honeydew away. Data were analysed using SAS Enterprise Guide (version 4.1 © 2003, Cary, NC, USA).
Results
Elevation and S. australis density
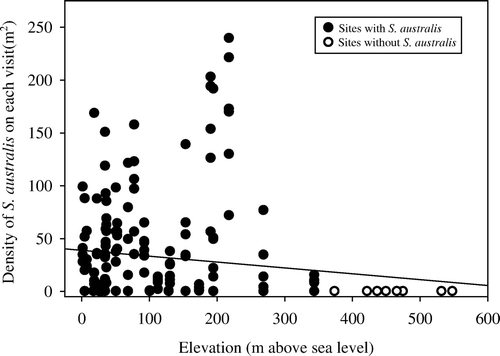
S. australis were not found at eight of the 40 transects. These transects were located at Moawhango (n=2), Taoroa (n = 1), Upper Kawhatau Valley (n=2) and Auputa (n = 1) in the Rangitikei region and Raurimu (n=2) in the Ruapehu region. All of these transects were at altitudes of 375–548 m above sea level. There was a significant but weak negative relationship (R 2=0.034, P=0.006) between combined nymph and adult S. australis density and elevation ().
Weather and honeydew
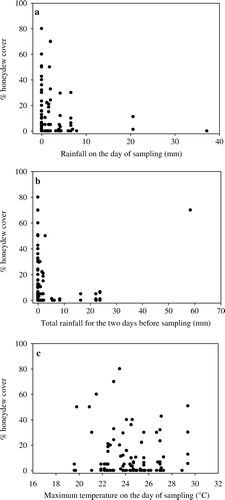
No significant correlations between the weather variables and percent honeydew cover were detected using the CORR procedure of SAS (Pearson correlation coefficients ranged from –0.008 to 0.102). However, the linear model (PROC GLM) found percentage honeydew leaf cover to be significantly affected by rainfall on the day of sampling, total rainfall for the 2 days prior to sampling and maximum temperature on the day of sampling (). Weather data collected over the week prior to sampling failed to predict percent honeydew cover altogether. Honeydew leaf cover differed significantly between transects (F (31,140)=5.62, P= < 0.001), but not between visits when these two factors were included as class effects.
Table 1 Summary of analysis of variance of weather (rainfall, maximum temperature [T max] and minimum temperature [T min]) and honeydew percent leaf cover for the 32 transects where S. australis were present
Graphical examination of the three weather variables with significant effects on honeydew cover show that in general, honeydew is most prevalent during warm conditions with little or no rainfall (). However, even significant amounts of rainfall both on the day of sampling (a) and combined rainfall for the 2 days prior to sampling (b) did not always result in the complete absence of honeydew. This finding is emphasised in b where the presence of an obvious outlier suggests either that heavy amounts of rain are not effective at washing honeydew away, or honeydew can re-accumulate to high levels soon after a significant rainfall event. In support of the latter, the field researchers observed honeydew reappearing at some high S. australis density sites within hours of rainfall ceasing. Honeydew was produced at any daily maximum temperature between 19 and 30°C (c).
Weather and honey bee presence
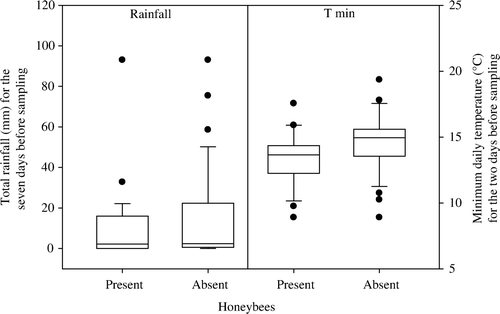
The model revealed a significant effect of weekly rainfall and average minimum daily temperatures for the 2 days prior to sampling on the presence of honey bees (). A significant effect of transect site on the presence of honey bees (F (31, 140)=2.57, P= < 0.001) was detected when this was included as a class effect.
Table 2 Summary of analysis of variance of weather (rainfall, maximum temperature [T max] and minimum temperature [T min]) and honey bee presence for the 32 transects where S. australis were present
Honey bees were slightly more likely to forage on honeydew during drier conditions (). However, it is likely the greater effect of weekly rather than daily rainfall is due to the fact that weekly rainfall was cumulative, increasing the chance of an absence of honey bees correlating with high amounts of rainfall. Although minimum daily temperature for the 2 days before sampling was found to significantly affect the presence of honey bees, graphical examination showed that honey bees were both present and absent from tutu bushes over a similar range of minimum temperatures ().
Honeydew and honey bee and wasp presence
Honey bees were present at sites with S. australis in 19% of the observations. An early, unexpected and significant finding was that wasps were present at these sites 68% of the time. This led to the modification of the second objective to include observation of wasp foraging activity. Overall, honeydew was present at transects with S. australis 42% of the time. The presence of honey bees and wasps was significantly affected by the presence of honeydew (honey bees: F (1, 170)=9.48, P=0.002; wasps: F (1, 169)=7.56, P=0.01). Of the times that honey bees were seen foraging at tutu bushes, honeydew was also present 67% of the time. For wasps, this was 48% of the time.
2008 toxic honey outbreak
The 2008 toxic honey outbreak occurred in late March; however, the honey was harvested on 20 January 2008. Weather conditions immediately leading up to the harvest time were warm and dry; rain had not fallen in significant quantities for about 6 weeks prior to harvest ().
Fig. 5 Daily rainfall and maximum (T max) and minimum (T min) temperatures recorded in Whangamata township (37 216S, 175 877E) by a local resident from 1 November 2007 to 31 January 2008. Toxic honey was harvested in the Whangamata region on 20 January 2008 (indicated by open circle).
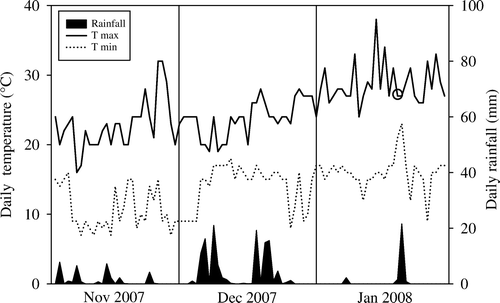
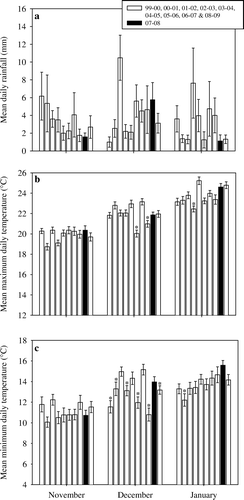
Weather conditions in the Whangamata area for the 2007–08 season were for the most part comparable to other years when toxic honey was not produced (). Mean daily rainfall for 2007–08 was low in November, December and January but not significantly lower than any of the other years. Mean daily maximum and minimum temperatures in each month also compared favourably with other years but generally grouped with those years with the highest temperatures. The total number of days without rain in January for the 2007–08 season was 26. For other years, this ranged from 13 days (2000–01) to 24 days (2004–05) (overall = 19.3±1.28 d [standard error]).
Fig. 6 Differences in mean rainfall and temperature conditions recorded by the Whitianga Aero AWS (36 834S, 175 677E) in November, December and January between 2007–08 (period that toxic honey was produced) and 1999–00 to 2008–09. Asterisks indicate significant differences (P<0.05) between the 2007–08 period and other years; error bars represent the standard error of the mean.
Discussion
The main findings of this study were that S. australis did not inhabit sites above 375 m altitude; honeydew abundance was affected by rainfall and temperature; honeydew reappeared soon after rainfall in densely populated sites; the presence of honey bees and wasps foraging at tutu bushes was positively affected by the presence of honeydew; and weather conditions leading up to the 2008 toxic honey outbreak were warm and dry but were not significantly warmer or drier than other years when toxic honey was not produced. The significance of these findings is discussed below.
Elevation
The results of this study support the findings of Cumber (Citation1966) in that S. australis do not populate higher altitude areas. Because this study was conducted over a 6-week period in the height of the adult activity season, high confidence can be placed on the absence of S. australis from the sites visited. However, only eight high elevation (>375 m) sites were studied and all were located in the Rangitikei and Ruapehu regions. Cumber (1996) experimentally demonstrated that eggs of S. australis require an over-wintering period for hatching to occur in the spring. Additionally, Gerard (Citation1985) found that eggs of S. australis require temperatures of at least 10°C to initiate eclosion. It is likely that cold winter temperatures at high altitudes limit the survival of eggs during the winter and are not sufficient to initiate hatching which is why S. australis are absent from many higher altitude areas, noting that Scolypopa is not particularly migratory. If this is the case, it is probable that S. australis populations in the warmer parts of New Zealand will not be restricted by elevation. Therefore, further analysis of altitudinal restrictions on S. australis populations should be conducted for specific regions in New Zealand. If this research is conducted, it may be possible for the NZFSA to reclassify some higher altitude areas of New Zealand as being low risk.
Weather and honeydew
Of the weather variables examined, honeydew abundance was most affected by rainfall on the day of sampling. Honeydew was most abundant on the day of sampling during conditions of little or no rainfall. Rainfall for the 2 days prior to sampling also significantly affected honeydew abundance, but graphical examination proved this relationship to be less rigorous. Some sites with high levels of rainfall 2 days before sampling still had high levels of honeydew present. In addition, the field researchers observed honeydew reappearing at some high S. australis density sites within hours of rainfall ceasing. Rapid reappearance of honeydew is an expected result when considering the feeding physiology of sap-sucking homopterans. When the bugs insert their stylets into the plants' sieve tubes, phloem is forced through their digestive tracts via turgor pressure and is subsequently released through the anus, creating a continuous flow of honeydew (Raven et al. Citation1999). Build-up of honeydew could also occur overnight if S. australis exhibit some nocturnal feeding activity as shown by the citrus flatid planthopper (Metclafa pruinosa) (Wilson & Lucchi Citation2007). To further assess the importance of rainfall for removing honeydew and reducing the risk of toxic honey being produced, future studies could make an attempt to quantify the rate of honeydew reappearance in relation to S. australis density by intensive monitoring of transects after rainfall. It should be noted that rainfall may not always result in the complete removal of honeydew from tutu leaves and stems. In New Zealand beech (Nothofagus spp.) forests, wasps (Vespula spp.) are known to forage on the diluted honeydew that results after a rainfall event (Harris et al. Citation1991).
Maximum daily temperature on the day of sampling also significantly affected honeydew abundance. Graphical examination showed that maximum temperatures between 19 and 30°C are all capable of facilitating high production of honeydew. Therefore, honeydew can be present within the normal temperature range during the summer in most parts of New Zealand as long as S. australis are also present. There is common belief that hot, dry summers allow for the greater accumulation of honeydew, resulting in an increased risk of toxic honey being produced. However, there is a possibility that honeydew excretion is actually reduced in high temperature, low humidity conditions. In sooty-beech scale (Ultracoelostoma spp.), honeydew droplets are evaporated in hot, dry conditions to the extent that further droplet formation becomes restricted (James et al. Citation2007).
Weather variables were collected from the nearest NIWA weather stations, and it is likely that the actual weather conditions at each transect differed somewhat from these. Future studies would benefit from the placement of automatic data loggers in closer proximity to transects to better investigate the relationship between weather and honeydew.
Weather and honey bees
Honey bee activity at tutu bushes with S. australis was relatively independent of temperature and rainfall conditions. Honey bee flight activity is known to be restricted by lower temperatures, foraging activity is low below 12–14°C and they do not fly below 9°C (Burrill & Dietz Citation1981; Kevan & Baker Citation1983; Vicens & Bosch Citation2000). Diurnal temperatures in this study were unlikely to have fallen below this temperature threshold, a possible explanation for the absence of a relationship between honey bee presence and temperature. Rainfall is known to prevent honey bees from foraging; bees either return to the hive when they detect impending rain or they remain in the hive while it is raining (Crailsheim et al. Citation1999) and so a strong effect of rainfall was expected. That this was not observed indicates that there are other factors contributing to the presence or absence of honey bees at tutu bushes. Other weather factors such as humidity, solar radiation, wind velocity and atmospheric pressure are all known to affect worker bee flight activity (Szabo Citation1980; Burrill & Dietz Citation1981; Kevan & Baker Citation1983; Willmer Citation1983; Vicens & Bosch Citation2000) and may be better predictors of activity in New Zealand honey bees.
Honey bees are assumed to prefer other sources of nectar to honeydew and so the abundance of these sources may also affect the presence of bees at tutu bushes. In support of this, Tan et al. (Citation2007) observed Indian honey bees (A. cerana) to prefer non-toxic nectar to the toxic nectar of the Chinese plant Tripterygium hypoglaucum. When the non-toxic nectar was removed, the bees readily accepted the toxic nectar (Tan et al. Citation2007). In this case however, the toxic nectar had a poisonous effect on honey bees, whereas honeydew from tutu is harmless to bees (Palmer-Jones Citation1947). Nectar preference has not been experimentally determined in New Zealand and what may constitute a ‘preferred food source’ is currently undefined. Another factor that is likely to affect the foraging behaviour of honey bees is temporal variation in the nutritional requirements of the hive. For example, different larval types and stages require different ratios of carbohydrates (from nectar and honeydew) and proteins (from pollen) in their diet (Crailsheim et al. Citation1992; Babendeier et al. Citation2004).
Honeydew and honey bees
Honey bees were most likely to forage on tutu bushes when honeydew was present. However, approximately 30% of the time, honey bees were observed foraging in the absence of honeydew. One explanation for this is that they were feeding on microscopic amounts of honeydew. This is supported by the fact that wasps were observed feeding when no observable honeydew was present 50% of the time. One of the options given by the NZFSA to demonstrate honey safety is the lack of honeydew on tutu bushes (NZFSA Citation2008c, Citationd). If honey bees do in fact feed on microscopic amounts of honeydew then there is a possibility that a perceived absence of honeydew could result in the production of toxic honey. The incorporation of minute amounts of honey into hives may not pose a large risk at all but it would be wise to err on the side of caution and assume that if S. australis are present then honeydew will be as well. To help clarify this issue, future research should focus on quantifying the level of foraging that would result in the storage of enough honeydew to produce toxic honey. This will depend on the quantity of honey stored in the hive, variations in the concentration of tutin in the honeydew and the rate of incorporation of honeydew into honey stores as well as geographic, environmental and temporal factors. This research could be carried out through observational studies of honey bee behaviour and the inclusion of toxicological honey testing from experimental hives. This study investigated honeybees in general and not the differences between the Italian and Carniolan races of A. mellifera that are used in New Zealand beekeeping. Future studies could investigate whether there are any significant differences in tutu foraging behaviour between these two races.
2008 toxic honey outbreak
Examination of the weather data during the time that toxic comb honey was produced showed that weather conditions were dry and warm leading up to the date that the honey was harvested. This supports the paradigm that these kinds of conditions are required for the production of toxic honey. However, the 2007–08 summer was on average no warmer or drier than the other 9 years that were studied (2000 to 2009) suggesting that there is potential for toxic honey to be produced in any typical New Zealand summer. During January 2008, there were a high number of days without rain. Perhaps it is long periods of no rainfall, rather than average rainfall that increases the risk of toxic honey production. However, the years 2000 and 2005 also had a high proportion of no-rain days in January and toxic honey poisonings were not reported in those years.
Following the 2008 toxic honey episode, the NZFSA carried out a tutin survey of 146 honey samples taken from seven regions of New Zealand (NZFSA Citation2008a). Of these samples, 46 (32%) contained tutin. The positive samples were from Northland, Coromandel, Eastern Bay of Plenty/Gisborne, Northern Hawke's Bay and Marlborough. The results of this snapshot survey suggest that the production of toxic honey is widespread and there is a chance that mild poisonings may actually occur frequently but go undetected and or unreported. It is unclear whether this situation was unique to the 2007–08 season, but similarities in weather conditions over the past 10 years suggest this may be the norm rather than the exception. Unfortunately, now that beekeepers are following the guidelines of the Food Standard, the results of this survey cannot be compared with future studies as the previous situation has in effect now been lost.
Conclusions and recommendations
Altitude is likely to be an important factor for the production of toxic honey in New Zealand as higher altitude regions do not appear to support populations of S. australis. However, a more widespread survey is required to confirm this relationship for specific regions in order for the NZFSA to increase the number of low-risk areas in New Zealand. Investigation of weather conditions showed that honeydew can be present in a wide range of temperatures and while rainfall was effective at washing honeydew from leaves and stems, this removal was only temporary. Therefore, honeydew is likely to be available to honey bees in a wide array of weather conditions. Honey bee presence was strongly linked to honeydew presence but bees were observed foraging in the absence of honeydew, suggesting they were feeding on very small honeydew deposits. This has implications for the Food Standard, as the absence of honeydew is one of the options given to beekeepers to prove honey safety. Due to the similarities in rainfall and temperature conditions over the past 10 years, it seems that inadequate management of hives, rather than exceptionally favourable weather conditions were responsible for the size and severity of the 2008 toxic honey outbreak. Further, a survey conducted by the NZFSA suggests the phenomenon of toxic honey may be more widespread than previously thought.
The present study focused mainly on the importance of environmental conditions for the production of honeydew rather than the production of toxic honey itself. Now that it has been demonstrated that honeydew is available in a wide range of circumstances, future research should focus on determining the conditions required for toxic levels of honeydew to be incorporated into hives. However, geographical and temporal variation in densities of tutu and S. australis populations, the activity and behaviour of honey bees in relation to many factors as well as variations in the concentration of tutin in honeydew are all likely to render this a difficult task. Future research could also investigate whether other vegetation in the locality affects the foraging demand for tutu honeydew.
It is generally regarded that the new requirements of the Food Standard are essential for the management of toxic honey in the areas where this has traditionally been a problem. It is possible that regions lacking a history of toxic honey poisonings may not require the same restrictions. These regions should be closely researched in the future to determine whether any can be reclassified as low risk.
Acknowledgements
We thank those members of the New Zealand Beekeepers Association of New Zealand Incorporated who provided funding for this research and acknowledge the financial contributions made by the New Zealand Honey Industry Trust and the New Zealand Honey Packers Association Inc. We also thank Kaye Seymour at the New Zealand Department of Conservation for creating the map of the study area. We are very grateful to Russell Blake at Whangamata, who provided us with local weather records for the 2007–08 summer.
References
- Babendeier , D , Kalberer , N , Romeis , J , Flur , P and Bigler , F . 2004 . Pollen consumption in honeybee larvae: a step forward in the risk assessment of transgenic plants . Apidologie , 35 : 293 – 300 .
- Burrill , RM and Dietz , A . 1981 . The response of honeybees to variations in solar-radiation and temperature . Apidologie , 12 ( 4 ) : 319 – 328 .
- Crailsheim , K , Riessberger , U , Blaschon , B , Nowogrodzki , R and Hrassnigg , N . 1999 . Short-term effects of simulated bad weather conditions upon the behaviour of food-storer honeybees during day and night (Apis mellifera carnica Pollmann) . Apidologie , 30 ( 4 ) : 299 – 310 .
- Crailsheim , K , Schneider , LHW , Hrassnigg , N , Buhlmann , G , Brosch , U , Gmeinbauer , R and Schoffmann , B . 1992 . Pollen consumption and utilization in worker honeybees: dependence on individual age and function . Journal of Insect Physiology , 38 : 409 – 419 .
- Cumber , RA . 1966 . Factors influencing population levels of Scolypopa australis Walker (Hemiptera-Homoptera: Ricaniidae) in New Zealand . New Zealand Journal of Science , 9 ( 2 ) : 336 – 357 .
- Gerard PJ 1985 The ecology of Scolypopa australis and its parasite Centrodora scolypopae Unpublished thesis, University of Waikato, Hamilton 257
- Gunduz , A , Turedi , S , Russell , RM and Ayaz , FA . 2008 . Clinical review of grayanotoxin /mad honey poisoning past and present . Clinical Toxicology , 46 ( 5 ) : 437 – 442 .
- Harris , RJ , Moller , H and Tilley , JAV . 1991 . Weather-related differences in attractiveness of protein foods to Vespula wasps . New Zealand Journal of Ecology , 15 ( 2 ) : 167 – 170 .
- Howes , FN . 1949 . Sources of poisonous honey . Kew Bulletin , 4 ( 2 ) : 167 – 171 .
- James , A , Dungan , R , Plank , M and Ito , R . 2007 . A dynamical model of honeydew droplet production by sooty-beech scale insects (Ultracoelostoma spp.) in New Zealand Nothofagus forest . Ecological Modelling , 209 ( 2–4 ) : 323 – 332 .
- Kevan , PG and Baker , HG . 1983 . Insects as flower visitors and pollinators . Annual Review of Entomology , 28 : 407 – 453 .
- Koca , I and Koca , AF . 2007 . Poisoning by mad honey: a brief review . Food and Chemical Toxicology , 45 ( 8 ) : 1315 – 1318 .
- Love , JL . 1990 . Toxic honey—a New Zealand story . Analytical Proceedings , 27 : 87 – 89 .
- NIWA 2009 . CliFlo National Climate Database . National Institute of Water and Atmospheric Research .
- NZFSA 2003 . Background on toxic honey . http://www.nzfsa.govt.nz/animalproducts/publications/info-pamphlet/bee-products/toxic-honey.htm (accessed 29 February 2009) .
- NZFSA 2008a . Tutin in honey . NZFSA public discussion paper . New Zealand Food Safety Authority , Wellington 23
- NZFSA 2008b . Summary of submissions received on NZFSA Public Discussion Document 09/08: Tutin in honey . Wellington , New Zealand Food Safety Authority 14 .
- NZFSA 2008c . Food (tutin in honey) Standard 2008 . Wellington , New Zealand Food Safety Authority 7 .
- NZFSA 2008d . Compliance guide: the Food (tutin in honey) Standard 2008 . Wellington , New Zealand Food Safety Authority 17 .
- Palmer-Jones , T . 1947 . A recent outbreak of honey poisoning. Part I historical and descriptive . The New Zealand Journal of Science and Technology Section A , 29 ( 3 ) : 107 – 114 .
- Raven , PH , Evert , RF and Eichorn , SE . 1999 . Biology of plants , 6th edn , New York : WH Freeman and Company .
- Saisho , K , Toyoda , M , Takagi , K , Satake , M , Takahashi , S , Yamamoto , Y , Kasai , K , Hashimoto , S and Saito , Y . 1994 . Identification of aconitine in raw honey that caused food poisoning . Journal of the Food Hygienic Society of Japan , 35 ( 1 ) : 46 – 50 .
- Sutlupmar , N , Mat , A and Satganoglu , Y . 1993 . Poisoning by toxic honey in Turkey . Archives of Toxicology , 67 ( 2 ) : 148 – 150 .
- Szabo , TI . 1980 . Effects of weather factors on honeybee activity and colony weight gain . Journal of Apicultural Research , 19 : 164 – 171 .
- Tan , K , Guo , YH , Nicolson , SW , Radloff , SE , Song , QS and Hepburn , HR . 2007 . Honeybee (Apis cerana) foraging responses to the toxic honey of Tripterygium hypoglaucum (Celastraceae): changing threshold of nectar acceptability . Journal of Chemical Ecology , 33 ( 12 ) : 2209 – 2217 .
- Vicens , N and Bosch , J . 2000 . Weather-dependent pollinator activity in an apple orchard, with special reference to Osmia cornuta and Apis mellifera (Hymenoptera: Megachilidae and Apidae) . Environmental Entomology , 29 ( 3 ) : 413 – 420 .
- Willmer , PG . 1983 . Thermal constraints on activity paterns in nectar-feeding insects . Ecological Entomology , 8 : 455 – 469 .
- Wilson , SW and Lucchi , A . 2007 . Feeding activity of the flatid planthopper Metcalfa pruinosa (Hemiptera: Fulgoroidea) . Journal of the Kansas Entomological Society , 80 ( 2 ) : 175 – 178 .