Abstract
Fruit dry matter content (DM) of kiwifruit, defined as the fruit dry weight (DW) expressed as a percentage of the fresh weight (FW), is used as an indicator of eating quality. A high DM at harvest results in increased consumer satisfaction. To determine the role of carbohydrate availability and sink competition in altering fruit size and DM at commercial harvest, we used an experimental model system based on phloem-girdled shoots to manipulate the number of source leaves, fruit and vegetative sinks on a shoot of ‘Hayward’ kiwifruit. Commercially produced kiwifruit fruits typically weigh 95–115 g and have a DM of 14–17 units (this is a percentage but the % sign has not been shown, to reduce confusion with changes in DM expressed as a % change). With our experimental system, this corresponded to the fruit growth observed with two to three leaves when there was no vegetative competition. With four source leaves and a single fruit, competition from a vegetative regrowth reduced fruit fresh weight by 28% and dry weight by 39%. With two fruit, and no vegetative sink, between-fruit competition had less severe effects on the fruit and dry weight. Doubling the number of fruit on a girdled shoot reduced average fruit fresh weight by 15%, dry weight by 23% and DM by 12%. With a high source supply (seven leaves with no regrowth competition) fruit growth was very high with a fresh weight of 174 g and 18.4 DM, substantially above that of commercial production.
Keywords:
Introduction
Consumers prefer kiwifruit that are large and have a high soluble solids content (SSC) when eating-ripe (Crisosto & Crisosto Citation2001; Lancaster Citation2002; Harker et al. Citation2009). Since kiwifruit are picked before the starch in the fruit has been fully converted to soluble sugars, it is not possible to measure the eating-ripe SSC at harvest. However, dry matter (DM) measured as dry weight (DW) or measured as a percentage of total fresh weight (FW) concentration of a fruit, provides a good estimate of ripe SSC (Richardson et al. Citation1997; Jordan et al. Citation2000). DW and FW are measured in grams, and DM is expressed as the percentage of the FW that is DW, that is 100×DW/FW which is often stated as a percentage. In this work we do not express DM with units of % as this can lead to confusion when comparing differences in treatment effects that are often expressed as a percentage change, so the two uses of percentage have the potential to be confused. In this work when percentage (%) is used, it refers to a treatment change expressed relative to the control. New Zealand kiwifruit orchardists are now paid a premium for fruit that meets the DM requirements of customers (Woodward et al. Citation2007). In commercial orchards, kiwifruit typically have a fresh weight (FW) of 95–115 g (Taylor et al. Citation2007) and DM of 14–17 (Burdon et al. Citation2004).
Kiwifruit vines produce excessive vegetative growth which competes with fruit growth for carbohydrate and other resources as well as creating shade. Modern canopy management methods are aimed at reducing vegetative growth and, over the past 15 years, these techniques have almost doubled the yield of high-quality fruit per vine. The physiological principles behind these changes are not well understood, so we do not know if we have reached the limit of improvements in harvest yield, or DM production.
Dry matter predominantly enters the fruit through the phloem while water enters via both the phloem and xylem and is lost by fruit transpiration. Hence DW is a measure of accumulated phloem flow, whereas DM is a complex mix of phloem, xylem and transpiration flows. An ecophysiological model of fruit growth and development of peach driven by phloem, xylem and transpiration flows has successfully reproduced important fruit traits including fruit FW and DW (Fishman & Génard Citation1998; Génard et al. Citation1998; Lescourret et al. Citation1998, Lescourret & Génard Citation2005) and recently has been used to describe genotypic variation within breeding populations (Quilot et al. Citation2005).
Higher plants consist of many sources of carbohydrate and many carbohydrate sinks. Growing plants orchestrate the balance between supply and demand to develop and mature in a way characteristic of the species. A single sink is prevented from dominating. In the case of more distributed reproductive sinks, there is more variability in fruit size, but this is still kept under remarkable control (Avery et al. Citation1979; Palmer Citation1992; Austin et al. Citation1999). Maximum fruit size is currently believed to be limited by its growth potential determined early in development, probably during an early stage of cell division (Grossman & DeJong Citation1995). Carbohydrate sinks are described by their sink strength, that is their ability to attract carbohydrate when supply is not limited (Waring & Patrick Citation1975; Grossman & DeJong Citation1994; Lacointe & Minchin Citation2008), and by their priority which describes their ability to compete when carbohydrate supply is limited. In the species that have been investigated to this detail, sink priority increases from roots to reproductive sinks in the following order (Wright Citation1989; Wardlaw Citation1990):
A high priority sink, for example the seeds, can have a small total requirement but when supply is reduced, it is the high priority sinks that get the limited resources. In contrast, roots have a low priority, such that at times of short supply they are starved of carbohydrate. Experiments with apple trees have shown that roots can die from lack of carbohydrate supply when they are over cropped (Palmer Citation1992).
The aim of this work was to quantify the effects of competition from vegetative sinks upon FW and DW accumulation with the fruit of kiwifruit vines. This information is needed for mechanistic understanding of these interactions and to determine what controls fruit DM.
Materials and methods
Experimental sites and treatments
Experimental work was carried out on the Te Puke Research Orchard, Bay of Plenty, New Zealand (37°49′S, 176°19′E) during the 2006–08 seasons. Mature (>10 year-old) Actinidia deliciosa (A. Chev.) C.F. Liang et A.R. Ferguson ‘Hayward’ kiwifruit trained on a pergola trellis were used.
The trial work was carried out on vines with an open canopy and with vigorous growth to avoid weak self-terminating shoots. This requirement meant that a different orchard block of kiwifruit vines was used each year. In year 1, the selected block had been managed to organic BioGro standards (BioGro Citation2009). In year 2, a different orchard block was used, with vines managed conventionally, which included use of hydrogen cyanamide to increase bud break and flowering.
Flowering was monitored on 20 tagged canes in the experimental block. The number of fully open flowers was assessed at about five-day intervals and the date of mid-bloom estimated as the 50% point of a fitted cumulative curve of the total number of open flowers verses date. Fruit growth is referenced as days after mid-bloom (DAMB).
When a growing kiwifruit shoot is pruned, the remaining most distal bud will usually break and develop into a secondary shoot, which is also known as a regrowth. We used shoot pruning as a method to induce a vegetative sink located near the fruiting zone to compete with a developing fruit.
Experimental shoots were initially pruned to length so that they contained seven leaves and two to four fruit. These leaves were mature and quite large (c. 130 cm2, diameter c. 13.0 cm [Snelgar & Thorp Citation1988]). All shoots were phloem girdled to restrict the flow of carbohydrate in or out of the shoot, and this girdle was kept open until fruit were harvested. In both years, shoots were pruned 10 days after flowering, thus stimulating regrowth. Girdles and the treatments were applied 10 days after that. In year 1, all unwanted regrowths were removed at about 50 DAMB and in year 2 at about 20 DAMB. An example of a girdled experimental shoot with four leaves, one fruit and a regrowth is shown in .
Fig. 1 A schematic diagram of the experimental unit for treatment 4L1FR showing the girdles at the base of the shoot. Initially the shoot has been pruned to length so that seven leaves and two fruits remained, and girdled to phloem isolate the experimental unit. About 20 days later, this was reduced to four leaves and one fruit. The initial pruning stimulated development of a vegetative regrowth and this was retained. Leaves were continually removed from the regrowth before they were fully expanded. In this way the regrowth acted as a net sink.
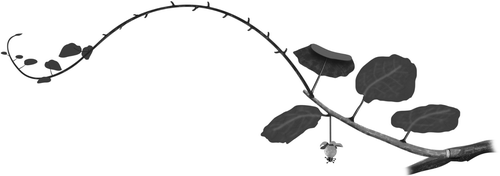
Each treatment was replicated 20 times, and all treatments were applied in a randomized block design. Sink/source relationships were manipulated by:
-
Adjusting source potential by setting the number of mature leaves on each shoot to two, four or seven. These are denoted 2L, 4L and 7L. Previous work has indicated that with six or more leaves, supply to the fruit was near saturation (Snelgar et al. Citation1986). We designed these experiments with most fruit supported by four leaves so as not to saturate growth of a single fruit. Under these source-limited conditions, any competing sink would be expected to significantly reduce fruit growth. In year 2, all shoots were pruned to four leaves. The number of fruit on the shoot is denoted by 1F or 2F. In year 1, only the 1F treatment was used; in year 2, both 1F and 2F treatments were used.
-
A vegetative sink was added by allowing a single vegetative regrowth to develop from the distal end of the primary shoot after pruning (see ). Since growth rates of vegetative shoots are typically 50 mm/day (Snelgar et al. Citation2005), these were expected to be strong sinks. To maintain the regrowth as a carbohydrate sink, leaves were removed once they reached about 50 mm in diameter. At this early stage of growth, the leaves were expected to be net sinks (Lai et al. Citation1988; Greer & Jeffares Citation1998; Greer Citation1999). These defoliated regrowths are denoted R. On all other shoots, where no vegetative sink was wanted, regrowths were removed before they were 100 mm long.
-
In year 2, treatments involving four leaves with one fruit in competition with a de-leafed regrowth were repeated. In addition, treatments with two competing fruit were added.
In year 1, the full set of treatments was 2L1F, 2LR, 4L1F, 4LR, 4L1FR, 7L1F, 7LR. In year 2 we repeated treatments 4L1F, 4L1FR, 4LR and added 4L2F.
Monitoring
Fruit growth was monitored non-destructively by measuring the fruit length (L) and the maximum (D1) and minimum (D2) fruit diameters in mm then calculating the product D1×D2×L/1000, which is denoted as LDD. LDD was converted into an estimate of the fruit FW using a calibration curve derived from destructive fruit harvests made at various times including fruit harvest (Snelgar et al. Citation1992). Non-destructive measurements were made at 10–30 day intervals throughout the growing season. A non-destructive measurement of regrowth was obtained by monitoring regrowth length.
Destructive sampling
In year 1, 20 samples of each treatment were harvested at 50 and 100 DAMB and at fruit maturity (about 180 DAMB). Linear dimensions of the fruit were used to obtain a relationship between LDD and fruit FW. Destructive measurements of FW and DW of the fruit and shoots were made. In addition, at 181 DAFB (days after full bloom) seed counts were made after extraction of the seeds from fruit softened with pectinase (Rohapect® D5L Special, AB Enzymes GmbH, Darmstadt, Germany). In year 2, destructive measurements were made at 180 DAMB.
Data analysis
The fruit growth rates were estimated by spline fitting to the FW data using R software. This method was used as it involves smoothing of the observed data which reduces noise in gradient estimation. Simpler differencing of data increased observation noise but did produce the same general patterns (not shown).
Means and their standard error were calculated using Microsoft Excel. Payton et al. (Citation2003) have shown that when the standard errors do not differ by more than a factor of two, the probability of overlap of 85.6% confidence intervals is 0.95, that is, non-overlap indicates p < 0.05. A 85.6% confidence interval corresponds to±1.46 se. So in this work we have used the rule that when the 1.5 standard errors do not overlap, the means are significantly different at p < 0.05. As we do comparisons within table columns as well as between data in different tables, we have chosen not to indicate non-significant differences within the tables, leaving the reader to compare the means±1.5 se which is a simple mental exercise and can be done on any pair of means taken from the same or different tables.
Results
Calibration of non-destructive fruit measurements
Snelgar et al. (Citation1992) demonstrated that the kiwifruit fresh weight is highly correlated with the production LDD of the three linear measurements of a fruit, and is best described by a power law. This work confirmed this, but with slightly different parameter values. There was very little difference between the power law and a simpler linear relationship (Minchin et al. Citation2003), so we used the linear relationship in this work, which was:
There was no indication of treatment differences, even though some treatments included regrowth competition.
Fruit and shoot growth patterns
lists the fruit data for year 1 measured at harvest (181 DAMB). Reducing the number of source leaves tended to increase variability in both FW and DW. FW, DW and DM all reduced with declining leaf number. Four leaves were not sufficient to saturate growth. In the presence of a regrowth, i.e. a vegetative sink, the fruit FW, DW and DM were all significantly reduced.
Table 1 Year 1. Effect of leaf number (L) and regrowth (R) on fruit growth, measured as fresh weight (FW), dry weight (DW) and dry matter content (DM), and on seed production, with standard errors in parenthesis. Means within a column are different at α = 0.05, except for seed numbers which are not different at this level. Each treatment shoot contained one fruit (1F)
A shows the time sequence of fruit FW with the estimated rates of fruit growth. The 4L1FR growth rate clearly shows two rapid growth phases, with the second starting at about 80 DAMB. It is usual to describe the first and second rapid growth phases as stage 1 and stage 3 with the intervening phase as stage 2 (Coombe Citation1976). This is also clear in the 4L1F data and possibly still evident in the 7L1F data but there is no sign of this multi-phase behaviour in the 2L1F.
Fig. 2 Time sequence of fruit and vegetative growth for treatments with varying numbers of leaves (L) and fruit (F) in year 1. (A) Estimated fruit fresh weights (FW) based upon LDD. Lines with data points and standard errors show the fruit FW, while the smooth lines alone are the corresponding growth rates calculated using cubic spline fitting to the size data using the R statistical language. (B) Measured regrowth lengths.
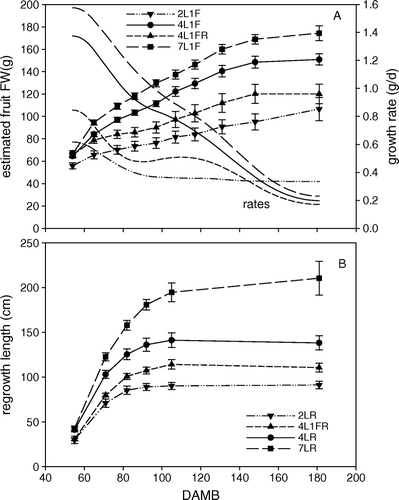
B shows the time sequence of regrowth lengths. Regrowth was completed by about 100 DAMB with no sign of a second growth phase. Regrowth length increased when more leaves were available to support growth, as seen for fruit FW. With two leaves, regrowth length was the least and with seven leaves the maximum. The presence of a fruit reduced regrowth length compared with that with the same number of leaves and with no competing fruit. Regrowth length with four leaves plus fruit was greater than with two leaves and a regrowth alone. Hence, the extra two leaves were enough to compensate for the presence of the fruit.
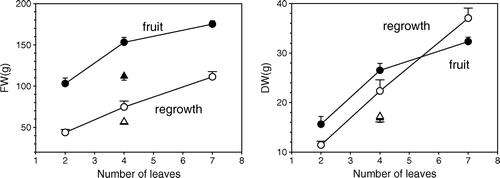
The effects of the number of source leaves on fruit and shoot FW and DW at 181 DAMB are presented in . Fruit FW and DW increase non-linearly with leaf number, increasing more rapidly with low leaf number and showing clear signs of approaching saturation with high leaf number. Regrowth FW and DW increases linearly with leaf number, with no indication of saturation. Regrowth DW increases at a similar rate to that of fruit DW for low leaf number and much more rapidly as leaf number increases.
Fig. 3 Effect of leaf number on fresh weight (FW) and dry weight (DW) at 181 DAMB of a single type of sink (fruit = filled symbols, regrowth = hollow symbols). The single data point for four leaves when there was also a sink of the other type competing for resources is shown as a triangle. Error bars show the standard error.
The FW and DW of fruit and vegetative shoots for year 1, from destructive harvests at 100 and 181 DAMB, are presented in . By 100 DAMB, a single fruit supplied by four leaves (4L1F) had accumulated 109.5 g FW and 16.2 g DW. During the same period, a regrowth supplied by the same number of leaves (treatment 4LR) accumulated less FW and less DW. The same was true for the entire 181 days. Thus, for both periods, the accumulation in both FW and DW of a vegetative sink alone was less than that of a fruit alone.
Table 2 Year 1. Average fruit and regrowth fresh weight (FW), dry weight (DW) and dry matter content (DM), with standard errors (se) in parenthesis, for treatments with four leaves and only a fruit sink (4L1F), only a vegetative regrowth sink (4LR), or both fruit and regrowth sinks (4L1FR). Means differ significantly (P < 0.05) when the difference is more than 1.5 times the sum of the standard errors (Payton et al. Citation2003)
Competition
With competition between the two sinks, in the first 100 days, fruit FW was reduced by 23% and DW reduced by 52%, compared with fruit alone. Regrowth FW was reduced by 15%, DW by 14%, compared with regrowth alone, with neither of these differences being statistically significant. Competition induced greater FW and DW loss in the fruit than in the regrowth. With competing fruit and regrowth, total FW and DW growth was considerably more than with either sinks alone, but less than that of the fruit alone plus regrowth alone. In the competing system, from 100 DAMB to harvest, fruit FW and DW continued to increase with DM also increasing. Regrowth FW growth ceased, while DW continued to increase, resulting in a large regrowth DM at 181 DAMB.
At 181 DAMB, vegetative competition reduced fruit FW from 152.7 to 112.8 g, a loss of 26% compared with a fruit alone. Fruit DW was reduced from 26.5 to 16.9 g, a loss of 36% compared with a fruit without a competing regrowth. Fruit DM was reduced from 17.3 to 14.9, a 14% reduction. Regrowth FW was reduced by 24%, while regrowth DW reduced by 23%. Hence, competition had a larger relative effect on fruit DW than on fruit FW, and the vegetative sink similarly suffered effects on both FW and DW. The relative reduction in FW of the fruit and regrowth were similar but the fruit suffered greater relative DW loss than regrowth.
In year 2, the fruit regrowth competition experiment was repeated, with the addition of two fruits in competition (). Comparison between the same treatments in year 1 and year 2, which involved different orchard blocks, showed no significant difference (p < 0.05) in fruit and vegetative FW and DW results at final harvest , ie, about 180 DAFB ( and ).
Table 3 Year 2, data at 180 DAMB. Average fruit and regrowth fresh weight (FW), dry weight (DW) and dry matter content (DM), with standard errors (se) in parenthesis, for treatments with four source leaves (4L), one or two fruits (1F, 2F), and regrowth (R) in year 2. Means differ significantly (P < 0.05) when the difference is more than 1.5 times the sum of the standard errors (Payton et al. Citation2003)
With two fruits, (year 2) at 181 DAMB each of the fruits had similar FW and DW. Competition between two fruits caused growth of each fruit to be reduced by 15% in FW and 23% in DW, resulting in 12% loss of DM. Competition of a single fruit with regrowth in year 2 resulted in a loss of fruit FW (28%) and DW (39%) resulting in a loss of 15% DM. Looked at in a different way, two fruits resulted in 268.0 g FW and 41.6 g DW while one fruit plus regrowth at harvest had a FW of 183.3 g and DW of 39.4 g. The total FW of two fruits was greater than that of one fruit plus a deleafed regrowth, but the DWs were not significantly different (p < 0.05).
Discussion
Lai et al. (Citation1989) and Hopping (Citation1990) reported that fruit weight of kiwifruit increases with the number of seeds in the fruit. In our experiments, the fruit were well pollinated, as fruit averaged more than 1000 seeds per fruit, considered adequate for commercial production. Seed counts per fruit taken at harvest did not differ significantly among the treatments (), indicating that the observed differences in fruit FW and DM were not caused by differences in seed counts. Previous studies have shown that seed number has much less influence on fruit size when fruit are supplied with adequate resources on girdled shoots (Snelgar & Thorp, Citation1988). Lai et al. (Citation1988) showed that phloem transport within a shoot of kiwifruit follows an orthostichy relation of the nth node supplying nodes n + 5 and n + 8, so we may expect this orthostichy relationship to affect the distribution of photosynthate within our treatments. However, Lai et al. found that on pruning, this relation was completely lost. Hence, because our shoot was pruned, issues of orthostichy are not expected to be relevant. We saw a consistent increase in fruit size with the number of source leaves ().
Source limitations
At harvest, one fruit supplied by seven leaves resulted in fruit with larger FW and DW than when supplied by four leaves (), confirming that four leaves were not sufficient to saturate fruit growth. Reducing the number of source leaves tended to increase the variability in FW and DW, seen by the increasing standard error of these measurements. This was not unexpected. With a specific number of leaves, source supply would be expected to vary between replicates due to differences in total leaf area and in light exposure. With a large number of leaves on a shoot, carbohydrate supply would approach saturation of sink utilization. Hence, variation in source supply between replicates of a treatment with a small number of leaves is expected to result in greater variability on FW and DW than between replicates with a larger number of leaves.
The effect of leaf number on fruit and regrowth FW and DW is shown in . The rate of increase of both fruit FW and DW fell with increasing leaf number, suggesting saturation as carbohydrate supply increased. Regrowth did not show signs of saturation, increasing linearly with leaf number. Since fruit FW and DW increased with leaf number at different rates, fruit DM must change with the number of supply leaves. Put another way, on increasing source supply (i.e. leaf number) a bigger fraction of the increased supply went into DM growth of the vegetative sink than went into DM growth of the fruit. The DM of a fruit increased with increasing number of leaves. The major component of FW is water, imported into a sink by phloem and xylem flow, but DW is predominantly imported by the phloem. These two flow systems are not independent. Their interaction is complex, depending on transpirational water loss through the fruit surface and osmotic effects within the fruit. The osmotic effects are dependent on photosynthate import, its route of phloem unloading and metabolic fate. Interactions between phloem and xylem flow are only just starting to be investigated (see Holbrook & Zwieniecki Citation2005) and to date this has probably best been done in the modelling of peach fruit growth (Fishman & Génard Citation1998; Génard et al. Citation1998; Lescourret et al. Citation1998; Lescourret & Génard Citation2005). This level of mechanistic detail has not been developed for other fruit.
Standard commercial orchard practice produces fruit of about 95–115 g FW and about 14–17 DM which corresponds to two to three leaves per fruit (see ) with a girdled shoot. Lai et al. (Citation1989) found that two leaves per fruit on a girdled shoot resulted in similar fruit size to fruit on non-girdled shoots, so this is consistent with our observations. Extremely large fruit can be produced by higher leaf numbers. With seven leaves we achieved a fruit FW of 174 g with 18.4 DM, demonstrating the potential of fruit size.
Fruit growth curves have often been interpreted in terms of three phases of growth (Coombe Citation1976), which are a direct consequence of double sigmoid growth, which has often been reported (Coombe Citation1976) and first reported for kiwifruit by Hopping (Citation1976). The three growth stages are: stage 1 and 3 when each growth period is at its peak and stage 2 being the time between the two growth periods when the cumulative growth appears to slow and then increase again. To a variable degree, the time courses of fruit FW growth showed the three stages of fruit growth. As pointed out by Coombe (Citation1976), description of fruit growth by means of sigmoid curves is problematic as the observed shape depends strongly on the method, and accuracy, of measurement as well as the delay between the individual times of more rapid growth which can obscure or emphasize phases 2 and 3. Coombe (Citation1976) suggested that a more rigorous description based on curve fitting should be used, but this has not been done in the fruit literature. Numerical differentiation of the observed growth curve can be revealing, but great care is needed with numerical differentiation as this amplifies the noise on what are already typically noisy observations. During anthesis there is some cell division and this rapidly increases immediately after anthesis, lasting for a limited period, and is associated with stage 1 of fruit growth (Coombe Citation1976). Then cell enlargement occurs which is traditionally associated with stage 3 of growth. The extent of stage 2 depends on the delay between the two stages of rapid growth. In our data, the two rapid growth stages were obvious only with four source leaves. With four leaves, growth rate showed a clear stage 2 and this was accentuated when the fruit were competing with the vegetative sink, and much less marked when the fruit was supplied by seven leaves. With two leaves, stage 1 growth was much reduced and there was no sign of stage 3 growth, with FW growth constant from about 90 DAMB up to harvest at 181 DAMB. The two-leaf treatment had higher growth rate at 180 DAMB than any of the other treatments.
The time course of regrowth extension is shown in B, with regrowth increasing with carbohydrate supply. At 181 DAMB, regrowth supported by seven leaves was about 52% greater than when supported by four leaves. Vegetative regrowth continued until about 100 DAMB, after which there was little extension growth. Elongation was reducing at the time of onset of phase 3 fruit growth. Hence, phase 3 of fruit growth may be a direct consequence of increasing availability of carbohydrate through reducing vegetative demand.
The pattern of regrowth extension was quite different from that of its DW and is quite misleading as to its time course of carbohydrate utilization. This is readily seen in . Here we see that between 100 and 181 DAMB, when in competition with a single fruit, regrowth FW did not significantly increase but there was a marked increase in its DW. Hence, although there was no length increase, there was a considerable increase in regrowth DW. This could not be accounted for by increase in regrowth diameter, as the time course of regrowth volume was the same as that of length (data not presented). By 100 DAMB, fruit DW growth had been considerably impaired by the competition from the regrowth (a loss of 8.5 g) and by 181 DAMB the loss was only slightly larger (9.6 g).
Sink competition
In the first 100 DAMB, vegetative growth was reduced less by the presence of a fruit than fruit growth was reduced by the presence of a regrowth (). That is, vegetative growth had a greater ability to attract growth substrates than did the fruit. When there were two fruits competing for available resources, the two fruits at 180 DAMB were indistinguishable (). The total DW growth resulting with two fruits was the same as one fruit plus a regrowth. Thus, DW growth was unaffected by the sink type even though the FW distribution between sink types was not equal. The vegetative sink had a higher priority for carbohydrate supply than did the fruit. While this describes the data, it does not explain the result.
Competition by a fruit sink is different from competition by a vegetative sink.
Currently, the only mechanistic explanation of differences in sink types is by differences in the Michaelis-Menton description of the saturatable kinetics of sink utilization of available carbohydrate (Minchin et al. Citation1993; Lacointe & Minchin Citation2008). In this description, sink function was described by saturatable Michaelis-Menton kinetics and differences in priority result for differences in Vmax. When the km of both sinks was assumed to be the same, the sink with the lower Vmax had the higher priority. If various sinks utilize the same carrier, or enzymology, then the effective km describing this will be the same as the Michaelis-Menton parameter km that describes carrier binding. For different sink types, the effective kms are expected to be different, so differences in Vmax may not be the sole cause of differences in sink priority between fruit and vegetative sinks. Equivalent sinks, that is sinks of the same physiological type, described by the same km and their individual Vm's, scaled according to their individual flow resistances resulted in this model having the same priorities (Minchin et al. Citation1993) i.e., with a change in supply the fraction of supply transported into each sink remained fixed. This is consistent with equal growth seen in the two fruits, but not between fruit and regrowth which would be modelled as non-equivalent sinks.
From about 100 DAMB, FW growth of the vegetative sink was small, practically zero in the presence of a competing fruit, and increasing by 15% without this competition (). But, both fruit and regrowth DW continued to increase, with the fruit gaining almost twice the DW increment of the regrowth. Consequently, the fruit DM showed a large increase between 100 and 181 DAMB.
Field interpretation
The defoliated regrowth used in this work is quite unrepresentative of normal vegetative growth, but was used here as a means of maintaining the vegetative growth as a sink. Normal vegetative growth starts as a net carbohydrate sink and as leaves mature becomes a net exporter and supplies the fruit: a series of experiments to explore the dynamics of this more complex system are currently being carried out.
This work investigated the competition between carbohydrate sinks in kiwifruit vines. We were interested in the plant responses at the organ level, and possible vine management issues. Girdling, as a means of inhibiting phloem transport, has been used as a horticultural practice for thousands of years (Goren et al. Citation2004) and is still not well understood. It appears to induce a complex interaction between changes in endogenous hormones and carbohydrate distribution (Goren et al. Citation2004; Theron & Steyn Citation2008). At this stage, we have not attempted to give a complete physiological interpretation to the reported responses. This will be done in the near future through application of a functional-structural-plant model of kiwifruit, incorporating phloem and xylem transport to model proposed mechanisms of sink competition (Lacointe & Minchin Citation2008; Cieslak et al. Citation2009).
Conclusions
The aim of this work was to investigate the interactions between fruit and between fruit and vegetative growth in ‘Hayward’ kiwifruit, to increase understanding of sink competition and how this can be used to optimize fruit FW and DM.
We have shown that fruit FW and DW at harvest are readily varied by carbohydrate supply level, but not equally. As supply increased, DW increases proportionally faster than FW, resulting in increased DM. We have shown that competition for available carbohydrate by a regrowth has a large detrimental impact on both fruit FW and DM. Pruning that stimulates regrowth can be expected to have a large negative impact on the fruit properties at harvest. A regrowth has a much bigger effect than an extra fruit. The total DW imported by two fruits or one fruit plus the defoliated regrowth were equal but a fruit had greater FW growth than that of a defoliated regrowth.
These quantitative data will provide important calibration data for both mechanistic models of source sink interactions as well as of fruit growth.
Acknowledgements
This work was funded by Plant & Food Research's internal kiwifruit reinvestment fund and FRST contract C06X0706.
References
- Austin , PT , Hall , AJ , Gandar , PW , Warrington , IJ , Fulton , TA and Halligan , EA . 1999 . A compartmental model of the effect of early-season temperature on potential size and growth of ‘Delicious’ apple fruits . Annals of Botany , 83 : 129 – 143 .
- Avery , DJ , Priestley , CA and Treharne , KJ . 1979 . “ Integration of assimilation and carbohydrate utilization in apple ” . In Photosynthesis and plant development , Edited by: Marcelle , R . 221 – 231 . The Hague : Junk .
- BioGro 2009 . BioBro Manual, BioGro Society , PO Box 9693, Marion Square, Wellington, NZ. http://www.biogro.co.nz/main.php?page=230
- Burdon , J , McLeod , D , Lallu , N , Gamble , J , Petley , M and Gunson , A . 2004 . Consumer evaluation of ‘Hayward’ kiwifruit of different at-harvest dry matter contents . Postharvest Biology and Technology , 34 : 245 – 255 .
- Cieslak M , Seleznyova AN , Hanan J 2009 . A functional-structural kiwifruit vine model . Proceedings of the Third International Symposium on Plant Growth Modelling, Simulation, Visualization and Applications . Beijing (China), 9–13 November 2009 .
- Coombe , BG . 1976 . The development of fleshy fruits . Annual Review of Plant Physiology , 27 : 507 – 528 .
- Crisosto , CH and Crisosto , GM . 2001 . Understanding consumer acceptance of early harvested ‘Hayward’ kiwifruit . Postharvest Biology and Technology , 22 : 205 – 213 .
- Fishman , S and Génard , M . 1998 . A biophysical model of fruit growth: simulation of seasonal and diurnal dynamics of mass. Plant . Cell and Environment , 21 : 739 – 752 .
- Génard , M , Lescourret , F , Ben Mimoun , M , Besset , J and Bussi , C . 1998 . A simulation model of growth at the shoot-bearing fruit level. II. Test and effect of source and sink factors in the case of peach . European Journal of Agronomy , 9 : 189 – 202 .
- Goren , F , Huberman , M and Goldschmidt , EE . 2004 . Girdling: physiological and horticultural aspects . Horticultural Reviews , 30 : 1 – 38 .
- Greer , DH . 1999 . Seasonal and daily changes in carbon acquisition of kiwifruit leaves with and without auxiliary fruit . New Zealand Journal of Crop and Horticultural Science , 27 : 23 – 31 .
- Greer , DH and Jeffares , D . 1998 . Temperature-dependence of carbon acquisition and demand in relation to shoot growth of kiwifruit (Actinidia deliciosa) vines grown in controlled environments . Australian Journal of Plant Physiology , 25 : 843 – 850 .
- Grossman , YL and DeJong , TM . 1994 . PEACH: A simulation model of reproductive and vegetative growth in peach trees . Tree Physiology , 14 : 329 – 345 .
- Grossman , YL and DeJong , TM . 1995 . Maximum fruit growth potential and seasonal patterns of resource dynamics during peach growth . Annals of Botany , 75 : 553 – 560 .
- Harker , FR , Carr , BT , Lenjo , M , MacRae , EA , Wismer , WV , Marsh , KB , Williams , M , White , A , Lund , CM , Walker , SB , Gunson , FA and Pereira , RB . 2009 . Consumer liking for kiwifruit flavour: A meta-analysis of five studies on fruit quality . Food Quality and Preference , 20 : 30 – 41 .
- Holbrook , NM and Zwieniecki , MA . 2005 . Vascular transport in plants , New York : Elsevier Academic Press .
- Hopping , ME . 1976 . Structure and development of fruit and seeds in Chinese gooseberry (Actinidia chinensis Planch.) . New Zealand Journal of Botany , 14 : 63 – 68 .
- Hopping , ME . 1990 . “ Floral biology, pollination, and fruit set ” . In Kiwifruit: science and management , Edited by: Warrington , IJ and Weston , GC . 71 – 96 . Auckland, NZ : Ray Richards .
- Jordan , RB , Walton , EF , Klages , KU and Seelye , RJ . 2000 . Postharvest fruit density as an indicator of dry matter and ripened soluble solids of kiwifruit . Postharvest Biology and Technology , 20 : 163 – 173 .
- Lacointe , A and Minchin , PEH . 2008 . Modelling phloem and xylem transport within a complex architecture . Functional Plant Biology , 35 : 772 – 780 .
- Lai , R , Wooley , DJ and Lawes , GS . 1988 . Patterns of assimilate transport from leaves to fruit within a kiwifruit (Actinidia deliciosa) lateral . Journal of Horticultural Science , 63 : 725 – 730 .
- Lai , R , Wooley , DJ and Lawes , GS . 1989 . Effect of leaf to fruit ratio on fruit growth of kiwifruit (Actinidia deliciosa) . Scientia Horticulturae , 39 : 247 – 255 .
- Lancaster , J . 2002 . What makes a good flavoured kiwifruit? . New Zealand Kiwifruit , 149 : 10 – 11 .
- Lescourret , F , Ben Mimoun , M and Génard , M . 1998 . A simulation model of growth at the shoot-bearing-fruit level. I Description and parameterisation for peach . European Journal of Agronomy , 9 : 173 – 188 .
- Lescourret , F and Génard , M . 2005 . A virtual peach fruit model simulating changes in fruit quality during the final stage of fruit growth . Tree Physiology , 25 : 1303 – 1315 .
- Minchin , PEH , Thorpe , MR and Farrar , JF . 1993 . A simple mechanistic model of phloem transport which explains sink priority . Journal of Experimental Botany , 44 : 947 – 955 .
- Minchin , PEH , Richardson , AC , Patterson , KJ and Martin , PJ . 2003 . Prediction of final weight for Actinidia chinensis ‘Hort16A’ fruit . New Zealand Journal of Crop and Horticultural Science , 31 : 147 – 157 .
- Palmer , JW . 1992 . Effects of varying crop-load on photosynthesis, dry matter production and partitioning of Crispin/M.27 apple trees . Tree Physiology , 11 : 19 – 33 .
- Payton ME , Greenstone MH , Schenker N 2003 . Overlapping confidence intervals or standard error intervals: What do they mean in terms of statistical significance? Journal of Insect Science 3 : 34 available online: insectscience.org/3:34
- Quilot , B , Génard , M , Lescourret , F and Kervelle , L . 2005 . Simulating genotypic variation of fruit quality in an advanced peach x Purus davidiana cross . Journal of Experimental Botany , 56 : 3071 – 3081 .
- Richardson , AC , McAneney , KJ and Dawson , TE . 1997 . Carbohydrate dynamics in kiwifruit . Journal of Horticultural Science , 72 : 907 – 917 .
- Snelgar , WP , Hall , AJ , Ferguson , AR and Blattmann , P . 2005 . Temperature influences growth and maturation of fruit on ‘Hayward’ kiwifruit vines . Functional Plant Biology , 32 : 1 – 12 .
- Snelgar , WP and Thorp , TG . 1988 . Leaf area, final fruit weight and productivity in kiwifruit . Scientia Horticulturae , 36 : 241 – 249 .
- Snelgar , WP , Thorp , TG and Patterson , KJ . 1986 . Optimal leaf: fruit ratios for fruit growth in kiwifruit . Acta Horticulturae , 175 : 115 – 120 .
- Snelgar , WP , Manson , PJ and Martin , PJ . 1992 . Influence of time of shading on flowering and yield of kiwifruit vines . Journal of Horticultural Science , 67 : 481 – 487 .
- Taylor , JA , Praat , JP and Bollen , AF . 2007 . Spatial variability of kiwifruit quality in orchards and its implications for sampling and mapping . HortScience , 42 : 246 – 250 .
- Theron , KI and Steyn , WJ . 2008 . Girdling: science behind the age-old technique . Acta Horticulturae , 800 : 51 – 60 .
- Waring , PF and Patrick , J . 1975 . “ Source-sink relations and the partition of assimilates in the plant ” . In Photosynthesis and productivity in different environments , Edited by: Cooper , JP . Cambridge, , UK : Cambridge University Press .
- Wardlaw , IF . 1990 . The control of carbon partitioning in plants . New Phytologist , 116 : 341 – 81 .
- Woodward , TJ , Green , TGA and Clearwater , MJ . 2007 . Between-vine variation in ‘Hayward’ kiwifruit vine income: The use of Lorenz curves and Gini coefficients to describe variation and assess the potential for zonal management . European Journal of Horticultural Science , 72 : 280 – 287 .
- Wright , CJ . 1989 . “ Interactions between vegetative and reproductive growth ” . In Manipulation of fruiting , Edited by: Wright , CJ . London, , UK : Butterworth .