Abstract
Selol is a mixture of selenitetriglycerides, obtained by esterification of pre-oxidated triglycerides from sunflower oil with selenous acid. Investigations have been carried out on the anticancer use of Selol and its antimitotic properties. The aim of our work was to compare the effect of two compounds (an organic and an inorganic one) containing selenium with oxidation number at +4: Selol and sodium selenate (IV), on plant cells of Allium test. Structural changes in cells, their mitotic activity and proportions of mitotic phases were subject to experimental analysis. Incubation in both selenium compounds led to changes in structure of chromosomes, consisting in their strong condensation and contraction (cc chromosomes), accompanied by gradual reduction or complete inhibition of cell division. High concentrations of Selol led to strong condensation of chromatin in interphase cells, while cells treated with high concentrations of sodium selenate (IV) displayed typical “pseudoapoptotic” bodies and totally fragmented nuclei. Both compounds caused inhibition of cell division; however, sodium selenate (IV) was more effective, while low concentrations of Selol stimulated division. Use of Selol resulted in changes in chromosome structure, condensation and contraction, which made it impossible to pass to further phases of cell division. The effect of sodium selenate (IV) consisted in raised prophase index, and high concentrations led to inhibition of divisions and arrest of cells at different stages of mitosis. The results obtained show markedly lower toxicity of Selol in comparison with sodium selenate, which makes it advisable to further investigate that compound as a potential safe anticancer drug.
1. Introduction
Neoplastic diseases, as well as cardiovascular diseases, pose a major threat to health. Their incidence and the resulting mortality are growing. Their causes as well as the continuous increase in incidence vary widely. They can be due to genetic conditions, infections with oncoviruses, or exposure to chemical, physical or environmental factors causing chromosome mutations leading to induction of cancers. Due to the growing incidence of neoplasms, great efforts are being made to find effective means of prevention and treatment. Therapy of neoplasms mainly involves traditional therapeutic methods: chemotherapy, radiation therapy and surgery. Unfortunately, the efficacy of these methods is often insufficient; also, chemotherapy and radiotherapy have a range of side-effects, many of them severe. For this reason, new means for preventing and treating the diseases are being introduced, including alternative therapies. Microelement preparations, especially those containing selenium, offer hope for treatment and prevention of neoplastic diseases.
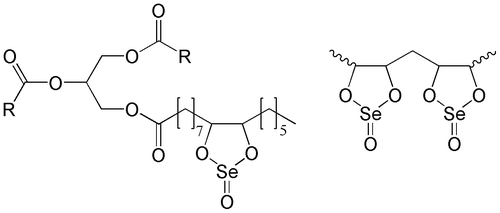
An inorganic compound containing selenium at +4 oxidation level is sodium selenate (IV) (Na2SeO3). In vitro investigations showed it to have very high anticancer efficacy, connected however with extreme toxicity (LD50 3.5 mg kg−1 body mass), which strongly limits its use in therapy. In spite of these limitations, sodium selenate (IV) is still used in research (Gasmi et al. Citation1997; Jiang et al. Citation2001; Van Zandwijk and Hirsch Citation2003; Wierzbicka et al. Citation2007). Due to the high degree of toxicity, new substances containing selenium are required with lower toxicity. Selol may meet these requirements. It was synthesized at the Department of Drug Analysis, Medical University of Warsaw almost 20 years ago, as the first item of series of organic compounds containing selenium at +4 oxidation level (Figure ) (Fitak et al. Citation1999). It is a mixture of selenitetriglycerides obtained on the basis of sunflower oil, whose fatty acids contain the most double bonds. It has been shown to have antioxidative properties (“free radical scavenger”), but is an oxidant itself. It also shows comparatively low toxicity and thus may be safely used in therapy in doses reaching several mg kg−1 of the body mass (Zagrodzki et al. Citation2000). The preparation is widely used in experimental and clinical investigations, even in the treatment of highly advanced stages of neoplasm, often having positive effects such as lengthening the patient’s life, or complete remission. However, there are still no basic experimental data on its effects on plant, animal and human cells. Thus it is necessary to perform wider and more thorough studies on its cytological effects, cytotoxicity and cytostatics, and to carry out a wider range of basic laboratory tests that might lead to registration of Selol as an anticancer drug.
Figure 1. A putative structure of Selol (Suchocki et al. Citation2003). The selenitetriglycerides containing 2% of Se (Selol 2%) have a structure with mostly single dioxaselenolane rings (left); the selenitetriglycerides containing 5% and more of Se (Selol 5%) have also double dioxaselenolane rings (right). R, polyunsaturated fatty acid moiety.
The aim of the current work was to compare the effects of Selol and sodium selenate (IV) using Levan’s test, more commonly known as the Allium test (Levan Citation1938). It is based on meristematic cells of onion (Allium cepa L.) root tips, the equivalent of animal stem cells. Its advantage is availability of large amounts of genetically homogeneous material, suitable for karyological studies. The small number (16) of large chromosomes allows easy analysis of aberrations and mutagenic changes. For this reason, it is often used in screening tests and analyses of potential anticancer drugs with respect to their cytotoxicity and cytostatics, and the effects of pathogens, nanoparticles and environmental pollutions (Majewska et al. Citation2000; Gabara et al. Citation2006; Kuraś et al. Citation2006; Oyeyemi and Bakare Citation2013; Pesnya Citation2013; Firbas and Amon Citation2014). Our investigations compared the effect of both forms of selenium on structure of nuclei and chromosomes and the mitotic activity of Allium test cells.
2. Materials and methods
2.1. Selenium compounds used in the study
2.1.1. Procedure of preparation of Selol 2%
Selol is a mixture of selenitetriglycerides containing selenium with oxidation number at +4. Synthesis of Selol is preceded by oxidation of a mixture of triglycerides (Suchocki et al. Citation2003) with saturated solution of KMnO4 in 0.5% H2SO4. The obtained emulsion is left for several days for breaking. Hydroxyl derivatives of triglycerides are subject to esterification with selenous acid (IV) (H2SeO3) in dioxane solution. A precise description of Selol 2% synthesis is available in Fitak et al. (Citation1999).
2.1.2. Procedure of preparation of sodium selenate (IV)
Eight hundred and eighty mg of sodiuim selenate (IV) (Na2SeO3 from Sigma-Aldrich, Germany) was dissolved in 100 ml of saline solution (0.9% NaCl), giving a solution of sodium selenate (IV) at concentration of 2 mg Se ml−1, used for preparation of its micellar fraction.
2.2. Obtaining micelles containing 2% Selol and sodium selenate (IV)
Selol is a lipohphilic compound. Its solubility in water posed some problems in our studies. The experiments presented herein required a form of Selol which would make a small-size fraction of micelles suspended in a water solution. The solution of sodium selenate (IV) was also prepared in the form of micelles. To 3 ml of Selol 2% and sodium selenate (IV), 0.5 g chicken egg lecithin was added; the ingredients were triturated in a porcelain mortar to a homogenous mixture, and, while constantly grinding, small amounts of saline solution were gradually added to obtain 50 ml of total volume of the suspension. After careful grinding, the solution was sonicated at 80°C for 20 min (Fisher Sonic Dismembrator Model 150, sonifying head power 500 W). Micelles of the preparations obtained this way were kept at –20°C. Before starting the experiment, they were heated in a thermostat up to 22°C, and the required concentrations were prepared.
2.3. Plant material (for tests) and procedure of preparation of the experiments
As the test plant in the investigations, meristems of fibrous roots of onion (Allium cepa L.) were used, of genetically selected Dawidowska cultivar obtained from the Gospodarstwo Warzywno-Rolne (Vegetable and Agricultural Holding) in Babice Stare near Warsaw. Onion fibrous roots were grown in distilled water in a 200 ml Erlenmeyer flask until reaching a length of 3 cm (±0.5 cm). Then they were incubated in 2% Selol, at concentrations of 25, 100, 400, 800 and 1600 μg Se ml−1, and in a solution of sodium selenate (IV) in 0.9% NaCl, at initial concentration of 2 mg Se ml−1, from which subsequent concentrations of 25, 100, 200 and 400 μg Se ml−1 were obtained. The samples were taken after 6, 24 and 48 h. For each concentration, roots of two onions were used for incubation, three single roots taken as samples after each period. Tips (2 mm long) were cut off and used for preparations squeezed in aceto-orcein, which were further analyzed using a NU Zeiss light microscope (Zeiss, Germany). At the same time, photographic documentation was made using a Nikon camera, and image analysis software type Lucia (Nikon, Japan).
The microscopic analysis comprised:
changes in structure of interphase cell nuclei and structure of chromosomes, disturbances in divisions of control root cells and ones incubated in solutions of Selol and sodium selenate (IV);
determination of the level of the mitotic activity, expressed as the mitotic index, i.e. the percentage of dividing cells per 1000 meristematic cells (Lopez-Saez and Fernandez-Gomez Citation1965); and
establishment of proportions of particular mitotic phases, i.e. the phase index, which means the percentage of particular mitotic phases among dividing cells.
3. Results
3.1. Structure of control cells of the Allium test, and cells treated with different concentrations of Selol and sodium selenate (IV)
3.1.1. Control cells
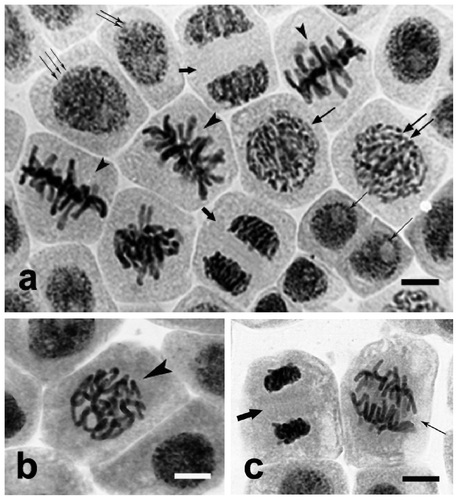
Control cells of the Allium test showed morphological and structural differentiation typical of meristematic tissue, resulting from particular phases of the division cycle. Interphase cells constituted 92% (±1%). Among them, a group of distinctly smaller post-telophase cells could be distinguished, probably at G0 or G1 stage (Figure a, cell marked with a single thin arrow). Another group consisted of slightly larger cells with nuclei of rarefied chromatin, probably at the end of the G1 phase and the beginning of the S phase (Figure a, cell marked with double thin arrows). Another and the most numerous group was made of large cells at the late S and G2 phases, containing large nuclei with rarefied chromatin (Figure a, cells marked with triple thin arrows). The remaining part 8% (±1%) consisted of cells at different stages of mitosis. At early prophase, chromatin in the cells was condensed, with emerging chromosome territories (Figure a, cells marked with a medium-sized arrow). At late prophase, chromatin took the form of thin tangled chromatin threads (Figure a, double medium-sized arrows), which became distinctly shortened and thickened by the end of the phase (Figure b, arrowhead). The chromosomes were further arrayed along the equator of the cell, forming the metaphase plate (Figure a, arrowhead). During anaphase, longitudinal division of chromosomes took place, and the newly formed oblong chromatids were pulled to opposite poles of the cell (Figure c, thin arrow). After telophase (Figure a, c, thick arrow), chromatin was despiralized and phragmoplast, the cell plate and middle lamella were formed, cytokinesis took place and cell division completed.
Figure 2. Allium test control cells. Squeezed preparation, stained with 2% aceto-orcein, observed under a light microscope; bar = 20 μm. (a) Different degrees of dispersion of chromatin in control cell interphase nuclei and at different stages of mitosis. Single thin arrows: condensed chromatin of cells at G0 and G1 phases. Two thin arrows: cells with nuclei containing rarefied chromatin, at G1 and initial S phases. Three thin arrows: cells at late S and G2 phases, with nuclei containing non-condensed chromatin. Single medium arrow: cells at early prophase, chromatin condensed with emerging chromosome territories. Two medium arrows: prophase proper; chromatin in a form of tangled threads. Arrowheads: control cells at metaphase stage. Thick arrows: control cells at telophase. (b) Control cell at the end of prophase, chromatin visible as thickened chromosome threads (arrowhead). (c) Chromosomes are pulled towards opposite cell poles during anaphase (thin arrow), and are despiralized in new cells after typical telophase (thick arrow).
3.1.2. Selol
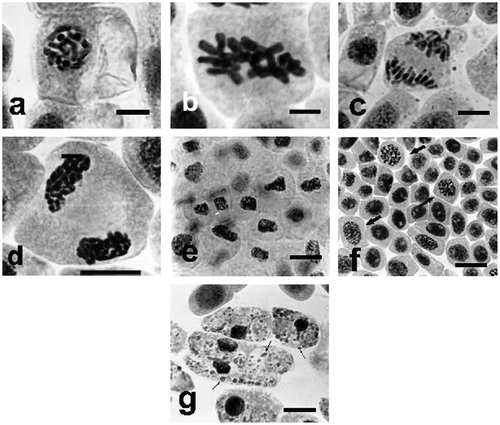
The effect of Selol on Allium test cells was seen only at higher concentrations, and it consisted only in turbagenic changes in chromosome morphology (thickening and shortening) (cc meaning “condensed and contracted”). In late prophase, the chromosomes were thickened and shortened, and clear patches of karyolymph were seen in the nucleus (Figure a), which gave typical cc prophases. Thickened metaphase chromosomes were arrayed in the metaphase plate (Figure b), and, during anaphase (Figure c), they underwent normal division, and cc-sister chromosomes were pulled towards the cell poles, forming irregular chromatin concentrations. Decondensation of chromatin took more time at the telophase stage (Figure d). This way Selol led to almost complete inhibition of mitotic activity. Selol caused no clastogenic changes, typical for sodium selenate (IV), as described below. The final effect of Selol was a change in morphological formation of nuclei, leading to inhibition of mitotic activity by turbagenic aberration, contraction and uneven condensation of chromatin (Figure e). Selol did not have mutagenic effects. During incubation at lower concentrations, accumulation of cells at polyploid interphase and the earliest mitotic phases took place, cell divisions retarded (Figure f, arrows). With further incubation, at the longest periods and at the highest concentration, gradual transition to apoptosis and formation of apoptotic bodies were observed (Figure g, arrows).
Figure 3. Changes in chromosome structure in Allium test cells following incubation in Selol solutions at concentration of 100 μg ml−1. Squeezed preparation, stained with 2% aceto-orcein, observation under a light microscope; bar = 10 μm. (a) Thickened and shortened chromosomes and light patches of karyolymph within the nucleus in cc-prophase, following 24 h incubation. (b) Turbagenic metaphase, chromosomes arranged in the metaphase plate. (c) Shortened chromosomes are normally separated during anaphase. (d) Chromatin decondensation at telophase stage. (e) Change of shape of cell nuclei (contraction and condensation of chromatin) following 48 h of incubation. (f) Stimulation of mitotic activity (arrows) after 24 h incubation in lower concentrations of Selol. (g) Transition to apoptosis and formation of apoptotic bodies (arrows) after prolonged incubation in higher concentrations of Selol.
3.1.3. Sodium selenate (IV)
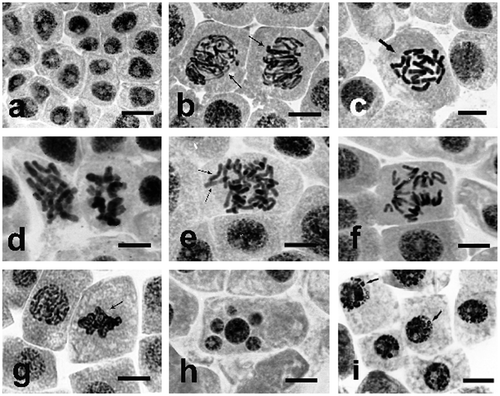
Incubation at different concentrations of sodium selenate (IV) clearly changed the structure of interphase nuclei and the morphology of their chromosomes. As early as after six hours of incubation, cell divisions disappeared, and the structure of interphase nuclei was thickened and homogeneous, which was probably due to their arrest at G2 phase (Figure a). In some cells, chromosomes formed atypical prophase–metaphase arrangements (Figure b, thin arrows; d) and C-metaphases, when chromosomes did not form a normal metaphase plate but were dispersed within the cell (Figure c). Instead of dividing, restitution of their structure took place, leading to formation of specific interphase polyploid (tetraploid) nuclei. These are turbagenic changes, not causing mutagenesis. However, in all the investigated concentrations, some of the cells arrested in their division developed aberrant (clastogenic) changes in chromosome structure, particularly distinct at metaphase and anaphase. A large part of meta- and anaphase chromosomes formed irregular multipolar systems (Figure e, f), triggering formation of clastogenic, i.e. mutagenic, aberrations. At anaphase stage, bridges, ruptures, and loss of portions of chromosomes or whole chromosomes (Figure e arrows) were observed. Such atypical chromosome structures blocked segregation and proper separation of chromosomes (Figure e, f). At telophase, the disturbed separation of chromosomes resulted in formation of uneven or undivided telophase nuclei (Figure g, arrow), which led to formation of polyploid cells, often containing micronuclei (Figure h). Such changes are clastogenic, and are lethal, especially at higher concentrations. Prolonged treatment with solutions of sodium selenate (IV), up to 72–96 h, led to more and more distinct degradation of chromatin and formation of its specific protrusions and “pseudoapoptotic” bodies (Figure i, arrows), further resulting in complete fragmentation of nuclei. As the result of the above, incubation in all the investigated concentrations of sodium selenate (IV) resulted in dramatic decrease of the mitotic index, even at low concentrations, as presented in the next section.
Figure 4. Changes in chromosome structure in Allium test cells following incubation in sodium selenate (IV) solutions at concentration of 100 μg Se ml−1. Squeezed preparation, stained with 2% aceto-orcein, observation under a light microscope; bar = 20 μm. (a) Homogenization of structure of Allium test cell nuclei following 6 h of incubation in sodium selenate (IV) at concentration of 100 μg Se ml−1, probably caused by their arrest at G2 phase. (b, c, d) Thickened and shortened chromosomes, forming atypical prophases (thin arrows), and C-metaphases (thick arrows). (e, f) Lost chromosomes and their atypical arrangement at anaphase. (g) Unseparated telophase nuclei (arrow). (h) A cell with micronuclei. (i) Typical protrusions of chromatin (arrows) formed in prolonged incubation (48 h) in sodium selenate (IV).
3.2. Mitotic activity of control cells of the Allium test, and cells treated with Selol and sodium selenate (IV)
3.2.1. Mitotic activity of Allium test cells treated with Selol
In control cells of the Allium test, mitotic activity expressed as the mitotic index oscillated around 12%. This value was treated as 100%, and was further used for presentation of changes caused by treatment with Selol and sodium selenate (IV) (Figure a, b).
Figure 5. (Color online) Changes of the mitotic index of the Allium test cells during incubation (a) in Selol solutions of different selenium concentrations; and (b) during incubation in sodium selenate (IV) solutions of different selenium concentrations.
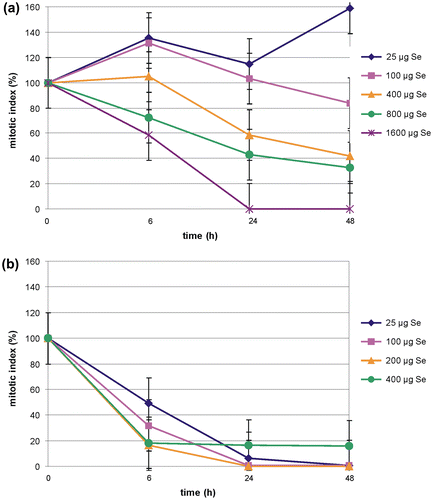
The effect of Selol depended on the concentration of its solution and on the period of incubation (Figure a). Incubation of roots in the lowest concentration of Selol (25 μg Se ml−1) resulted in distinct stimulation of divisions during the whole experiment. After 6 h, the index rose to 138%, and after 48 h, in spite of a temporary slight decrease, it rose to 159%. Incubation in Selol solution at a concentration of 100 μg Se ml−1 also induced initial raise of the mitotic index up to 131%, but this value dropped to the control level after 24 h, and further decreased to 84% after 48 h. The two higher concentrations (400 and 800 μg Se ml−1) caused gradual decrease of the mitotic index during incubation. The lower concentration reduced the divisions less effectively, starting only from 24 h of incubation. The higher one resulted in a decrease of the mitotic index to 73% after 6 h. Incubation in the highest concentration of selenium (1600 μg ml−1) caused rapid decrease of the mitotic index to 60% after 6 h of incubation, and to 0 after 24 h, with this level maintained until the end of the experiment (Figure a).
3.2.2. Mitotic activity of Allium test cells treated with sodium selenate (IV)
The influence of sodium selenate (IV) on the Allium test cells was different to that of Selol. In all concentrations used, rapid decrease of mitotic activity during incubation was observed. Even incubation in the mildest solution (25 μg Se ml−1) for 6 h caused a decrease of the mitotic activity by 40% in comparison to the control. After 24 h, the mitotic index was only 17%, and total inhibition of divisions was observed after 48 h. Two higher concentrations (100 and 200 μg Se ml−1) led to complete inhibition of divisions after 24 h of incubation. Incubation in the selenium solution of 400 μg ml−1 caused a decrease of the mitotic index to 20%, and that value persisted until the end of the experiment. This was caused by the extremely strong toxic effect of this concentration of selenium in cells, which resulted in their arrest at the stage of the cell cycle they were at after 6 h of incubation (Figure b).
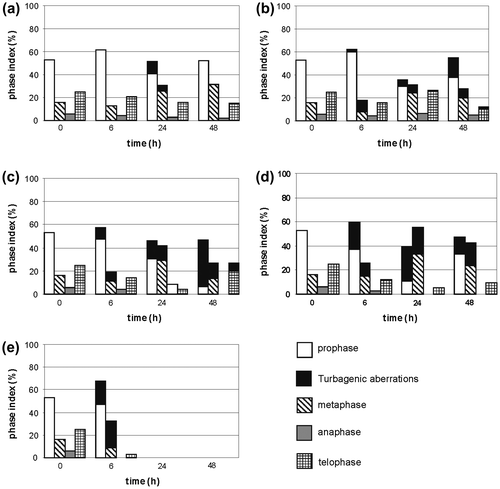
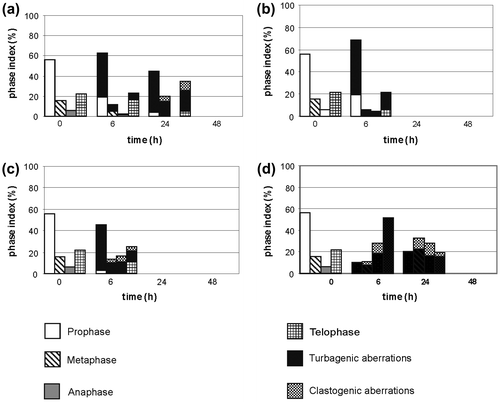
3.3. Changes in phase index of the Allium test cells treated with different concentrations of Selol and sodium selenate (IV)
3.3.1. Changes in the phase index following treatment with Selol
In the control, the phase index remained at a comparatively stable level, and its respective values were: 57% for prophases, 17% for metaphases, 5% for anaphases, and 21% for telophases. The chromosomes of dividing cells in all phases looked normal. Incubation in concentrations of Selol changed proportions between the phases and caused formation of changed mitoses.
Incubation in a solution of the lowest concentration (25 μg Se ml−1) did not result in a significant change to the phase index, and changed divisions (cc prophases and cc metaphases) appeared only transiently after 24 h of incubation (Figure a). In the solution of 100 μg Se ml−1, a distinct decrease of the prophase index was observed after 24 h, accompanied by an increase of the metaphase and telophase index. During further incubation, the prophase index rose and the telophase index was reduced. Increased percentage of changed mitoses was observed as well (17% prophases and 8% metaphases) (Figure b). After 6 h of incubation at concentrations of 400 and 800 μg Se ml−1, the prophase index rose and telophase index decreased; then, after 24 h, the prophase index was distinctly reduced and the metaphase index rose to 56% in the concentration of 800 μg Se ml−1 (Figure d). These phases also displayed a higher percentage of turbagenic changes (Figure c, d). This became more distinct at increasing concentrations of Selol. The most changed divisions were observed after 48 h of incubation in solution of 400 μg Se ml−1 (40% prophases, 13% metaphases, 7% telophases), and after 24 h of incubation in 800 μg Se ml−1 (28% prophases and 22% metaphases) (Figure d). In the solution of 1600 μg Se ml−1, after 6 h of incubation, the prophase index rose to 68%, and the metaphase index to 32%. At the same time, about 30% prophases and 70% metaphases displayed cc chromosomes. Anaphases were absent, and telophases were scarce – only about 3% (Figure e).
3.3.2. Changes in the phase index following treatment with sodium selenate (IV)
After 6 h of incubation in solutions at the two lower concentrations (25 and 100 μg Se ml−1) of sodium selenate (IV), the prophase index rose (to 63% and 69%), while the metaphase and anaphase indices dropped. During further incubation at 25 μg Se ml−1, the prophase index decreased and the metaphase and telophase indices rose (telophases constituted 35% of all divisions) (Figure a). After 24 h of incubation, almost all divisions were abnormal. The solution of 100 μg Se ml−1 caused total disappearance of divisions after 24 h of incubation, which lasted until the end of the experiment (Figure b). Six hours of incubation at 200 μg Se ml−1 led to decrease of prophase percentage and a slight reduction of metaphases, with growth of anaphase and telophase indices; almost all observed divisions were changed (Figure c). A 6 h incubation in sodium selenate (IV) solution at 400 μg Se ml−1 caused a decrease of the prophase and metaphase indices to about 10% each, and growth of the anaphase and telophase indices (to 29% and 51%, respectively). During further incubation, percentages of particular phases became relatively equal. An important fact is that all the observed divisions, starting from 6 h of incubation, were abnormal, and remained arrested in this form until the end of the experiment (Figure d).
4. Discussion
Selenium is one of the most interesting trace elements. Very small amounts are extremely important for many life processes, while large doses are strongly poisonous. More than 30 selenium compounds have been identified in living organisms. In the 1970s, the first reports were published regarding the connection between selenium and the risk of development of cancerous diseases. Among many drugs proposed by alternative medicine, much hope is put in introducing selenium preparations in prophylaxis and treatment.
The bioavailability of selenium in diet supplements is strictly related to its chemical form. Selenoamino acids (e.g. selenomethionine, selenocysteine) are easier to absorb and less toxic than inorganic compounds of selenium (such as sodium selenate (IV)) (Rayman Citation2000). For this reason, we compared the effect of an organic (Selol) and inorganic (sodium selenate (IV)) form of selenium, using the Allium plant test. The test is widely used in arrays of cytotoxicity of various substances, such as heavy metals, hormones, nanoparticles or potential anticancer drugs (Grant Citation1982; Oyeyemi and Bakare Citation2013; Pesnya Citation2013; Firbas and Amon Citation2014). Cells of the test contain a small number (16) of large chromosomes, which facilitates measurements of cytological parameters such as cell mitotic activity, type and frequency of aberrations and disturbances of cell divisions (Nielsen and Rank Citation1994). Moreover, published data indicate that Allium test cells are compatible with other biological tests, including cancer cell lines (Fiskesjö Citation1985). Our studies compared the effects of organic (Selol) and inorganic (sodium selenate (IV)) selenium compounds with oxidation number at +4, on structure of nuclei and chromosomes and on mitotic activity of the Allium test cells.
Cytological analyses show inhibition of the mitotic activity following treatment with sodium selenate (IV). This was correlated with a change in structure of chromosomes, their thickening and shortening (cc-chromosomes). The process was more intensive in cells treated with sodium selenate (IV) at the concentrations of 25, 100 and 400 μg Se ml−1. At the concentration of 200 μg Se ml−1, the toxic effect on cells during mitoses was very distinct. After 24 and 48 h of incubation, progressive degradation of chromosomes was apparent. In contrast to the above, in cells incubated in solutions of Selol 2%, the condensation of chromosomes was milder, and its intensity depended on concentration of selenium and duration of the experiment. After 24 h of incubation in solutions of 25 and 100 μg Se ml−1, condensation of cc chromosomes occurred only in prophase and metaphase cells. At a concentration of 400 μg Se ml−1, cc chromosomes were observed in prophase and metaphase throughout the experiment, also appearing in telophase cells after 48 h. The two highest concentrations (800 and 1600 μg Se ml−1) caused formation of cc chromosomes in prophase and metaphase. Chromatin of interphase cells incubated both in a solution of sodium selenate (IV) and of Selol was condensed. However, only the highest concentrations of sodium selenate (IV) caused fragmentation of nuclei and appearance of “pseudoapoptotic” bodies within cells. Based on these analyses, it can be stated that, unlike sodium selenate (IV), Selol causes changes only in the two initial phases of the division cycle and does not induce formation of apoptotic bodies. This may result from larger amounts of selenium in roots incubated in sodium selenate (IV), as this compound penetrates plant cells more easily than Selol. High doses of selenium are toxic to plants, and larger amounts damage proteins and disturb cell metabolism (Wierzbicka et al. Citation2007).
The process of inhibition of proliferation caused by Selol was proportional to its concentration. Only the concentration of 1600 μg Se ml−1 brought total inhibition of the mitotic activity after 24 h of incubation. Together with the mitotic index, the phase index was changed, in which a higher concentration of cells at the stage of metaphase and telophase was observed as concentrations of sodium selenate (IV) and Selol were increased, and incubation time prolonged. Sodium selenate (IV) solution at concentration of 100 μg Se ml−1 caused aggregation of cells at the prophase stage. It is remarkable that sodium selenate (IV) caused much deeper changes in the course of cell divisions (clastogenic, i.e. mutagenic ones), which confirms its higher toxicity.
According to published data, sodium selenate (IV) is one of the easiest selenium compounds for plants to absorb. It is known to penetrate inside cells and move inside cells by symplastic transportation (Wierzbicka et al. Citation2007). It is further transformed, among others, to Se-methyl-selenocysteine (Wierzbicka et al. Citation2007). The synthesized selenoamino acid is a so-called non-protein amino acid. It is also known that selenocysteine and selenomethionine are synthesized in plant cells as well. Those two amino acids are non-specifically incorporated into newly synthesized proteins, replacing cysteine and methionine, which often blocks their functions (Whanger Citation2002). This is the main mechanism of the toxic effect of selenium.
The results obtained herein prove the toxic effect of sodium selenate (IV) on the Allium cepa L. cells. Interestingly, cells treated with Selol stopped dividing, but the structure of their interphase nuclei remained unchanged. Only a four-fold higher concentration of the compound caused changes in the structure of the nuclei. This might mean that Selol acts much more specifically than sodium selenate (IV), and does not cause general poisoning of cells. It only affects cell cycle proteins, which is indicated by the fact that even the lowest concentrations of Selol solution (25 and 50 μg Se ml−1) induced changes in chromosome structure (their thickening and condensation). Stabilization of higher-order chromatin structures depends on histone proteins, including histone H1, and a range of non-histone proteins (Van Holde Citation1989; Wolffe Citation1994). The strongly thickened and shortened (cc) chromosomes at prophase, metaphase and further division stages, which were observed by us especially following incubation in Selol, indicate that it may modify the organization of chromatin by affecting proteins controlling its proper structure. As a result of experimentally lowering and raising proportions of histone proteins of H1 group in tobacco, structure of meiotic chromosomes was disturbed, or somatic cell nuclei displayed heterochromatinization (Prymakowska et al. Citation1999; Ślusarczyk et al. Citation1999, Citation2003).
Unlike animals, plants do not synthesize selenoproteins (Whanger Citation2002; Wierzbicka et al. Citation2007), or proteins with different functions, including antioxidant. However, it is possible that administering Selol to plants induces a specific activity of the intracellular pathway responsible for inhibition of cell cycle proteins or damage to proteins controlling the proper mechanism of the cycle. In case of sodium selenate (IV), this mechanism seems to be different, as, apart from chromosome condensation (cc), clastogenic, i.e. mutagenic changes also appear (C-metaphases, lost chromosomes). This indicates disturbances in the process of division, e.g. by inhibition of microtubule polymerization. According to investigations by Leynadier et al. (Citation1991), sodium selenate (IV) at a concentration of 10 μM affects microtubule proteins. The compound caused inhibition of microtubule polymerization. It is likely that selenium from sodium selenate (IV) replaces sulfur in disulfide bridges connecting tubulin. Presumably, selenium affects cancer cells and the human immune system through selenoenzymes, which eliminate uncontrolled excess of free radicals, thus repairing cell membranes and DNA structural defects (Huanq et al. Citation2002). The arrest of cells in mitosis observed in this study suggests degradation of the mechanism allowing the completion of mitotic divisions, i.e. transition to the G1 or G0 phases (e.g. disruption of actin and tubulin cytoskeleton). However, in order to confirm this hypothesis, it would be necessary to carry out immunocytochemical analysis of form and modifications of tubulin and actin cytoskeleton, together with spectrometry analysis.
The results indicate that Selol 2% may become useful as an anticancer drug, due to its cytostatic properties and low toxicity. However, the precise mechanism of its action is yet to be explained. Further stages of studies on Selol should include similar experiments which would allow its efficacy to be established in comparison with other preparations containing selenium. According to existing data, a dose of 1 mg Se kg−1 of body weight stops the cancer process, and a dose of 2 mg Se kg−1 of body weight causes remission of cancerous lesions. For this reason, it is necessary to carry out further experiments which would allow a more precise analysis of the action of this compound.
References
- Firbas P, Amon T. 2014. Chromosome damage studies in the onion plant Allium cepa L. Caryologia. 67(1):25–35.
- Fiskesjö G. 1985. The Allium test as standard in environmental monitoring. Hereditas. 102(1):99–112.
- Fitak B, Grabowski M, Suchocki P. 1999. Polski Patent. Pol. Pl 176530 (Cl. A61K31/095), 30 June 1999, Appl. 304046, 29 June 1994; p. 6.
- Gabara B, Kalwinek J, Koziróg A, Żakowska Z, Brycki B. 2006. Influence of N, N-bis (aminopropylo)dodecyloamine on the ultrastructure of nuclei in Aspergillus niger mycelium and on cell proliferation and mitotic disturbances in Allium cepa L. root meristem. Acta Biol Cracov Ser Bot. 48(1):45–52.
- Gasmi A, Garnier R, Galliot-Guilley M, Gaudillat C, Quartenoud B, Buisine A, Djebbar D. 1997. Acute selenium poisoning. Vet Hum Toxicol. 39(5):304–308.
- Grant WF. 1982. Chromosome aberration assays in Allium. A report of the US Environmental Protection Agency Gene – Toxicology Programm Mutat. Res. 99(3):73–91.
- Huanq K, Liu H, Chen Z, Xu H. 2002. Role of selenium in cytoprotection against cholesterol oxide-induced vascular damage in rats. Atherosclerosis. 162(1):137–144.
- Jiang C, Wang Z, Ganther H, Lu J. 2001. Caspases as key of methyl selenium-induced apoptosis (Anoiks) of DU-145 prostate cancer cells. Cancer Res. 61(7):3062–3070.
- Kuraś M, Nowakowska J, Śliwińska E, Pilarski R, Ilasz R, Tykarska T, Zobel A, Gulewicz K. 2006. Changes in chromosome structure, mitotic activity and nuclear DNA kontent from cells of Allium test induced by bark water extract of Uncaria tomentosa (Willd.) DC. J Ethnopharmacol. 107(2):211–221.
- Levan A. 1938. The effect of colchicine on root mitoses in Allium. Hereditas. 24(4):471–486.
- Leynadier D, Peyrot V, Codaccioni F, Briand C. 1991. Selenium: inhibition of microtubule formation and interaction with tubulin. Chem-Biol Interact. 79:91–102.
- Lopez-Saez JF, Fernandez-Gomez E. 1965. Partial mitotic index and phase indices. Experientia. 21:591–592.
- Majewska A, Furmanowa M, Śliwińska E, Głowniak K, Guzewska J, Kuraś M, Zobel A. 2000. Influence of extract from shoots of Taxus baccata var. “elegantissima” on mitotic activity of meristematic cells of Allium cepa L. roots. Acta Soc Bot Polon. 69(3):185–192.
- Nielsen MH, Rank J. 1994. Screening of toxicity and genotoxicity in wastewater by the use of the Allium test. Hereditas. 121(3):249–254.
- Oyeyemi IT, Bakare AA. 2013. Genotoxic and anti-genotoxic effect of aqueous extracts of Spondias mombin L., Nymphea lotus L. and Luffa cylindrica L. on Allium cepa root tip cells. Caryologia. 66(4):360–367.
- Pesnya DS. 2013. Cytogenetic effects of chitosan-capped silver nanoparticles in the Allium cepa test. Caryologia. 66(3):275–281.
- Prymakowska-Bosak M, Przewłoka M, Ślusarczyk J, Kuraś M, Lichota J, Kiliańczyk B, Jerzmanowski A. 1999. Linker histones play a role in male meiosis and the development of pollen grains in tobacco. Plant Cell. 11(12):2317–2329.
- Rayman MP. 2000. The importance of selenium to human health. Lancet. 356(9225):233–241.
- Ślusarczyk J, Prymakowska-Bosak M, Przewłoka M, Jerzmanowski A, Kuraś M. 1999. Ultrastructural organization of leaves of transgenic tobacco overexpressing histone H1 from Arabidopsis thaliana. Ann Bot. 84(3):329–335.
- Ślusarczyk J, Wierzbicki A, Przewłoka M, Tykarska T, Kuraś M. 2003. Influence of change in the proportion of H1 histone variants on microsporogenesis and development of male gametophyte in transgenic plants of tobacco (Nicotiana tabacum L.). Acta Soc Bot Polon. 72(1):25–35.
- Suchocki P, Jakoniuk D, Fitak BA. 2003. Specific spectrophotometric method with trifluoroacetic acid for the determination of selenium (IV) in selenitetriglycerides. J Pharmaceut Biomed Anal. 32(4):1029–1036.
- Van Holde KE. 1989. Chromatin. New York: Springer.
- Van Zandwijk N, Hirsch FR. 2003. Chemoprevention of lung cancer: current status and future prospects. Lung Cancer. 42(2):71–79.
- Whanger PD. 2002. Selenocompounds in plants and animals and their biological significance. J Am Coll Nutrit. 21(3):223–232.
- Wierzbicka M, Pyrzyńska K, Bulska E. 2007. Selen – pierwisatek ważny dla zdrowia, fascynujący dla badacza. Warsaw: Malamut Press.
- Wolffe AP. 1994. Regulation of chromatin structure and function. Austin: RG Landes.
- Zagrodzki P, Bik D, Fitak B, Suchocki P, Niemczuk K. 2000. Selenoenzymes in animal tissues after supplementation with Selol. Bull Vet Inst Pulawy. 44(2):215–220.