Abstract
Bacteriophages isolated from free-range chickens were tested as a therapeutic agent for reducing the concentration of Salmonella enterica serovar Enteritidis phage type 4 (S. Enteritidis PT4) in caeca of broilers. One-day-old broilers infected with S. Enteritidis PT4 by a seeder bird method were orally treated on the seventh day of age with a mixture of 1011 plaque-forming units of each of three bacteriophages. Five days after treatment the bacteriophage-treated group showed a reduction of 3.5 orders of magnitude on colony-forming units of S. Enteritidis PT4 per gram of caecal content. Samples collected at 10, 15, 20 and 25 days after treatment revealed that treated birds still had lower colony-forming units of S. Enteritidis PT4 per gram of caecal content. These data gave us compelling evidence that a mixture of bacteriophages may be efficacious in reducing S. Enteritidis PT4 concentration in broilers’ caeca and therefore reducing contamination of poultry products by this food-borne pathogen.
Le traitement oral avec des bactériophages réduit la concentration de Salmonella Enteritidis PT4 dans les contenus cæcaux des poulets de chair
Les bactériophages isolés de poulets élevés en plein air ont été testés comme agent thérapeutique pour réduire la concentration de Salmonella enterica sérovar Enteritidis phage type 4 (S. Enteritidis PT4) dans les cæca de poulets de chair. Des poulets de chair, âgés d'un jour, infectés par l'intermédiaire d'animaux excréteurs de S. Enteritidis PT4, ont été traités oralement à l’âge de 7 jours avec un mélange de trois bactériophages tirant 1011 plages formant unité (PFU). Cinq jours après le traitement, le lot ayant reçu les bactériophages a présenté une réduction de l'ordre de 3,5 du nombre de colonies formant unité de S. Enteritidis PT4 par gramme de contenu cæcal (CFU/g). Les échantillons récoltés à 10, 15, 20 et 25 jours après le traitement ont révélé que les animaux traités avaient encore les plus faibles CFU/g. Ces données mettent en évidence que le mélange de bactériophages peut être efficace en réduisant la concentration de S. Enteritidis PT4 dans les cæca de poulets de chair et par conséquent réduire la contamination des produits avicoles par cet agent pathogène d'origine alimentaire.
Orale Behandlung mit Bakteriophagen reduziert die Konzentration von Salmonella Enteritidis PT4 im Zäkuminhalt von Broilern
Bakteriophagen aus im Auslauf gehaltenen Hühnern wurden als Therapeutikum zur Reduktion der Konzentration von Salmonella enterica serover Enteritidis Phagentyp 4 (S. Enteritidis PT4) im Zäkum von Broilern getestet. Boilerküken, die mittels eines Trägertieres mit S. Enteritidis PT4 am 1. Lebenstag infiziert worden waren, wurden am 7. Lebenstag mit einer Mischung von jeweils 1011 Plaque bildenden Einheiten (PFU) von drei Bakteriophagen oral behandelt. Fünf Tage nach der Behandlung zeigten die mit Bakteriophagen behandelten Gruppen eine Reduktion der Kolonie bildenden Einheiten von S. Enteritidis PT4 je Gramm Zäkalinhalt (CFU/g) in einer Größenordnung von 3,5. Proben, die 10, 15, 20 und 25 Tage nach der Behandlung gesammelt wurden, zeigten, dass die behandelten Tiere immer noch niedrigere CFU/g aufwiesen. Diese Daten gaben uns den zwingenden Beweis, dass eine Bakteriophagenmischung effektiv die S. Enteritidis PT4-Konzentration in Broilerzäka verringern und damit die Kontamination von Geflügelprodukten mit diesem durchs Futter übertragenen Erreger reduzieren kann.
El tratamiento oral con bacteriófagos reduce la concentración de Salmonella Enteritidis PT4 en contenido cecal de pollos de engorde
Se probaron bacteriófagos obtenidos de pollos en extensivo como agentes terapéuticos para reducir la concentración de Salmonella enterica serovar Enteritidis fagotipo 4 (S. Enteritidis PT4) en ciegos de pollos de engorde. Pollos de engorde infectados con S. Enteritidis PT4 mediante un método de ave semilla fueron tratados oralmente al 7o día de edad con una mezcla de 1011 unidades formadoras de placa (UFP) de cada uno de los tres bacteriófagos. Tras cinco días de tratamiento, el grupo tratado con los bacteriófagos mostró una reducción de 3.5 órdenes de magnitud de unidades formadoras de colonias de S. Enteritidis PT4 por gramo de contenido cecal (UFC/g). Las muestras recogidas a los 10, 15, 20 y 25 días tras el tratamiento revelaron que las aves tratadas aún tenían menores UFC/g. Estos datos evidencian que una mezcla de bacteriófagos pueden ser eficaces para reducir la concentración de S. Enteritidis en ciegos de pollos de engorde y, en consecuencia, para reducir la contaminación de productos derivados del pollo por parte de este patógeno alimentario.
Introduction
Concerns related to drug-resistant bacteria have stimulated interest in alternative treatments of bacterial infections (Cohen, Citation1994). Among these therapies, a special interest has been given to phage therapy, the use of bacteriophages to kill or otherwise control the bacterial population in infected hosts (Lorch, Citation1999; Sulakvelidze et al., Citation2001; Joerger, Citation2003).
Early studies by Smith and Huggins with Escherichia coli (Smith & Huggins, Citation1982, Citation1983; Smith et al., Citation1987) demonstrated that phage therapy can be as efficient as antibiotics. After these breakthrough studies had been presented, several other publications reported success of experimental phage therapy with different bacteria, Acinetobacter baumanii, Pseudomonas aeruginosa and Staphylococcus aureus (Soothill, Citation1992), Vibrio vulnificus (Cerveny et al., Citation2002), Enterococcus faecium (Biswas et al., Citation2002), and Pseudomonas aeruginosa (Soothill, Citation1994); and even Escherichia coli in other animals such as calves (Barrow et al., Citation1998) and chickens (Huff et al., Citation2002a,Citationb).
Major risks factors for food poisoning caused by salmonellae are related to poultry, such as eating raw or undercooked eggs (Molbak & Neiman, Citation2002). Food poisoning caused by salmonellae in man results in reduction of productivity, discomfort, expenditure on medication and sometimes death (Persson & Jendteg, Citation1992; Mead et al., Citation1999). Contamination of frozen carcasses of broilers with salmonellae can reach rates as high as 53.5% (Santos et al., Citation2000). Among the most important salmonellae associated with chicken meat and eggs is Salmonella enterica serovar Enteritidis (S. Enteritidis) (Chung et al., Citation2003). This is a murine serotype that has entered the intensive poultry industry and can cause infection and contamination of poultry products in the absence of a clinical disease (Gast & Beard, Citation1990). S. Enteritidis phage type 4 (S. Enteritidis PT4) is the salmonella of higher frequency in broilers in Brazil (dos Santos et al., Citation2003; Fernandes et al., Citation2003; Nunes et al., Citation2003) and one of the most frequently isolated salmonella in other poultry-producing countries as well (Capita et al., Citation2003; Cogan & Humphrey, Citation2003; Esaki et al., Citation2004).
Control of salmonellae at the pre-harvest stage is of paramount importance. It can prevent the introduction of this bacterium into the food chain and consequently reduce food poisoning among consumers (Wegener et al., Citation2003). Control of salmonellae at farm level is more likely to be effective through a multi-factorial approach. Good agricultural practices based on hazard analysis and critical control points (Rose et al., Citation2002; Nayak et al., Citation2003), vaccination (Zhang-Barber et al., Citation1999; Yamame et al., Citation2000; Cogan & Humphrey, Citation2003), probiotics, prebiotics and synbiotics (Van Immerseel et al., Citation2002) have all been used as preventive measures when salmonella infection is likely to occur in broilers.
However, the use of antimicrobial drugs is often still required for reducing shedding and intestinal carriage of salmonellae, especially when preventive measures have not been sufficiently efficient (Davies et al., Citation2003). In such cases, new concerns are born due to selection of resistant bacteria (Velonakis et al., Citation2001; Molbak et al., Citation2002; Chung et al., Citation2003; Malorny et al., Citation2003; Busani et al., Citation2004), the possibility that residues of antimicrobial drugs may contaminate chicken meat and eggs consumed by man (Donoghue, Citation2003), and finally because antibiotic therapy alone will is unlikely to clear salmonellae from infected chickens (Fernandez et al., Citation2001).
Alternatives to antimicrobial drugs are required for the control of salmonellae within poultry production. Besides reducing the use of antibiotics, these methods could also be used in conjunction with good agricultural practices in the multi-factorial approach for improvements on salmonella's control at a pre-harvest level. Bactericidal bacteriophages may provide a natural, non-toxic, feasible and non-expensive component for the pre-harvest control of salmonellae in poultry. Some preliminary work has indicated that salmonellae can be controlled by the use of bacteriophages (Barrow et al., Citation1987; Berchieri et al., Citation1991; Sklar & Joerger, Citation2001), although additional work has to be done in order to achieve more optimistic results.
In the study reported here we infected 1-day-old broilers with S. Enteritidis PT4 and orally treated them with a mixture of three bacteriophages isolated from faeces of free-range chickens.
Materials and Methods
Salmonella
S. Enteritidis PT4 isolate P125589, used throughout this study, was kindly provided by Dr Paul Barrow, from the Institute for Animal Health, England (Barrow & Lovell, Citation1991). This isolate was originally obtained from the Central Public Health Laboratory, London, UK following a case of human food poisoning. S. Enteritidis PT4 was grown on nutrient broth (NB) (Oxoid; 1 g/l beef extract, 2 g/l yeast extract, 5 g/l peptone, 5 g/l sodium chloride) or nutrient agar (NA) (NB was added at 15 g/l bacteriologic agar) at 37°C and kept frozen at −80°C unless stated. When used for bacteriophage isolation, S. Enteritidis PT4 was grown on NB or NA with added 5 mM MgSO4 (NB-MgSO4 or NA-MgSO4). Inocula doses were measured by conventional plate counting on Brilliant Green Agar (BGA) (10 g/l proteose peptone, 3 g/l yeast extract, 10 g/l lactose, 10 g/l sucrose, 5 g/l sodium chloride, 0.08 g/l phenol red, 0.0125 g/l brilliant green, 12 g/l bacteriologic agar, pH 6.9). An antibiotic-resistant strain of salmonella was not necessary due to the high number of colony-forming units (CFU) present in the caecal contents, which allowed one to enumerate S. Enteritidis PT4 in dilutions rid of contaminants.
Bacteriophages
Bacteriophages lytic to S. Enteritidis PT4, denominated CNPSA1, CNPSA3 and CNPSA4, were isolated from free-range layers in Brazil and were morphologically characterized as described elsewhere (Fiorentin et al., Citation2004). When stocks of equivalent titres were tested in overlay cultures to lyse S. Enteritidis PT4, these bacteriophages showed very similar performance and were chosen to be used as a combination to avoid selection of resistant S. Enteritidis PT4. Bacteriophage stocks were kept at −80°C in SM buffer with 7% dimethyl sulfoxide and 1% chloroform for long-term use. When used for treating birds, frozen stocks of bacteriophages were expanded by inoculation into 20 ml S. Enteritidis PT4 cultures in exponential grow in NB-MgSO4, followed by incubation for approximately 18 h at 37°C with shaking (200×g), treated with 5% chloroform to lyse all bacteria, centrifuged (12 000×g for 5 min) and stored at 4°C as a supernatant with 1% chloroform. Inocula were titrated for plaque-forming units per millilitre (PFU/ml) in agar overlay cultures of S. Enteritidis PT4 as described by Kudva et al. (Citation1999). Agar overlays were prepared by inoculating 10 μl of 10-fold dilutions of bacteriophage stocks in SM buffer (5.8 g/l NaCl, 2 g/l MgSO4–7H2O, 0.05 M Tris–HCl, pH 7.5, 0.1 g/l gelatin) into 250 μl S. Enteritidis PT4 culture in the exponential phase of growth, which was incubated for 20 min at 37°C and poured into 7 ml melted NB (45°C) containing 0.7% agarose. The melted agarose containing bacteria and diluted bacteriophage was then laid over a 10 cm diameter sterile NA-MgSO4 Petri dish to solidify and consequently be incubated for 24 h at 37°C. Plaques were identified as clear spots of lyse measuring approximately 1 mm in diameter.
Experimental infection with S. Enteritidis PT4
Three groups of 1-day-old chicks of the broiler-type Embrapa 021 breed were separately housed in air-filtered wire-mesh floor isolator cabinets, with freely available drug-free feed and water for 32 days (). Cloacal swabs collected from all birds as well as 10 g feed were negative for both salmonellae and bacteriophages lytic to S. Enteritidis PT4 on the first day of the experiment.
Table 1. Experimental design
Infection with S. Enteritidis PT4 was performed under a seeder bird method as to simulate natural conditions. On the first day of life, five birds were marked with metal rings in their wings and orally inoculated with 100 μl fresh S. Enteritidis PT4 culture (108 CFU per bird), while 30 birds were kept for infection by contact. On the third day post-infection (p.i.) all birds inoculated with the S. Enteritidis PT4 culture were eliminated and cloacal swabs were taken from the remaining birds to test for S. Enteritidis PT4 shedding.
Treatment with bacteriophages
On the seventh day of life (7 days p.i. of seeder birds), five birds of each group were necropsied to confirm the salmonella-free status of controls and to obtain frequencies of liver and spleen positive for S. Enteritidis PT4 as well as CFU S. Enteritidis PT4 per gram of caecal content (CFU/g) before treatment. All remaining birds from the treated group (Group 3) orally received 1011 PFU of each of the three bacteriophages denominated CNPSA1, CNPSA3 and CNPSA4 diluted together in 300 μl SM buffer. Bacteriophages were administered as crude lysates of S. Enteritidis PT4 stored at 4°C and diluted 10 or 100 times in SM buffer to reach the necessary oral dose.
Addressing the effect of the treatment
Five birds of each group were necropsied at 5-day intervals, corresponding to days 0, 5, 10, 15, 20 and 25 post-treatment (p.t.) with bacteriophages or to 12, 15, 17, 22, 27 and 32 days of life, the equivalent to days p.i. of seeder birds. Fragments of about 5 mm from the liver, spleen and caeca (including the tonsils) were inoculated in 3 ml Rappaport–Vassiliadis Soya Peptone broth (Oxoid; 4.5 g/l soya peptone, 7.2 g sodium chloride, 1.26 g/l potassium dihydrogen phosphate, 0.18 g/l di-potassium hydrogen phosphate, 13.58 g/l magnesium chloride anhydrous, 0.036 g/l malachite green, pH 5.2) and incubated overnight at 42°C. Loopfuls were then transferred to BGA and incubated at 37°C for an additional 48 h to identify colonies by morphology and serum agglutination with polyvalent anti-somatic serum (Probac). Caecal contents were weight, diluted 10-fold in phosphate-buffered saline (pH 7.4) and aliquots of 100 μl inoculated onto BGA plates and incubated at 37°C for 24 h to obtain the CFU/g.
Fragments of the liver, spleen and caeca were also subjected to attempts of bacteriophage isolation by incubation in a culture of S. Enteritidis PT4. Fragments of tissues of approximately 5 mm were incubated overnight at 37°C with 2 ml S. Enteritidis PT at the exponential phase of growth on NB-MgSO4. The culture was then made to 5% chloroform, vortexed, centrifuged at 12 000×g for 5 min and 5 μl supernatant inoculated over a fresh lawn of S. Enteritidis PT4 grown on NB-MgSO4 solidified with 0.7% agarose. The presence of bacteriophages was identified by transparent spots of the bacterial lawn on sample sites 24 h after incubation at 37°C. Quantitative analysis of bacteriophages on caeca was addressed by 10-fold dilutions of their contents in SM buffer followed by inoculation of 10 μl each dilution into 250 μl S. Enteritidis PT4 in the exponential phase of growth. This mixture was then incubated for 20 min at 37°C and poured into melted NB-MgSO4 with added 0.7% agarose for preparing overlay cultures as already described. Cultures were incubated overnight at 37°C to obtain the PFU per gram (PFU/g).
Statistical analysis
Frequencies of S. Enteritidis PT4 isolation from the spleen, liver and caecal fragments of Group 2 and Group 3 were compared using Fisher's exact test. Means of CFU/g obtained from caecal contents of birds from Group 2 and Group 3 were subjected to variance analysis and compared with the Student t test weighted by the inverse of the mean standard errors. Analyses were performed using the SAS-GLM procedure (SAS Institute Inc., Citation2001).
Results
S. Enteritidis PT4 infection
Cloacal swabs collected from contact birds on the third day of life allowed us to isolate S. Enteritidis PT4 from 28/30 and 30/30 birds in Group 2 and Group 3, respectively. In the face of the apparently different levels of infection detected we treated Group 3 with bacteriophages, keeping the group with lower level of S. Enteritidis PT4 shedding as a control. Neither clinical sign nor mortality was observed in any group during the whole experiment. The control group remained both salmonella-free and bacteriophage-free all through the experiment.
Concentration of bacteriophages in caecal contents
Counting of total bacteriophages was addressed by direct isolation from caecal contents after bacterial lysis using 5% chloroform. Samples were diluted in SM buffer and aliquots mixed with S. Enteritidis PT4, which was then poured into melted NB-MgSO4 containing 0.7% agarose and plated over NA-MgSO4 to form overlay cultures. Because all three bacteriophages showed morphologically similar plaques we could not obtain individual counts. No bacteriophage was isolated from any sample of birds from Group 1 (uninfected and non-treated control) or Group 2 (S. Enteritidis PT4-infected and non-treated control). Bacteriophages were present in caecal contents collected at necropsies performed on Group 3 from 5 to 20 days after treatment ( and ). All samples of the liver and spleen from all three groups were negative for bacteriophages.
Table 2. Log10 bacteriophage counting on caecal contents of treated birdsa
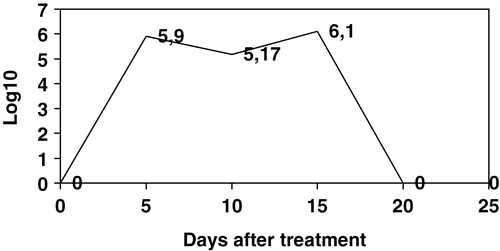
Figure 1. Log10 PFU/g bacteriophages isolated from caecal contents of birds from Group 3. Data are the mean of five birds. On day 20 post-treatment all birds were positive in a qualitative assay but under the limit of detection for the technique used for enumeration. On day 25 all birds were negative in both qualitative and quantitative tests.
The effect of bacteriophages over S. Enteritidis PT4 concentration on caecal contents
Group 3 had higher levels of infection by S. Enteritidis PT4 compared with Group 2, as addressed by the number of positive cloacal swabs after 3 days on contact with seeder birds as well as CFU/g caecal content at day 0 of bacteriophage treatment (). Five days after treatment, both groups had a similar mean of CFU/g, representing approximately a 3.5 times reduction (from 1682×107 to 486×107 CFU/g) of CFU evidently caused by the bacteriophages. Caecal contents collected at days 10, 15, 20 and 25 p.t. showed higher P values for differences between means between means in a comparison done with the Student t test (), thus not being statistically significant. However, samples collected 20 days p.t. showed a difference with the marginal P value of 0.06, thus indicating a tendency for the treated group to have lower levels of contamination on an extended period of time.
Table 3. Log10 means of S. Enteritidis PT4 on caecal contents of birds from Group 2 and Group 3a
Invasion of S. Enteritidis PT4
We isolated S. Enteritidis PT4 from the liver () and spleen () in both treated and infected control groups from 5 days p.i. until the end of the experiment. Although P values were high there was a tendency for a lesser number of positive birds among the treated group in some instances, like the frequency of positive spleens observed on day 15 p.i. The high frequency of isolation of S. Enteritidis PT4 from caecal tonsils () was in accordance with the presence of the bacterium in caecal contents as verified by the quantitative test used for counting CFU/g ().
Table 4. Frequency of S. Enteritidis PT4-positive livers per group
Table 5. Frequency of S. Enteritidis PT4-positive spleens per group
Table 6. Frequency of S. Enteritidis PT4-positive caecal tonsils per group
Discussion
The aim of this study was to see whether bacteriophages, originally isolated from free-range chickens, could be of any help in reducing the concentration of S. Enteritidis PT4 in caeca of experimentally infected broilers. The results obtained by us showed that bacteriophages acted as a factor inducing reduction in CFU/g caecal content. Results of CFU/g also showed that bacteriophages possibly reduced the cross-contamination that occurred within the isolator cabinets, indicating that benefits from the treatment may extend beyond the direct action of the oral dose administered.
Under the conditions in which this experiment was conducted, an apparent heavy infection occurred. Three days after inoculation of seeder birds almost every contact bird was shedding S. Enteritidis PT4, thus indicating that bacteriophages would act as therapeutic agents not prophylactic agents. We also detected a high frequency of liver and spleen positive for S. Enteritidis PT4 throughout the experiment, which indicates a high frequency of systemic infection. According to experiments conducted by others, S. Enteritidis PT4 is highly invasive for 1-day-old chicks (Barrow & Lovell, Citation1991; Berchieri et al., Citation2001; Roy et al., Citation2001) and oral infection with low doses of S. Enteritidis PT4 would not cause a systemic infection (Duchet-Suchaux et al., Citation1997), thus indicating that the seeder birds method used by us resulted in high infection dose for contact birds. In this context, it is understandable that bacteriophages did not clear S. Enteritidis PT4 from infected chicks. However, the 3.5 orders of magnitude reduction caused on CFU/g treated birds clearly indicates that bacteriophages had an ‘in vivo’ effect towards reduction of S. Enteritidis PT4 in caecal contents. In a similar work conducted by Sklar & Joerger (Citation2001) the reduction obtained on CFU/g by the means of bacteriophage was 1.3 orders of magnitude. The use of a single dose with a high PFU of bacteriophages was probably the reason why we obtained a better result, in contrast to the continuous administration of bacteriophages used in the feed by Sklar & Joerger (Citation2001). Continuous administration of bacteriophages may lead to resistant salmonellae. We used different bacteriophages, and that can also account for some of the difference. However, reduction with several logs of magnitude will be necessary to make this methodology viable as a practical standpoint. This will probably be achieved with additional research.
The almost complete elimination of bacteriophage from faeces 15 days p.t. could indicate a selection for S. Enteritidis PT4 strains resistant to the bacteriophages. We did not test for this purpose in this experiment because in a previous work all S. Enteritidis PT4 isolates obtained from faeces as long as 15 days after inoculation of bacteriophages remained sensitive (Fiorentin et al., Citation2004). The use of three bacteriophages was also chosen to reduce the possibility of selection for resistance against one specific bacteriophage. Clearing of bacteriophages from faeces is more likely to be related to poor multiplication inside the chicken alimentary tract due to less contact with salmonella cells and the continuous expelling caused by intestinal motility. The scope of this experiment was to study a single flock because continuous use of bateriophage in the same site could select resistant strains of salmonella (Sklar & Joerger, Citation2001).
Samples collected at 5 and 10 days after treatment showed a drop in total counts of bacteriophages per gram, while at day 15 after treatment the results showed an increase in total bacteriophage counting (). This observation coincides with the increase on CFU/g S. Enteritidis PT4 in both Group 2 and Group 3 (), probably representing multiplication of bacteriophages inside the alimentary tract of the bird due to the increased salmonella population originated from cross-contamination within the isolators. The technique used for bacteriophage counting had a dilution factor of 1000, thus leading to the conclusion that after 20 days of treatment the bacteriophages were present in the caecal content at levels lower than 103 PFU/g. As an attempt to increase the sensitivity of our method, qualitative tests were performed inoculating 1 ml S. Enteritidis PT4 culture with fragments of caeca, followed by incubation at 37°C for 24 h to allow bacteriophages to multiply, and identifying their presence by plating over a fresh lawn of S. Enteritidis PT4 grown in NB with 7% agarose. This technique would theoretically detect a single particle of bacteriophages and allowed us to isolate bacteriophages from caecal contents of birds collected at day 20 after treatment while on the 25th day all samples were confirmed as negative (). In previous work (Fiorentin et al., Citation2004) we noticed that these bacteriophages did not multiply in the alimentary tract of salmonella-free birds, thus not using any bacteria from the physiologic flora as a target. Taken together, the results obtained from counting bacteriophages and CFU/g S. Enteritidis PT4 suggest that bacteriophages will best be effective in a short period after administration and only in birds with high CFU/g. The fact that bacteriophages disappear will help to avoid resistant salmonellae arising.
The concentration of S. Enteritidis PT4 in the caecal content naturally decreases a few weeks after inoculation of 1-day-old chicks (Desmidt et al., Citation1997; Berchieri et al., Citation2001). A peak in CFU/g we observed 15 days p.i. probably represents cross-contamination within the isolator cabinets. In this view, bacteriophages not only speeded up the natural drop in CFU/g, but also opposed cross-contamination once treated birds had a lower peak ().
Figure 2. Log10 CFU/g S. Enteritidis PT4 enumerated from caecal contents of birds from Group 2 and Group 3. Data are the mean of five birds as displayed in . On the x axis are days post-treatment, starting on day 7 post-infection by contact. Birds on Group 2 (dashed line) represent infected non-treated controls while birds of Group 3 were infected by contact and orally treated with bacteriophages. Uninfected and non-treated birds (Group 1) were negative for salmonellae in all tests. See for P values for differences between means.
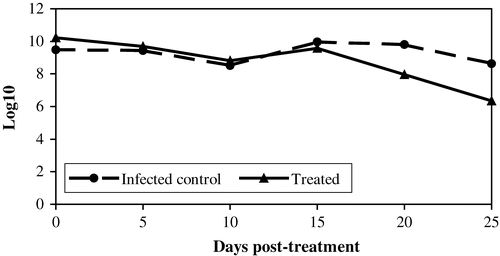
The reduction in CFU/g was more evident 5 days after treatment, when both control and treated groups showed similar means even though treated birds had higher initial counts at day 0. We are tempted to conclude that the high multiplicity of infection, the number of particles of bacteriophage per cell of salmonella, is necessary for dropping caecal CFU. When PFU/g dropped over time the difference between means of CFU/g was no longer statistically significant. Further studies with higher PFU per dose in several treatments spaced 5 days apart may result in one even more evident drop in CFU/g.
We could not calculate the multiplicity of infection because it is impossible to estimate how many particles of bacteriophages from the oral dose actually reached the caeca. However, this number was probably high at day 1 p.t. because we administered an individual dose with about 18 times more bacteriophages than the CFU/g S. Enteritidis PT4 identified in the caeca of treated birds. Goode et al. (Citation2003) observed that S. Enteritidis PT4 counting was reduced in experimentally contaminated chicken skin when the multiplicity of infection was as low as one, which has probably been achieved at the caecal level in our experiment.
This was our first attempt to control S. Enteritidis PT4 using these bacteriophages. New experiments with a higher number of birds, lower variability among birds and repeated treatments with the bacteriophages in a 5-day interval may show statistical differences beyond 5 days p.t. Overall, the results obtained with this experiment gave us an optimistic view over the possibilities of controlling S. Enteritidis PT4 by the use of bacteriophages once it confirms reduction of CFU/g at the caecal level.
Translations of the abstract in French, German and Spanish are available on the Avian Pathology website.
References
- Barrow , PA . (1991) . Experimental infection of chickens with Salmonella enteritidis . Avian Pathology , 20 : 145 – 153 .
- Barrow , PA and Lovell , MA . (1991) . Experimental infection of egg-laying hens with Salmonella enteritidis phage type 4 . Avian Pathology , 20 : 335 – 348 .
- Barrow , PA , Tucker , JF and Simpson , JM . (1987) . Inhibition of colonization of the chicken alimentary tract with Salmonella typhimurium gram-negative facultatively anaerobic bacteria . Epidemiology and Infection , 98 : 311 – 322 .
- Barrow P Lovell MA Berchieri A Jr (1998) Use of lytic bacteriophage for control of experimental Escherichia coli septicemia and meningitis in chickens and calves Clinical and Diagnostic Laboratory Immunology 5 294 298
- Berchieri A Jr Lovell MA Barrow PA (1991) The activity in the chicken alimentary tract of bacteriophages lytic for Salmonella typhimurium Research Microbiology 142 541 549
- Berchieri A Jr Wigley P Page K Murphy CK Barrow PA (2001) Further studies on vertical transmission and persistence of Salmonella enterica serovar Enteritidis phage type 4 in chickens Avian Pathology 30 297 310
- Biswas , B , Adhya , S , Washart , P , Paul , B , Trostel , AN , Powell , B , Carlton , R and Merril , CR . (2002) . Bacteriophage therapy rescues mice bacteremic from a clinical isolate of vancomycin-resistant Enterococcus faecium . Infection and Immunity , 70 : 204 – 210 .
- Busani , L , Graziani , C , Battisti , A , Franco , A , Ricci , A , Vio , D , Digiannatale , E , Paterlini , F , D'Incau , M , Owczarek , S , Caprioli , A and Luzzi , I . (2004) . Antibiotic resistance in Salmonella enterica serotypes Typhimurium, Enteritidis and Infantis from human infections, foodstuffs and farm animals in Italy . Epidemiology and Infection , 132 : 245 – 251 .
- Capita , R , Alvarez-Astorga , M , Alonso-Calleja , C , Moreno , B and del Camino Garcia-Fernandez , M . (2003) . Occurrence of salmonellae in retail chicken carcasses and their products in Spain . International Journal of Food Microbiology , 81 : 169 – 173 .
- Cerveny , KE , DePaola , A , Duckworth , SH and Gulig , PA . (2002) . Phage therapy of local and systemic disease caused by Vibrio vulnificus in iron-dextran-treated mice . Infection and Immunity , 70 : 6251 – 6262 .
- Chung , YH , Kim , SY and Chang , YH . (2003) . Prevalence and antibiotic susceptibility of Salmonella isolated from foods in Korea from 1993 to 2001 . Journal of Food Protection , 66 : 1154 – 1157 .
- Cogan , TA and Humphrey , TJ . (2003) . The rise and fall of Salmonella Enteritidis in the UK . Journal of Applied Microbiology , 94 (Suppl) : 114S – 119S .
- Cohen , ML . (1994) . Antimicrobial resistance: prognosis of public health . Trends in Microbiology , 2 : 422 – 425 .
- Davies , R , Liebana , E and Breslin , M . (2003) . Investigation of the distribution and control of Salmonella enterica serovar Enteritidis PT6 in layer breeding and egg production . Avian Pathology , 32 : 225 – 237 .
- Desmidt , M , Ducatelle , R and Haesebrouck , F . (1997) . Pathogenesis of Salmonella enteritidis phage type four after experimental infection of young chickens . Veterinary Microbiology , 56 : 99 – 109 .
- Donoghue , DJ . (2003) . Antibiotic residues in poultry tissues and eggs: human health concerns? . Poultry Sciences , 82 : 618 – 621 .
- dos Santos , LR , do Nascimento , VP , de Oliveira , SD , Rodrigues , DP , dos Reis , EM , Seki , LM , Ribeiro , AR and Fernandes , SA . (2003) . Phage types of Salmonella enteritidis isolated from clinical and food samples, and from broiler carcasses in southern Brazil . Revista do Instituto de Medicina Tropical de São Paulo , 45 : 1 – 4 .
- Duchet-Suchaux , M , Mompart , F , Berthelot , F , Beaumont , C , Lechopier , P and Pardon , P . (1997) . Differences in frequency, level, and duration of caecal carriage between four outbred chicken lines infected orally with Salmonella enteritidis . Avian Diseases , 41 : 559 – 567 .
- Esaki , H , Morioka , A , Ishihara , K , Kojima , A , Shiroki , S , Tamura , Y and Takahashi , T . (2004) . Antimicrobial susceptibility of Salmonella isolated from cattle, swine and poultry (2001–2002): report from the Japanese Veterinary Antimicrobial Resistance Monitoring Program . Journal of Antimicrobials and Chemotherapy , 53 : 266 – 270 .
- Fernandes , SA , Ghilardi , AC , Tavechio , AT , Machado , AM and Pignatari , AC . (2003) . Phenotypic and molecular characterization of Salmonella Enteritidis strains isolated in São Paulo, Brazil . Revista do Instituto de Medicina Tropical de São Paulo , 45 : 59 – 63 .
- Fernandez , A , Lara , C , Loste , A , Calvo , S and Marca , MC . (2001) . Control of Salmonella enteritidis phage type 4 experimental infection by fosfomycin in newly hatched chicks . Comparative Immunology, Microbiology & Infectious Diseases , 24 : 207 – 216 .
- Fiorentin , LF , Vieira , ND and Barros , S . (2004) . ‘In vivo’ characterization and ‘in vivo’ properties of Salmonellae lytic bacteriophages isolated from free-range layers . Brazilian Journal of Poultry Sciences , 6 : 105 – 112 .
- Gast , RK and Beard , CW . (1990) . Production of Salmonella enteritidis-contaminated eggs by experimentally infected hens . Avian Diseases , 34 : 438 – 446 .
- Goode , D , Allen , VM and Barrow , PA . (2003) . Reduction of experimental Salmonella and Campylobacter contamination of chicken skin by application of lytic bacteriophages . Applied Environmental Microbiology , 69 : 5032 – 5036 .
- Huff , WE , Huff , GR , Rath , NC , Balog , JM and Donoghue , AM . (2002a) . Prevention of Escherichia coli infection in broiler chickens with a bacteriophage aerosol spray . Poultry Sciences , 81 : 1486 – 1491 .
- Huff WE Huff GR Rath NC Balog JM Xie H Moore PA Jr Donoghue AM (2002b) Prevention of Escherichia coli espiratory infection in broiler chickens with bacteriophages (SPR02) Poultry Sciences 81 437 441
- Joerger , RD . (2003) . Alternatives to antibiotics: bacteriocins, antimicrobial peptides and bacteriophages . Poultry Sciences , 82 : 640 – 647 .
- Kudva , IT , Jelacic , S , Tarr , PP , Youderian , P and Hovne , CJ . (1999) . Biocontrol of Escherichia coli O157 with O157-specific bacteriophages . Applied and Environmental Bacteriology , 65 : 3767 – 3773 .
- Lorch , A . (1999) . Bacteriophages: an alternative to antibiotics? . Biotechnology and Development Monitor , 39 : 14 – 17 .
- Malorny , B , Schroeter , A , Guerra , B and Helmuth , R . (2003) . Incidence of quinolone resistance in strains of Salmonella isolated from poultry, cattle and pigs in Germany between 1998 and 2001 . Veterinary Record , 153 : 643 – 648 .
- Mead , PS , Slutsker , L , Dietz , V , McCaig , LF , Bresee , JS , Shapiro , C , Griffin , PM and Tauxe , RV . (1999) . Food-related illness and death in the United States . Emerging Infectious Diseases , 5 : 607 – 625 .
- Molbak , K and Neimann , J . (2002) . Risk factors for sporadic infection with Salmonella enteritidis, Denmark, 1997–1999 . American Journal of Epidemiology , 156 : 654 – 661 .
- Molbak , K , Gerner-Smidt , P and Wegener , HC . (2002) . Increasing quinolone resistance in Salmonella enterica serotype Enteritidis . Emerging Infectious Diseases , 8 : 514 – 515 .
- Nayak , R , Kenney , PB , Keswani , J and Ritz , C . (2003) . Isolation and characterization of Salmonella in a turkey production facility . British Poultry Sciences , 44 : 192 – 202 .
- Nunes , IA , Helmuth , R , Schroeter , A , Mead , GC , Santos , MA , Solari , CA , Silva , OR and Ferreira , AJ . (2003) . Phage typing of Salmonella enteritidis from different sources in Brazil . Journal of Food Protection , 66 : 324 – 327 .
- Persson , U and Jendteg , S . (1992) . The economic impact of poultry-borne salmonellosis: how much should be spent on prophylaxis? . International Journal of Food Microbiology , 15 : 207 – 213 .
- Rose , BE , Hill , WE , Umholtz , R , Ransom , GM and James , WO . (2002) . Testing for Salmonella in raw meat and poultry products collected at federally inspected establishments in the United States, 1998 through 2000 . Journal of Food Protection , 65 : 937 – 947 .
- Roy , P , Dhillon , AS , Shivaprasad , HL , Schaberg , DM , Bandli , D and Johnson , S . (2001) . Pathogenicity of different serogroups of avian salmonellae in specific-pathogen-free chickens . Avian Diseases , 45 : 922 – 937 .
- Santos DMS Berchieri A Jr Fernandes SA Tavechio AT Amaral LAdo (2000) Salmonella em carcaças de frangos congeladas Pesquisa Veterinária Brasileira 20 39 42
- SAS Institute Inc. (2001) System for Microsoft Windows Release 8.2 (1999–2001, CD-ROM) Cary NC SAS Institute Inc
- Sklar , IB and Joerger , RD . (2001) . Attempts to utilize bacteriophages to combat Salmonella enterica serovar Enteritidis in chickens . Journal of Food Safety , 21 : 15 – 29 .
- Smith , HW and Huggins , MB . (1982) . Successful treatment of experimental Escherichia coli infections in mice using phage: its general superiority over antibiotics . Journal of General Microbiology , 128 : 307 – 318 .
- Smith , HW and Huggins , MB . (1983) . Effectiveness of phage in treating experimental Escherichia coli diarrhoea in calves, piglets and lamb . Journal of General Microbiology , 129 : 2659 – 2675 .
- Smith , HW , Huggins , MB and Shaw , KM . (1987) . The control of experimental Escherichia coli diarrhoea in calves by the means of bacteriophages . Journal of General Microbiology , 133 : 1111 – 1126 .
- Soothill , JS . (1992) . Treatment of experimental infections of mice with bacteriophages . Journal of Medical Microbiology , 37 : 258 – 261 .
- Soothill , JS . (1994) . Bacteriophage prevents destruction of skin grafts by Pseudomonas aeruginosa . Burns , 20 : 209 – 211 .
- Sulakvelidze A Alavidze Z Morris JG Jr (2001) Bacteriophage therapy Antimicrobial, Agents and Chemotherapy 45 649 659
- Van Immerseel , F , Cauwerts , K , Devriese , LA , Haesenbrouck , F and Ducatelle , R . (2002) . Feed additives to control Salmonella in poultry . World's Poultry Science Journal , 58 : 501 – 513 .
- Velonakis , EN , Markogiannakis , A , Kondili , L , Varjioti , E , Mahera , Z , Dedouli , E , Karaitianou , A , Vakalis , N and Bethimouti , K . (2001) . Evolution of antibiotic resistance of non-typhoidal salmonellae in Greece during 1990–97 . Euro Surveillance , 6 : 117 – 120 .
- Wegener , HC , Hald , T , Wong , DL , Madsen , M , Korsgaard , H , Bager , F , Gerner-Smidt , P and Molbak , K . (2003) . Salmonella control programs in Denmark . Emerging Infectious Diseases , 9 : 774 – 780 .
- Yamame , Y , Leonard , JD , Kobatake , R , Awamura , N , Toyota , Y , Ohta , H , Otsuki , K and Inoue , T . (2000) . A case study on Salmonella enteritidis (SE) origin at three egg-laying farms and its control with an S. enteritidis bacterin . Avian Diseases , 44 : 519 – 526 .
- Zhang-Barber , L , Turner , AK and Barrow , PA . (1999) . Vaccination for control of Salmonella in poultry . Vaccine , 17 : 2538 – 2545 .